Abstract
Aim:
Inflammatory myeloid lineage cells mediate neotissue formation in tissue-engineered vascular grafts, but the molecular mechanism is not completely understood. We examined the role of vasculogenic PDGF-B in tissue-engineered vascular graft neotissue development.
Materials & methods:
Myeloid cell-specific PDGF-B knockout mice (PDGF-KO) were generated using bone marrow transplantation, and scaffolds were implanted as inferior vena cava interposition grafts in either PDGF-KO or wild-type mice.
Results:
After 2 weeks, grafts from PDGF-KO mice had more remaining scaffold polymer and less intimal neotissue development. Increased macrophage apoptosis, decreased smooth muscle cell proliferation and decreased collagen content was also observed.
Conclusion:
Myeloid cell-derived PDGF contributes to vascular neotissue formation by regulating macrophage apoptosis, smooth muscle cell proliferation and extracellular matrix deposition.
Keywords: : extracellular matrix, macrophage, myeloid cell, neotissue development, platelet-derived growth factor, scaffold degradation, smooth muscle cell, TEVG, tissue-engineered vascular graft
Progress in tissue engineering continues to promise a solution to the lack of tissue available for reconstructive and replacement surgery. Our lab has worked over the past several years developing a tissue-engineered vascular graft (TEVG) for use in congenital heart and vascular surgery. Traditionally used prosthetic materials in the form of Dacron® or Gore-Tex® pose significant risk for thromboembolic and infectious complications and also lack growth capacity, making them less ideal for use in the pediatric population. Our ability to create a TEVG with growth capacity [1,2] has therefore been an important advancement in the field, but graft stenosis continues to be a major limitation to widespread clinical application. We have therefore concentrated our efforts on understanding the underlying processes of TEVG neotissue formation and stenosis development.
We developed a murine model of TEVG stenosis [3] and found that seeding the grafts with bone marrow-derived mononuclear cells prior to implantation increases graft patency [4] in a dose responsive manner [5], but the seeded cells disappear shortly after implantation [6,7]. We thus hypothesized that a paracrine mechanism, rather than proliferation of seeded cells, was responsible for the remodeling of the TEVG and then demonstrated that host endothelial and smooth muscle cells from the adjacent vessel migrate to form the TEVG neotissue.
Host monocytes and macrophages were identified as key mediators of graft remodeling. They infiltrate the graft in large numbers shortly after implantation, and excessive infiltration leads to graft stenosis [8]. Macrophage depletion, using a liposomal formulation of the bisphosphonate clodronate, resulted in decreased graft stenosis, but also attenuated the formation of viable vascular neotissue [8]. While our work has demonstrated that monocytes and macrophages are key mediators of graft remodeling, the molecular mechanisms by which they elicit their response are not completely understood.
The PDGF family is comprised of four subtypes, including PDGF-A and -B, which form hetero and homodimers (-AA, -AB and -BB), as well as -C and -D, which only form homodimers (-CC and -DD), that bind to cell membrane tyrosine kinase receptors. PDGF is a potent regulator of angiogenesis, inflammation, and smooth muscle cell (SMC) migration and proliferation [9–12], and is produced by several cell types, macrophages and monocytes being a major source [13]. The PDGF-B subtype, specifically, has been shown to be important for vasculogenesis [12]. In this study we examine the role of myeloid cell-derived PDGF-B on graft performance in a chimeric mouse model to investigate the hypothesis that this growth factor contributes to the complex process of neotissue formation in the TEVG and may be a key modulator of TEVG stenosis.
Materials & methods
Animal ethics statement
All animals received humane care in compliance with the NIH Guide for the Care and Use of Laboratory Animals (2010). The Institutional Care and Use Committee at Yale University approved and monitored the use of all animals and procedures described herein. PDGF-B +/- heterozygote mice were a kind gift from Christer Betsholtz [14] and used to generate donor mice for all adoptive cell transfer procedures. 8- to 12-week old female C57BL/6 wild-type (WT) mice (n = 48) were purchased from Jackson Laboratories (ME, USA) and were the recipients of adoptive cell transfers and/or graft implantations.
Generation of myeloid cell-specific PDGF-B knockout
Breeding pairs of PDGF-B +/- heterozygote mice were used to generate timed pregnancies. At day E14 the pregnant mice were euthanized using carbon dioxide in an approved container. The uterine horns were removed and placed in a sterile tissue culture dish containing phosphate-buffered saline with 10% fetal bovine serum and 1% penicillin and streptomycin. Embryos were separated into the wells of a tissue culture plate and washed with buffer. The liver was dissected and moved to a new well. A tissue sample was reserved for genotyping. Liver tissue was homogenized using a pipette and passed through a cell strainer. Cells were resuspended in Iscove’s Modified Dulbecco’s Medium (Life Technologies, CA, USA) with 10% fetal bovine serum, and kept at 4°C.
The tissue samples were lyzed using Direct PCR reagent (Qiagen, Hilden, Germany) according to the manufacturer’s directions. A multiplexed PCR assay was used to genotype the tissue samples. A total of 25 μl of Choice taq Mastermix (Denville Scientific, MA, USA) was combined with 5 μM of the three PDGF-B primers, 2 μl of template and water to total 50 μl. The three primers were: 5′ TTT GAA GCG TGC AGA ATG CC 3′, 5′ GGA ACG GAT TTT GGA GGT AGT GTC 3′ and 5′ GGG TGG GAC TTT GGT GTA GAG AAG 3′. The PCR product was run on 1% agarose gel. The WT band migrated at 265 bp and the KO band migrated at 624 bp, corresponding to the neo cassette used to promote excision of the PDGFB gene.
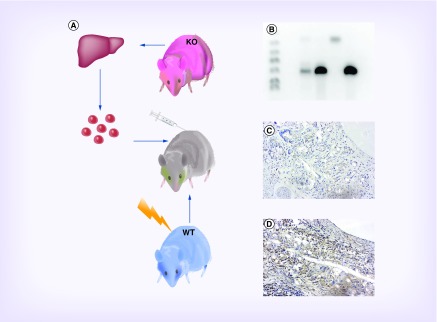
To generate the myeloid cell-specific PDGF-B knockout mice, recipient C57BL/6 mice received a lethal dose of 10 Gy x-ray radiation (Marietta model PXR 014). After irradiation, experimental mice received a tail vein injection of 1 × 106 -/- fetal liver cells from PDGF-B -/- donors (PGDF-KO), while the control group received 1 × 106 fetal liver cells from PDGF-B +/+ donors (WT). Mice were then allowed 4 weeks for reconstitution before implant surgery. Successful bone marrow transplantation was confirmed using PCR prior to implant (See Figure 1A for schematic).
Figure 1. . Development of PDGF knockout mouse.
Nonviable PDGF-B -/- mice were generated by breeding heterozygous PDGF knockout mice, then removing uterine horns from gravid mice and extracting pup livers. Adoptive transfer of homozygous PDGF-B -/- hematopoietic liver cells was performed via tail vein injection into lethally irradiated C57Bl/6 mice. (A) Multiplex PCR was used to genotype PDGF knockout mice prior to graft implantation. Lane 1: ladder; Lane 2: blank; Lane 3: ear of WT mouse after reconstitution demonstrating chimerism (products for neomycin cassette at 624 bp and PDGF-B at 265 bp); Lane 4: bone marrow of WT mouse after reconstitution demonstrating insertion of the neomycin cassette; Lane 5: negative control ear of WT mouse demonstrating lack of neomycin cassette and presence of PDGF-B; Lane 6: positive control liver from PDGF-B -/- indicating successful replacement of the PDGFB gene with a neomycin cassette. (B) Photomicrographs of immunohistochemical staining for PDGF-BB in explanted tissue-engineered vascular graft sections in PGDF-KO (C) and WT control (D) mice demonstrating an absence of PDGF-BB in PGDF-KO vascular neotissue. Scale bar = 50 μm.
KO: Knockout; WT: Wild-type.
Scaffold fabrication
Unseeded, biodegradable scaffolds were fabricated using a dual-chamber method as previously described [3,15]. In brief, a nonwoven polyglycolic acid felt (Biomedical Structures, RI, USA) was tubularized around a 19 G stainless steel needle and sealed with a 50:50 copolymer solution of ϵ-caprolactone and l-lactide (P[CL/LA]) (263.8 kD, Absorbable Polymers International, AL, USA). The constructs (length: 3.0 mm; inner diameter: 1.1 mm; outer diameter 1.45 mm; wall thickness 0.175 mm; Supplementary Figure 1) were snap frozen at -20°C for 30 min, lyophilized for 24 h and sterilized under UV light for 12 h prior to implantation.
Surgical implantation
Scaffolds were implanted as abdominal inferior vena cava interposition grafts using standard microsurgical technique as previously described [3,15]. Mice were administered a pre-anesthetic analgesic dose of ketoprofen (5 mg/kg, intraperitoneal) and were anesthetized with a cocktail of ketamine (100 mg/kg) and xylazine (10 mg/kg, intraperitoneal). After achieving a surgical plane of anesthesia, the abdomen was shaved, prepped, draped and opened via midline incision. A self-retaining retractor was placed and the intestines were eviscerated and wrapped in moist gauze. Blunt dissection exposed a 3.0 mm segment of the infrarenal inferior vena cava and vascular control was obtained. The inferior vena cava was cross clamped, divided, and scaffolds were implanted as interposition grafts via end-to-end anastomoses with running 10–0 nylon suture. Cross clamps were removed, hemostasis obtained, and the patency of the anastomoses evaluated. Intestines were returned to the abdomen, which was closed in two layers with 6–0 silk. Animals received routine postoperative care, including postoperative analgesia with Motrin® (30 mg/kg) in their drinking water for 2 days, and were maintained for the duration of the study without further therapeutic intervention.
Histology & immunohistochemistry
After 2 weeks, animals were euthanized by overdose cocktail of ketamine (300 mg/kg) and xylazine (30 mg/kg) followed by induction of pneumothorax. TEVGs were fixed via systemic perfusion with normal saline followed by 10% neutral-buffered formalin. Grafts were explanted and fixed overnight in neutral-buffered formalin at 4°C prior to dehydration, delipidation, paraffin embedding and serial sectioning (4 μm thick sections). Representative sections of each graft were stained with hematoxylin and eosin (H&E), Masson’s trichrome and Picro-sirius red following standard methods.
For immunohistochemical (IHC) analysis, slides were deparaffinized, rehydrated and blocked for endogenous peroxidase activity (0.3% H2O2 in MeOH) and nonspecific background staining (Background Sniper, BioCare Medical, CA, USA). Antigens were retrieved with the citrate buffer method (pH 6.0, 90°C) and slides were incubated overnight at 4°C with the following primary antibodies: rabbit anti-PDGF-BB (1:200, ab23914; Abcam, MA, USA), rabbit anti-CD31 (1:50, ab28364; Abcam), rabbit anti-vWF (1:750, A0082; Dako, CA, USA), rabbit anticollagen I (1:500, ab34710; Abcam), rabbit anticollagen III (1:500, ab7778; Abcam), rat anti-CD107b (MAC-3,1:75, 550292; BD Bioscience, CA, USA), rabbit anticalponin (1:200, ab46794; Abcam), mouse antismooth muscle actin (SMA; 1:500, M0851; Dako). Primary antibody binding was detected by subsequent incubation with species appropriate biotinylated IgG (Vector, CA, USA), followed by streptavidin-horse radish peroxidase (Vector) and chromogenic development with 3,3-diaminobenzidine (Vector). Nuclei were counterstained with Gill’s hematoxylin (Vector), and slides were dehydrated and cover slipped. For immunofluorescent analysis, slides were rehydrated and antigen retrieval was performed as described above. Sections were blocked for nonspecific background staining (3% normal goat serum) and incubated overnight at 4°C with additional primary antibodies including rabbit antiproliferating cell nuclear antigen (PCNA; 1:500, ab2426; Abcam) and rabbit anticleaved caspase-3 (Casp-3; 1:200, 96642; Cell Signaling Technology, MA, USA). Antibody binding was detected by incubation with cocktails of goat antimouse Alexa-Fluor® 647 (1:300; Life Technologies), goat antirat Alexa-Fluor® 594 (1:300; Life Technologies) and goat antirabbit Alexa-Fluor® 488 (1:300; Life Technologies) followed by nuclear counterstaining with 4,6-diamidino-2-phenylindole (DAPI; SlowFade® Gold antifade reagent with DAPI; Life Technologies). Photomicrographs were acquired with a Zeiss Axio Observer Z.1 microscope, 89-North PhotoFluor LM-75 light source with appropriate filters and a Zeiss Axiocam 503 (monochrome) or 105 (color) digital cameras.
Histomorphometry and semiquantitative immunohistochemistry
ImageJ (NIH, MD, USA) was used to quantify lumen area, neotissue area and TEVG area from low magnification (5×) images of H&E slides. Remaining scaffold polymers were identified with polarized light imaging of H&E sections and remaining polymer area was quantified with ImageJ. IHC stains were analyzed with ImageJ by conversion to the hue, saturation and lightness color space followed by pixel-specific thresholding to determine the cells or matrix constituents with positive staining. Reported area fractions correspond to the number of pixels satisfying the threshold requirements relative to the total number of pixels in the region of interest analyzed. Five high-powered field (HPF) images of one representative section from each animal were analyzed for every stain. Coincidence of positive immunolabeling and nuclear staining in fluorescent images was manually counted for each HPF.
Statistical analysis
Data are represented as mean ± standard error of the mean. Statistical significance was determined using unpaired, two-tailed Student’s t-test with Welch’s correction. Multiple t-tests were performed for multiple comparisons with Bonferroni–Holm correction. Fisher’s exact test was used for dichotomous variables with Bonferroni–Holm correction. Alpha was restricted to 0.05 for single comparisons. p-values of ≤0.05 were considered statistically significant. Statistical analyses were performed with GraphPad Prism 6.0, (GraphPad Software, CA, USA).
Results
Successful generation of PGDF-KO mice
A multiplexed PCR assay was used to confirm the success of the bone marrow transplantation (Figure 1B). Samples from KO donors and from WT recipients migrated as single bands at 624 and 265 bp, respectively. To confirm reconstitution, the bone marrow was isolated from a WT mouse that received an injection of KO cells after irradiation. Bands from reconstituted bone marrow samples migrated identically to those from KO donor tissue. An ear tissue sample from the same mouse showed both WT and KO bands, indicating successful generation of a chimera.
Surgical outcomes & adverse events analysis
Of the 50 mice that underwent implantation (n = 25/group), 96% (n = 48) survived to the 2-week study end point. One death occurred from each group, both within 24 h of graft implantation; these animals could not tolerate surgery due to cachexia secondary to incomplete immune reconstitution after irradiation and adoptive transfer. These animals were excluded from further analysis.
No difference observed in morphology between WT & PGDF-KO TEVGs
No differences in the cellular morphology of graft neotissue between PGDF-KO and WT mice were observed when comparing H&E stained slides (Figure 2 A–F). TEVGs from both groups demonstrated appropriate cellular infiltration within the scaffold architecture characterized by an abundance of monocytes, neutrophils, fibroblasts and macrophages including multinucleated foreign body giant cells. Luminal tissue was comprised of a laminated and circumferentially organized neomedia lined by a monolayer of endothelial cells (Supplementary Figure 2).
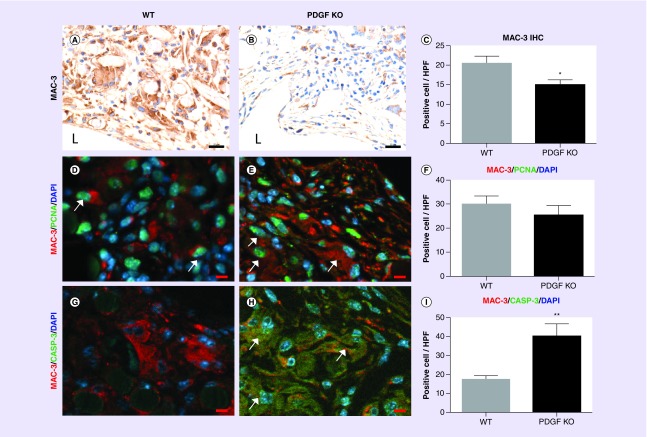
Figure 2. . Graft Histomorphometry.
Unseeded scaffolds were implanted in PDGF knockout mice and wild-type controls for 2 weeks. Representative hematoxylin and eosin stained tissue sections of stenotic (A, D) and patent (B, E) grafts from wild-type (A, B) and PGDF-KO (knockout) (D, E) mice (scale bar = 500 μm). High magnification images of representative patent TEVGs (tissue-engineered vascular grafts) from each group (C, F) demonstrate increased neointimal tissue formation in the WT mice (scale bar = 50 μm). Histomorphometry of all TEVG samples compared TEVG area, lumen area, remaining polymer area and neotissue area (G). PGDF-KO grafts were found to have greater TEVG area, more remaining polymer and reduced neotissue area compared with WT controls (H; *p < 0.05).
KO: Knockout; TEVG: Tissue-engineered vascular graft; WT: Wild-type.
PGDF-KO mice demonstrate reduced intimal neotissue formation
Of the mice that survived until the 2-week end point, 79% (n = 19) of PGDF-KO grafts were patent at time of explant versus 91% (n = 22) of grafts were taken from WT mice (Figure 2; p = 0.42). Similarly, no significant difference in lumen diameter was identified between the two groups (PGDF-KO: 0.64 mm2 vs WT: 0.76 mm2; p = 0.24; Figure 2H). Interestingly, the total TEVG area, extending from the border of the adventitia to the graft lumen, was greater in PGDF-KO versus WT grafts (p = 0.009; Figure 2H). To further explore this difference, the total remaining polymer area and the intimal neotissue area were both quantified. PGDF-KO mice demonstrated significantly more remaining polymer area (p = 0.01) and less neotissue area (p = 0.01, Figure 2H). These data suggest that myeloid cell-derived PDGF-B may impact both intimal proliferation and cell-mediated scaffold degradation.
PDGF-BB expression is reduced in TEVG neotissue of PGDF-KO mice
Graft sections were stained with an antibody against the PDGF-BB subtype to confirm that gene knockout effectively abrogated the expression of PDGF-B from myeloid cells at the protein level. Qualitative assessment confirms that PGDF-KO mice resulted in an obvious reduction of PGDF-BB expression in the neotissue of implanted grafts after 2 weeks (Figure 1C–D).
Macrophages from PGDF-KO mice experience increased apoptosis
To explore the potential causes of the observed difference in remaining polymer area, IHC characterization of macrophages was performed. Macrophages are the primary cell type involved in polymer degradation and are potent mediators of neovessel formation [8]. Quantification of Mac3 positive cells demonstrated significantly more macrophages in the grafts from WT compared with PGDF-KO mice (p = 0.028; Figure 3A–C). Immunofluorescent colabeling of Mac3 with PCNA, a marker of cell proliferation, or cleaved caspase-3, an apoptosis marker, was performed to investigate whether differences in cell turnover could explain the variance in macrophage residence within the TEVGs. No significant difference in the number of Mac3/PCNA double positive macrophages per HPF between WT and PGDF-KO samples (p = 0.39, Figure 3D–F) was found, suggesting myeloid-derived PDGF may not affect macrophage proliferation in the TEVG. However, PGDF-KO grafts were found to have significantly greater amounts of Mac3/caspase-3 double positive macrophages (p = 0.0041; Figure 3G–I), supporting an autocrine role for PDGF in mediation of macrophage apoptosis within the TEVG.
Figure 3. . Macrophage characterization.
Representative MAC-3 stained TEVG sections from WT (A) and PGDF-KO (B) mice (scale bar = 20 μm). Significantly more MAC-3+ macrophages/HPF were found in WT grafts (C; *p < 0.05). Immunofluorescent colabeling was used to identify proliferating (D, E) or apoptotic (G, H) MAC-3+ macrophages in WT (D, G) and PGDF-KO (E, H) graft sections. No significant difference in the number of proliferating macrophages/HPF was identified between the two groups (F), however, significantly more apoptotic macrophages/HPF were identified in PGDF-KO graft sections (I; **p < 0.005). White arrows identify representative double positive cells and ‘L’ denotes the graft’s lumen. D, E, G, H, scale bar = 10 μm.
HPF: High-powered field; IHC: Immunohistochemical; KO: Knockout; PCNA: Proliferating cell nuclear antigen; TEVG: Tissue-engineered vascular graft; WT: Wild-type.
Myeloid-derived PDGF regulates proliferation of vascular SMCs
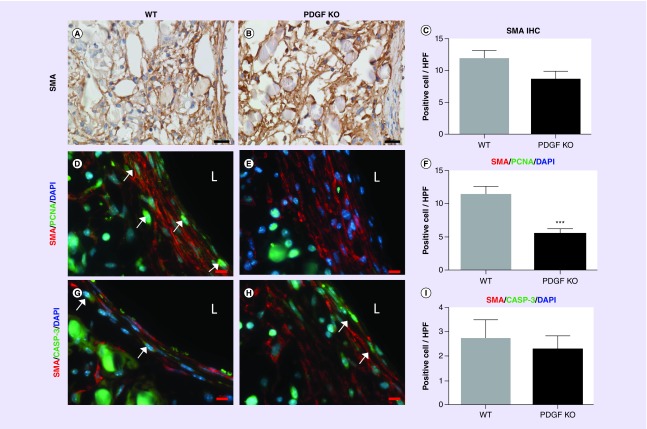
To explore the reduced neointimal tissue area observed in PGDF-KO grafts, SMCs, the primary component of neovessel media and intima, were identified and quantified. IHC staining for α-SMA positive SMCs within graft neotissue did not demonstrate any significant difference between the two groups (Figure 4A–C). In parallel with our macrophage analysis, we performed immunofluorescent colabeling of α-SMA and PCNA or caspase-3. The numbers of double positive α-SMA/PCNA cells per HPF were significantly decreased in the PGDF-KO group compared with WT controls (p = 0.0005; Figure 4D–F), indicating that myeloid-derived PDGF may have a proproliferative effect on vascular SMCs in the TEVG. No significant difference was found in the quantity of α-SMA/caspase-3 double positive cells between the two groups (p = 0.66; Figure 4G–I).
Figure 4. . Smooth muscle characterization.
Representative α-SMA (smooth muscle actin) stained sections from WT (A) and PGDF-KO (B) graft sections (scale bar = 20 μm); semiquantitative immunohistochemistry revealed no significant difference in positive area fraction between groups (C). Quantification of immunofluorescent colabeling of α-SMA/PCNA (D, E) and α-SMA/caspase-3 (G, H) in WT (D, G) and PGDF-KO (E, H) graft sections demonstrated significantly less proliferating α-SMA+ cells/HPF in the PGDF-KO mice (F; ***p < 0.0005). No difference was found in the number of apoptotic α-SMA+ cells/HPF (I). White arrows identify representative double positive cells from both experiments, and ‘L’ denotes the graft’s lumen. D, E, G, H, scale bar = 20 μm.
HPF: High-powered field; IHC: Immunohistochemical; KO: Knockout; PCNA: Proliferating cell nuclear antigen; SMA: Smooth muscle actin; WT: Wild-type.
Collagen deposition reduced in PGDF-KO TEVGs
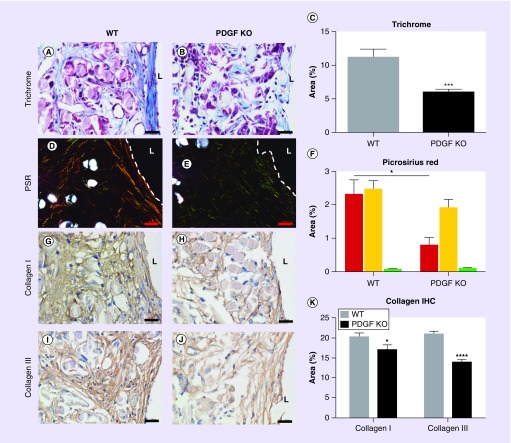
The evolving biomechanical properties of a TEVG are defined by the deposition, organization and maturation of extracellular matrix (ECM) constituents and reflect both macrophage and SMC function within the graft. Total collagen content was characterized by quantifying total positive area of Masson’s Trichrome staining. Significantly more collagen deposition in WT grafts compared with PGDF-KO grafts was observed (p = 0.0006; Figure 5A–C). To evaluate differences in mature (thick fibers) versus immature (thin fibers) collagen subtypes, picrosirius red staining was visualized under polarized light. WT grafts demonstrated significantly more thick (red) collagen fibers compared with those from PGDF-KO mice (p = 0.0067; Figure 5D–F). There was no significant difference in thin (green–yellow) collagen fibers. To confirm these data, collagen I (mature) and III (immature) content was assessed using IHC. Significantly more collagen I and III staining was present in WT compared with PGDF-KO grafts (collagen I, p = 0.047; collagen III, p = 0.0001; Figure 5G–K).
Figure 5. . Extracellular matrix characterization.
Masson’s Trichrome (A, B) and picrosirius red (D, E) stainings of WT (A, D) and PGDF-KO (B, E) were completed to characterize the extracellular matrix of explanted tissue-engineered vascular grafts. Computer-assisted image analysis revealed significantly less total collagen in PGDF-KO grafts (C; ***p < 0.0005) which was attributed to a significant difference in thick collagen content identified via polarized light microscopy of picrosirius red sections (F; *p < 0.05). These data were confirmed by immunohistochemical evaluation of mature collagen I (G, H) and immature collagen III (I, J) in WT (G, I) and PGDF-KO (H, J) graft sections. Tissue-engineered vascular grafts from PGDF-KO mice demonstrated a significant reduction in the amount of collagen I and III compared with WT controls (K; *p < 0.05, ****p < 0.0001). ‘L’ denotes the graft lumen, which is delineated in dark field images by a white dash. Scale bar = 20 μm.
IHC: Immunohistochemistry; KO: Knockout; PSR: Picro Sirius Red; TEVG: Tissue-engineered vascular graft; WT: Wild-type.
Discussion
By creating a chimeric mouse knockout model using previously described methods [16,17], we successfully disrupted the production of PDGF-B in myeloid lineage cells prior to implantation of a cell-free scaffold. Grafts taken from PGDF-KO mice demonstrate a larger total TEVG area, likely explained by a higher amount of remaining scaffold as well as a decreased neointimal area, and decreased ECM deposition after 2 weeks compared with WT controls. We attribute these differences to both autocrine and paracrine effects of PDGF-B on infiltrating macrophages and SMCs, respectively. These data provide novel insight into one potential molecular mediator of neotissue formation in TEVGs.
Degradation of the biodegradable polyglycolic acid/(P[CL/LA]) scaffold used in this study occurs by hydrolysis [18]. Monocytes and macrophages are among the earliest cell types to infiltrate the scaffold upon implantation, and are understood to be critical to the process of scaffold degradation and neotissue formation. The process by which macrophages enter the scaffold is dependent on several different factors, including graft characteristics, such as, porosity, pore size and fiber diameter, but also various cell signaling mechanisms which are poorly understood. By eliminating the production of PDGF-B from myeloid lineage cells, we sought to specifically probe the activity of PDGF-B in this cell type. Graft specimens from knockout mice demonstrated a decreased quantity of infiltrating Mac3-positive macrophages, which was supported by immunofluorescent colabeling with caspase-3, demonstrating increased macrophage apoptosis in the PGDF-KO group. Our findings suggest that PDGF-B secreted by myeloid lineage cells may have an autocrine role in maintenance of macrophage residence in the TEVG scaffold at 2 weeks. These data support further research to identify the kinetics of PDGF activity over the time course of neotissue formation, perhaps by using an inducible conditional knockout model. It is equally necessary to identify cofactors that synergize with PGDF during this time course to regulate or enhance its activity upon myeloid cells. Further, it may be that PDGF-B secretion from myeloid cells is specific to a particular macrophage phenotype. For example, some studies have demonstrated that macrophage and monocyte phenotype plays an important role in secretion of angiogenic molecules, and that their selective secretion at various time points may also be important. Spiller et al. demonstrated that M1 or ‘classically activated’ macrophages secrete VEGF to recruit endothelial cells and initiate angiogenesis, whereas alternatively activated macrophages secrete PDGF, causing pericyte migration and vessel maintenance [19]. Our next experiments are therefore aimed at applying FACS-based approaches to elucidate the autocrine effect of PDGF-B on monocyte and macrophage phenotype at varying times during neotissue formation.
Another significant finding was the decreased neotissue area in the PGDF-KO mice compared with controls. After the initial inflammatory phase incited by TEVG implantation, an endothelial lining forms along the graft lumen, and SMCs migrate from the adjacent vessel to populate the graft [20], forming what will eventually become the neointimal and neomedial layers of the vessel, respectively [6,15,21]. Interestingly, our results did not demonstrate any differences in the area fraction of α-SMA+ SMCs in 2-week specimens, but did identify decreased SMC proliferation in the PGDF-KO group. The decreased proliferation in PGDF-KO mice is consistent with literature demonstrating the paracrine role of PDGF in promoting SMC migration and proliferation [10,22–25]. One potential reason that no difference was observed in the total quantity of SMCs between the two groups is that we specifically targeted only the PDGF-B subtype. There are several other PDGF subtypes with varying biologic roles as well as other growth factors, such as TGF-β, that are known to regulate vascular SMC proliferation. PDGF-BB is the primary subtype involved in vasculogenesis [9,26,27], but PDGF-CC is also expressed in vascular SMCs in various organs [28] and plays a role in blood vessel protection and survival in models of vascular degeneration [29]. What role it may play in the development of TEVG neotissue, especially in the absence of PDGF-BB, is unclear. Nonetheless, the differences in neointimal area could be related to the decreased amount of collagen deposition in the PGDF-KO versus the WT TEVGs. While α-SMA staining is comparable between the two implant groups, the area fraction of both collagen I and III was decreased in the PGDF-KO compared with WT grafts. One hypothesis is that myeloid-derived PDGF stimulates vascular SMC and/or myofibroblast secretion of ECM constituents in the TEVG via a paracrine mechanism. Similarly, myeloid-derived PDGF could affect the rate of ECM turnover in TEVG neotissue. Further research to identify the relationship between ECM remodeling and PDGF-B signaling in the TEVG is thus warranted.
The current study demonstrated that the in vivo effects of myeloid cell-specific PDGF-B knockout was not able to significantly impact the incidence of stenosis compared with WT mice. One reason for this is simply that PDGF alone is unable to regulate the mechanisms that lead to critical stenosis. PDGF’s importance in angiogenesis and vascularization of tumors is well established [30–32], but the process of developing a living vessel from a biodegradable scaffold is likely multifactorial, involving a variety of cytokines, chemokines, and growth factors that act in concert at specific times to orchestrate the complex process of scaffold resorption and neotissue development. If we are operating under the assumption that stenosis occurs as a result of dysregulation or overactivity of the processes leading to neotissue formation, one would believe that disruption of PDGF signaling would play a significant role in decreasing stenosis given its proven role in vasculogenesis. Therefore, the data do again raise the question of whether other PDGF subtypes play a compensatory role in neotissue development in the absence of PDGF-B.
Our study is limited in that we performed a bone marrow transplant using disaggregated hematopoietic fetal PDGF-B -/- livers, therefore, our model is actually one in which all myeloid lineage cells (e.g., megakaryocytes, mast cells and neutrophils) do not produce PGDF, rather than one specific to macrophage-derived PDGF. However, our previous studies have demonstrated that monocytes and macrophages are the primary myeloid cell types inhabiting the neovessel at early time points. Our focus has mainly been on the role that monocytes and macrophages play in TEVG development, and we posit that the design of future studies may benefit from utilization of Cre/Lox conditional knockouts specific to one cell type. Finally, we evaluated grafts at 2-week postimplantation. Our previous work shows that if stenosis is to occur, it usually occurs by 2 weeks [8]. We chose the 2-week time point because the study was originally designed to test the effect of PDGF-B on graft stenosis, but our analysis is now limited to this singular time point in the natural history of neovessel formation. It is unclear what effects PDGF may have earlier in the development of TEVG neotissue, or what long-term effects may result from its depletion.
Conclusion
Here we demonstrate that myeloid cell-derived PDGF-B contributes to the inflammation-mediated process of vascular neotissue formation in both an autocrine and paracrine fashion by regulating macrophage apoptosis, SMC proliferation and ECM synthesis. We submit that this protein may be a useful target in combination with other signaling events during the time course of scaffold degradation and neovessel formation.
Future perspective
Congenital heart defects continue to be a leading cause of death in the newborn period. Tantamount to the successful treatment of these patients is our ability to generate new tissue for use in reconstructive surgical procedures. We currently possess the ability to create TEVGs with growth potential to be used as conduits in surgeries, such as the Fontan procedure [2,33], but the technology is still limited by stenosis development. An understanding of the molecular mechanisms driving neotissue formation in our graft will provide strategies to better direct this process, thereby abrogating the development of stenosis and leading to the development of a second-generation graft ready for widespread clinical application. Our current study identifies the growth factor, PDGF, as one important mediator in the development of TEVG neotissue. Disrupting the production of myeloid cell-derived PDGF-B was not in itself enough to eliminate stenosis in our mouse model, but identification of this molecule’s importance provides important insight into the way in which the various cell types that comprise the graft interact with the TEVG scaffold and with one another. It also provides important next steps in terms of the targeting of other PGDF subtypes for study as well as other molecules important to the process of vasculogenesis. Stenosis development, after all, is likely a multifactorial event, and an understanding of how these molecules interact together can allow targeted therapy against stenosis development and a functional vascular conduit with growth potential free from risk of stenosis, thrombosis or infection.
Summary points.
Comparison of cellular morphology & histomorphometry
Grafts taken from wild-type and PDGF-B knockout (PDGF-KO) mice demonstrated no difference in terms of cellular morphology, stenosis rates or lumen diameter.
Grafts taken from KO mice demonstrated lower amounts of intimal neotissue formation.
Grafts taken from KO mice demonstrated higher amount of remaining scaffold polymer.
Macrophage analysis & linkage to difference in remaining polymer
Following implantation of scaffold, macrophages invade and degrade polymer by hydrolysis.
Immunohistochemical analysis demonstrated lower amounts of macrophages in grafts taken from KO mice compared with wild-type controls.
Immunofluorescent analysis of grafts colabeled with Mac3 plus either PCNA for proliferation, or caspase-3 for apoptosis, showed a higher amount of apoptotic macrophages in grafts from KO mice, explaining the lower macrophage number in this group. The lower number leads to the decreased polymer degradation.
Smooth muscle cell analysis & linkage to differences in intimal neotissue
Following macrophage infiltration, smooth muscle cells (SMCs) invade the grafts, proliferate and form the intimal neotissue in tissue-engineered vascular grafts. Grafts taken from PGDF-KO mice demonstrated lower amounts of neotissue development.
Staining for SMC did not reveal an overall difference in number at the 2-week time phase, but the amount of SMCs undergoing active proliferation was lower in grafts taken from KO mice.
Extracellular matrix deposition
Analysis of extracellular matrix was performed to determine whether the differences in macrophage or SMCs translated to differences in extracellular matrix deposition. Grafts taken from KO mice showed less overall collagen deposition, as identified by Trichrome staining.
Collagen I and collagen III content was decreased in KO grafts compared with wild-type.
KO mice also demonstrated less mature collagen, as identified by picrosirius red staining.
Supplementary Material
Acknowledgements
The authors thank the Morphology Core at Nationwide Children’s Hospital for performing tissue processing, sectioning and histologic stainings.
Footnotes
Financial & competing interests disclosure
This work was supported by the NIH, grant #s R01 HL098228, R01 HL128847 and R01 HL128602. Research reported in this publication was also supported by the National Institutes of Allergy and Infectious Diseases of the NIH under Award Number T32AI106704. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH and T32AI106704. C Breuer receives research funding from Gunze Ltd. Breuer is on the scientific advisory board and receives funding from Cook Biomedical. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
All animals received humane care in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (2010). The Institutional Care and Use Committee at Yale University approved and monitored the use of all animals and procedures described in this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Patterson JT, Gilliland T, Maxfield MW, et al. Tissue-engineered vascular grafts for use in the treatment of congenital heart disease: from the bench to the clinic and back again. Regen. Med. 2012;7(3):409–419. doi: 10.2217/rme.12.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibino N, McGillicuddy E, Matsumura G, et al. Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 2010;139(2):431–436. 436–432. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]; •• Initial pilot study of tissue-engineered vascular graft (TEVG) implantation in Japanese cohort of 25 patients. Long-term results revealed graft stenosis to be major complication of TEVG implantation.
- 3.Lee Y-U, Yi T, Tara S, et al. Implantation of inferior vena cava interposition graft in mouse model. J. Vis. Exp. 2014;(88) doi: 10.3791/51632. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This video article explains the process of making the miniaturized scaffolds used by our group for murine experiments. Article also describes surgical implantation of scaffolds as inferior vena cava interposition grafts.
- 4.Mirensky TL, Hibino N, Sawh-Martinez RF, et al. Tissue-engineered vascular grafts: does cell seeding matter? J. Pediatr. Surg. 2010;45(6):1299–1305. doi: 10.1016/j.jpedsurg.2010.02.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Y-U, Mahler N, Best CA, et al. Rational design of an improved tissue-engineered vascular graft: determining the optimal cell dose and incubation time. Regen. Med. 2016;11(2):159–167. doi: 10.2217/rme.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roh JD, Sawh-Martinez R, Brennan MP, et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl Acad. Sci. USA. 2010;107(10):4669–4674. doi: 10.1073/pnas.0911465107. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the inflammatory cell-mediated process of TEVG development. Host monocytes are early responders to graft implantation, followed by infiltration by endothelial and smooth muscle cells.
- 7.Harrington JK, Chahboune H, Criscione JM, et al. Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J. 2011;25(12):4150–4161. doi: 10.1096/fj.11-185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibino N, Yi T, Duncan DR, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 2011;25(12):4253–4263. doi: 10.1096/fj.11-186585. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Using the bisphosphonate, clodronate, to deplete macrophages, Hibino et al. demonstrated the critical role of these cells in neotissue development. Grafts implanted in mice depletion of macrophages show decreased stenosis, but also have impaired neotissue development, as evidenced by the absence of endothelial cells, smooth muscle cells and collagen.
- 9.Battegay EJ, Rupp J, Iruela-Arispe L, Sage EH, Pech M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 1994;125(4):917–928. doi: 10.1083/jcb.125.4.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grotendorst GR, Seppä HE, Kleinman HK, Martin GR. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc. Natl Acad. Sci. USA. 1981;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Claesson-Welsh L. Platelet-derived growth factor receptor signals. J. Biol. Chem. 1994;269(51):32023–32026. [PubMed] [Google Scholar]
- 12.Hellström M, Kalén M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126(14):3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- 13.Badgett A, Bonner JC, Brody AR. Interferon-gamma modulates lung macrophage production of PDGF-BB and fibroblast growth. J. Lipid Mediat. Cell Signal. 1996;13(1):89–97. doi: 10.1016/0929-7855(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 14.Levéen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8(16):1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- 15.Roh JD, Nelson GN, Brennan MP, et al. Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials. 2008;29(10):1454–1463. doi: 10.1016/j.biomaterials.2007.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaminski WE, Lindahl P, Lin NL, et al. Basis of hematopoietic defects in platelet-derived growth factor (PDGF)-B and PDGF beta-receptor null mice. Blood. 2001;97(7):1990–1998. doi: 10.1182/blood.v97.7.1990. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer AJ, Tian W, Saucier-Sawyer JK, et al. The effect of inflammatory cell-derived MCP-1 loss on neuronal survival during chronic neuroinflammation. Biomaterials. 2014;35(25):6698–6706. doi: 10.1016/j.biomaterials.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In this article, Sawyer et al. describes the process by which an MCP-1 deficient mouse is created using the same bone marrow transplantation method that was used to create the myeloid cell-derived specific PDGF-B knockout in our current manuscript.
- 18.Naito Y, Williams-Fritze M, Duncan DR, et al. Characterization of the natural history of extracellular matrix production in tissue-engineered vascular grafts during neovessel formation. Cells Tissues Organs. 2012;195(1–2):60–72. doi: 10.1159/000331405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35(15):4477–4488. doi: 10.1016/j.biomaterials.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hibino N, Villalona G, Pietris N, et al. Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 2011;25(8):2731–2739. doi: 10.1096/fj.11-182246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brennan MP, Dardik A, Hibino N, et al. Tissue-engineered vascular grafts demonstrate evidence of growth and development when implanted in a juvenile animal model. Ann. Surg. 2008;248(3):370–377. doi: 10.1097/SLA.0b013e318184dcbd. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Brennan et al. describes the results of TEVG implantation in an ovine model. Grafts demonstrate neotissue development as well as growth capacity.
- 22.Morisaki N, Koyama N, Kawano M, et al. Human macrophages modulate the phenotype of cultured rabbit aortic smooth muscle cells through secretion of platelet-derived growth factor. Eur. J. Clin. Invest. 1992;22(7):461–468. doi: 10.1111/j.1365-2362.1992.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee KP, Lee K, Park W-H, Kim H, Hong H. Piperine inhibits platelet-derived growth factor-BB-induced proliferation and migration in vascular smooth muscle cells. J. Med. Food. 2015;18(2):208–215. doi: 10.1089/jmf.2014.3229. [DOI] [PubMed] [Google Scholar]
- 24.Cospedal R, Abedi H, Zachary I. Platelet-derived growth factor-BB (PDGF-BB) regulation of migration and focal adhesion kinase phosphorylation in rabbit aortic vascular smooth muscle cells: roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinases. Cardiovasc. Res. 1999;41(3):708–721. doi: 10.1016/s0008-6363(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee C-K, Lee HM, Kim HJ, et al. Syk contributes to PDGF-BB-mediated migration of rat aortic smooth muscle cells via MAPK pathways. Cardiovasc. Res. 2007;74(1):159–168. doi: 10.1016/j.cardiores.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Cospedal R, Abedi H, Zachary I. Platelet-derived growth factor-BB (PDGF-BB) regulation of migration and focal adhesion kinase phosphorylation in rabbit aortic vascular smooth muscle cells: roles of phosphatidylinositol 3-kinase and mitogen-activated protein kinases. Cardiovasc. Res. 1999;41(3):708–721. doi: 10.1016/s0008-6363(98)00232-6. [DOI] [PubMed] [Google Scholar]
- 27.Rolny C, Nilsson I, Magnusson P, et al. Platelet-derived growth factor receptor-beta promotes early endothelial cell differentiation. Blood. 2006;108(6):1877–1886. doi: 10.1182/blood-2006-04-014894. [DOI] [PubMed] [Google Scholar]
- 28.Fang L, Yan Y, Komuves LG, et al. PDGF C is a selective alpha platelet-derived growth factor receptor agonist that is highly expressed in platelet alpha granules and vascular smooth muscle. Arterioscler. Thromb. Vasc. Biol. 2004;24(4):787–792. doi: 10.1161/01.atv.0000120785.82268.8b. [DOI] [PubMed] [Google Scholar]
- 29.He C, Zhao C, Kumar A, et al. Vasoprotective effect of PDGF-CC mediated by HMOX1 rescues retinal degeneration. Proc. Natl Acad. Sci. USA. 2014;111(41):14806–14811. doi: 10.1073/pnas.1404140111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuge R, Kitadai Y, Shinagawa K, et al. mTOR and PDGF pathway blockade inhibits liver metastasis of colorectal cancer by modulating the tumor microenvironment. Am. J. Pathol. 2015;185(2):399–408. doi: 10.1016/j.ajpath.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Kuzmanov A, Hopfer U, Marti P, Meyer-Schaller N, Yilmaz M, Christofori G. LIM-homeobox gene 2 promotes tumor growth and metastasis by inducing autocrine and paracrine PDGF-B signaling. Mol. Oncol. 2014;8(2):401–416. doi: 10.1016/j.molonc.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Wang Z, Dai X, et al. Glioma initiating cells contribute to malignant transformation of host glial cells during tumor tissue remodeling via PDGF signaling. Cancer Lett. 2015;365(2):174–181. doi: 10.1016/j.canlet.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Shin’oka T, Matsumura G, Hibino N, et al. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 2005;129(6):1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.