Abstract
Purpose
To test the hypothesis that delayed dark adaptation in patients with macular degeneration is due to an excess of free unliganded opsin (apo-opsin) and a deficiency of the visual chromophore, 11-cis retinal, in rod outer segments.
Methods
A total of 50 human autopsy eyes were harvested from donors with and without macular degeneration within 2–24 hrs. postmortem. Protocols were developed which permitted dark adaptation of normal human eyes after death and enucleation. Biochemical methods of purifying rod outer segments were optimized and the concentration of rhodopsin and apo-opsin was measured with UV-visible scanning spectroscopy. The presence of apo-opsin was calculated by measuring the difference in the rhodopsin absorption spectra before and after the addition of 11-cis retinal.
Results
A total of 20 normal eyes and 16 eyes from donors with early, intermediate and advanced stages of macular degeneration were included in the final analysis. Dark adaptation was achieved by harvesting whole globes in low light, transferring into dark (light-proof) canisters and dissecting the globes using infrared light and image converters for visualization. Apo-opsin was readily detected in positive controls after the addition of 11-cis retinal. Normal autopsy eyes showed no evidence of apo-opsin. Eyes with macular degeneration also showed no evidence of apo-opsin, regardless of the severity of disease.
Conclusions
Methods have been developed to study dark adaptation in human autopsy eyes. Eyes with age-related macular degeneration do not show a deficiency of 11-cis retinal or an excess of apo-opsin within rod outer segments.
INTRODUCTION
DEFECTS IN VISUAL CHEMISTRY IN MACULAR DEGENERATION PATIENTS
Age-related macular degeneration is the most common cause of visual loss in patients over the age of 75 years in the United States and other developed countries. The etiology is multifactorial and is linked to environmental insults, cardiovascular disease and genetic polymorphisms involving the innate immune system.1–4 Ninety percent of patients suffer from the dry form of the disease, which is characterized by the accumulation of drusen, basal laminar and basal linear deposits, thickening of Bruch’s membrane, atrophy of retinal pigment epithelial cells and photoreceptor degeneration.1,5–7
Patients with macular degeneration describe numerous symptoms that point to abnormalities in the chemistry of vision. Poor night vision and transient scotomas, glare and light intolerance, loss of discrimination and resolution, visual illusions and hallucinations, are symptoms which fall into the categories of impaired dark adaptation,8 impaired light adaptation,9 abnormal synaptic remodeling,10 and disrupted feedback inhibition.11
Visual abnormalities caused by biochemical defects in the visual cycle and the visual transduction cascade have been described in detail using suction pipette recordings and ERGs in amphibians,12–14 primarily because of the larger size and accessibility of their photoreceptor cells, and in the mouse, whose genome can be genetically modified.15 However, the biochemical roadblocks which cause abnormalities in macular degeneration patients have not been identified. This is due to the lack of a good animal model of age-related macular degeneration that replicates the human condition and the inherent difficulty studying biochemical processes in living patients. Human autopsy tissues are available, but there are numerous challenges, including the lack of methodology for collecting and maintaining tissue in a viable state for study. In spite of these challenges, understanding the biochemical defects causing visual symptoms in patients with age-related macular degeneration could reveal important details about the pathophysiology and assist with the development of new therapies. For this reason, one of the goals of this proposal was to develop better methods for studying the biochemistry of vision in autopsy eyes from patients with macular degeneration.
DARK ADAPTATION AND MACULAR DEGENERATION
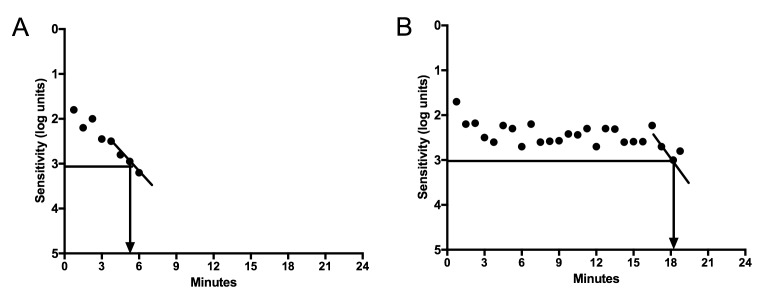
One of the earliest symptoms in patients with macular degeneration is difficulty adjusting to dim lighting after exposure to bright lights.16 This adjustment from bright to dark lighting conditions or dark adaptation requires the restoration of rod photoreceptor sensitivity, which is decreased in proportion to the extent of light exposure. While there is a normal delay in the ability of the healthy eye to recover vision after exposure to bright light, patients with macular degeneration have an excessive delay before they can adjust to low light conditions, on the order of 3–5 fold or even longer (Figure 1). In a normal healthy eye, the ability to regain a moderate level of visual sensitivity in darkness will occur within 6 minutes, whereas in a patient with macular degeneration, the time varies in proportion to the stage and severity of the disease.17 Delays more than 30 minutes are relatively common in patients with advanced disease.
FIGURE 1.
Dark Adaptation Curves using the AdaptDxTM dark adaptometer from patients without (A) and with macular degeneration (B). The time at which the scotopic vision recovers to a sensitivity of 3 log units [5×10−3 scot cd/m2] is 5.3 minutes in the normal eye and over 18 minutes in the eye with macular degeneration.
Why do patients with macular degeneration have prolonged delays in the time required to adjust to low light conditions? Restoring photoreceptor cell sensitivity requires regeneration of the visual pigment rhodopsin in the rod photoreceptor cells. This reaction occurs when the chromophore, 11-cis retinal, binds to apo-opsin in the outer segments of rod photoreceptor cells. The reason for the delay in dark adaptation in macular degeneration patients is not known, but is likely due to an abnormality in one or more of the pathways that govern the regeneration of rhodopsin: (1) Restocking the supplies of chromophore, 11-cis retinal, in the visual cycle, (2) Release and decay of the light-activated chromophore, all-trans retinal, (3) Turnoff of the active photopigment Meta II, and/or (4) Regeneration of the unphosphorylated form of rhodopsin. A brief review of the basic biochemistry of these three processes will be described in the following sections.
Human Visual Pigments
The human eye perceives light in the visible spectrum using four visual pigments. The visual pigments consist of a low light receptor, rhodopsin, which is located in the outer segments of rod photoreceptor cells, and three distinct cone pigments, red, green and blue, which reside in separate cone photoreceptor cells. The visual pigments are comprised of four related G protein-coupled receptors (GPCRs), known as opsins, and a single chromophore, 11-cis retinal, that binds to all opsins through a protonated Schiff base linkage. 18,19 Although the cone pigments use the same chromophore, 11-cis retinal, the absorption spectrum of each cone pigment is different and is determined by the interaction of 11-cis retinal with the unique amino acid sequences within the chromophore binding pocket.20 This feature, spectral tuning, allows each visual pigment to absorb light within a specific yet different range of wavelengths. Following light absorption, the pigments are described as “bleached”, a historical term which refers to the color change when the low light receptor, rhodopsin, changes from a deep red color to an orange and later pale yellow color.
11-cis retinal is unique as a ligand for GPCRs because it functions as an inverse agonist that blocks signaling through the G protein transducin in the fully dark-adapted state yet activates signaling following isomerization by light. The ability of 11-cis retinal to lock the visual pigments into an inactive state is a key mechanism for maintaining a high degree of visual sensitivity in the retina, particularly in rod photoreceptors, where they can respond to a single photon of light.12
The Visual Transduction Cascade
In the presence of light, 11-cis retinal isomerizes into all-trans retinal. In spite of the relatively small size of retinal, the isomerization reaction has significant structural consequences on the larger opsin moiety, which forms a rapid series of structurally related photo-intermediates that progress to the fully active structure known as meta-rhodopsin II (Meta II), and an inactive storage form, known as Meta III.21 Meta II binds a heterotrimeric G-protein transducin, leading to a catalytic exchange of GDP for GTP in the alpha subunit of transducin which activates the cyclic nucleotide phosphodiesterase (PDE6) [For reviews, see9,22]. The resulting effect is a decrease in free cGMP that leads to closure of the cGMP-gated ion channels in the plasma membrane, a decrease in intracellular Ca+2 levels and a decrease in the circulating dark current that controls neurotransmitter release at the photoreceptor synapse.
An important regulatory event in the visual transduction cascade is the rapid inactivation of the active photoproduct Meta II, which occurs either by formation of the inactive photoproduct Meta III21 or through an energy dependent process that involves ATP-mediated phosphorylation by rhodopsin kinase and secondary binding by arrestin.23
Shutting off the Visual Transduction Cascade
Like all GPCRs, the active state of Meta II rhodopsin is quickly quenched to turn off the signal. This regulatory mechanism attenuates the response so that rapid changes in the environment can be detected.24 Abnormalities in the reactions that quench Meta II and the reactions that reverse these changes will negatively impact the kinetics of dark adaptation.25,26 In the case of rhodopsin, Meta II is quenched by the phosphorylation of serine and threonine residues by rhodopsin kinase (Grk1) and subsequent arrestin (Arr1) binding.27–31 Mice that fail to quench active Meta II either due to impaired rhodopsin kinase activity or arrestin binding exhibit delayed dark adaptation kinetics.26,32 Likewise, conditions that fail to dephosphorylate rhodopsin will slow complete dark adaptation and decrease photoreceptor cell sensitivity.25
Stiles-Crawford and the Equivalent Background Intensity Theory of Dark Adaptation
In 1932, Stiles & Crawford proposed a theory of dark adaptation which equated the decrease in photosensitivity after bright light exposure, termed desensitization, to a steady background of light that was described as a luminous veil.33,34 This phenomenon of equivalent luminance and dark adaptation was studied using psychophysical methods in humans by multiple investigators.33–35 Various hypotheses were put forth to explain the cause of the background light response, including neural adaptation and a decrease in rhodopsin levels.36 Eventually, interest focused on a long lasting photoproduct of bleaching, a molecule which activates the visual transduction cascade at low levels until it decays.37–42 One of these putative photoproducts was free unliganded opsin or apo-opsin.
Constitutively Active Opsin
In the absence of bound 11-cis retinal, the apo-opsin molecule is constitutively active, capable of activating transducin at a low level in the absence of ligand and independent of light.43–45 Single cell electrophysiologic studies have demonstrated that bleached rods contain constitutively active apo-opsin which remains desensitized to light until the addition of 11-cis retinal regenerates rhodopsin.46,47 While the efficiency of visual transduction by apo-opsin is very low (10−6) compared to the activity of active meta-rhodopsin II46,48, the constitutive activity of significant amounts of apo-opsin will activate the visual transduction cascade to a sufficient degree to decrease the circulating current. This will cause the cell to behave as if it is light adapted, raising the rod threshold and decreasing night vision.48,49 Fain and Lisman originally proposed that constitutively active apo-opsin could cause the photoreceptor degeneration seen in severe cases of Vitamin A deficiency and certain forms of retinitis pigmentosa.37–39 This popular hypothesis gathered additional support when the RPE65−/− mouse, which contains unliganded opsin due to a secondary deficiency in 11-cis retinal, showed light adapted characteristics, with poor rod photosensitivity, decreased circulating current and secondary photoreceptor cell degeneration.49
11-cis Retinal and The Visual Cycle
Dark adaptation requires restoring rod photoreceptor cell sensitivity to low levels of light. This process is dependent on multiple biochemical steps needed to reestablish the dark current. Equally important, this process is also dependent on the regeneration of rhodopsin, which requires a new supply of 11-cis retinal from the visual cycle (see reviews50,51). The synthesis and recycling of 11-cis retinal is a highly cooperative process involving multiple enzymes and transporters in both the photoreceptor cell and the RPE cell. After rhodopsin is activated by light to form meta-rhodopsin II, all-trans retinal is released from the retinal binding pocket of opsin into the intradiscal space where it binds to a retina-specific ATP binding cassette transporter (ABCR)52,53 that transports the bound retinoid across the disc membrane into the cytoplasmic space, where it is reduced to all-trans retinol (vitamin A) by all-trans retinol dehydrogenase (RDH12).54 It is then transported to the RPE55,56 where it is esterified by lecithin retinol acyltransferase 57 to form all-trans retinyl esters, an important storage form for vitamin A.58 In the next step, 11-cis retinol is released from all-trans retinyl esters by isomerization and hydrolysis by RPE65.59–62 11-cis retinol is oxidized to the chromophore 11-cis retinal by retinol dehydrogenase 5 and then transported back to the photoreceptor cell to combine with apo-opsin and regenerate rhodopsin. Mutations in specific genes involved in the processing and transport of retinoids between the photoreceptors and RPE disrupts the visual cycle, leading to numerous retinal degenerations, including retinitis pigmentosa, congenital stationary night blindness, Leber’s congenital amaurosis, retinitis punctate albescens and Stargardt’s disease (see review63).
VITAMIN A DEFICIENCY-HISTORICAL OVERVIEW AND CLINICAL INSIGHTS
To understand the potential relationship between impaired dark adaptation, 11-cis retinal insufficiency and macular degeneration, it is useful to review the studies of Vitamin A deficiency and the visual defects, pathophysiology and therapeutic insights that are relevant to this thesis.
George Wald, John Dowling and others described the pathologic and functional consequences of vitamin A deficiency in mice, rats and cats. 64,65 Using animals kept in cyclic light, they showed that abnormal dark adaptation did not develop until vitamin A stores were significantly depleted from the liver. Thereafter, abnormal ERG changes progressed rapidly. Within two months of vitamin A deficiency, rhodopsin declined to 2% of normal levels. Apo-opsin could be measured in retinal extracts for the next 2–3 weeks. Thereafter, progressive outer segment degeneration occurred and the structural integrity of the outer retina was permanently damaged.64,66 By six months of vitamin A deficiency, complete outer segment degeneration and significant inner segment degeneration occurred and by ten months, there was extensive, permanent retinal degeneration.64 In addition, the mortality rate of vitamin A deficient animals was high, as the majority of mice died within 9–10 months.
Noell reported that the functional effects of vitamin A deficiency directly correlated with the lighting conditions in the animal facility.67 He showed that vitamin A deficient rats would maintain normal ERGs and normal rhodopsin levels for 5–6 months if the rats were maintained in complete darkness, whereas those exposed to cyclic light would progress to severe retinal degeneration in the same time frame. These studies support the idea that occupancy of the retinal binding pocket in rhodopsin is an important requirement for normal photoreceptor function and survival.
Important clinical insights have been gained from examining the outcomes of vitamin A supplementation in animals with vitamin A deficiency. Animals supplemented during the early stages of deficiency are capable of regenerating outer segments and recovering normal ERGs. However, by the time inner segment degeneration begins, the recovery of the outer nuclear layer is limited. Photoreceptor outer segments can regenerate from surviving cells, but the thickness of the outer nuclear layer cannot be restored to normal if outer nuclear thickness has already decreased.
These findings explain the variable recovery seen in patients with vitamin A deficiency. While some patients with mild vitamin A deficiency recover completely, others recover only partially. The recovery appears to be related to the underlying structural integrity of the photoreceptors before the replacement therapy is initiated, just as the restoration of normal ERG thresholds and structural morphology in the animal models correlate with the severity of photoreceptor degeneration that occurs before vitamin A is restored to the diet.64,65,68
In summary, the historical observational studies with vitamin A deficient animals indicates that the lack of 11-cis retinal leads to prolonged dark adaptation, abnormal photoreceptor cell morphology and ultimately irreversible photoreceptor degeneration. The correlation between delayed dark adaptation, 11-cis retinal deficiency and photoreceptor degeneration supports the hypothesis that deficiencies in 11-cis retinal could be related to the pathologic and functional defects seen in patients with macular degeneration.
Animal Models with 11-cis Retinal Deficiency
Mouse models with genetic mutations have provided opportunities to study the mechanisms that lead to visual impairment and photoreceptor degeneration in the setting of 11-cis retinal deficiency. The RPE65(−/−) mouse does not synthesize 11-cis retinal, and the photoreceptors have an excess of unliganded opsins. In the absence of 11-cis retinal, the opsins in the rod photoreceptors are constitutively active, stimulating the visual transduction cascade at levels which mimics the effect of low light.43–45,49 This model develops photoreceptor degeneration and demonstrates that occupancy of the retinal binding pocket with 11-cis retinal is important for normal photoreceptor cell survival.69,70
Certain genetic models of retinitis pigmentosa contain mutations in the retinal binding pocket of opsin that lead to constitutive activity of the phototransduction cascade.48,71–73 The most notable examples are the rhodopsin mutations at Lys-296 and Glu-113, the amino acids that bind to 11-cis retinal to form the protonated Schiff base and the salt bridge which locks rhodopsin into an inactive configuration. Mutations at either of these sites disrupt normal binding, leading to rhodopsin structures that are highly constitutively active.71,74 Mice harboring the constitutively active K296E mutation show severe photoreceptor degeneration as a result of mistrafficking of rhodopsin, which is heavily phosphorylated and bound to arrestin.73
Human Diseases Associated with 11-cis retinal Deficiency
A deficiency in 11-cis retinal has been linked to a number of human retinal diseases associated with dark adaptation defects, including vitamin A deficiency from liver disease, colon resections or dietary nutritional shortages and mutations in genes involved in the visual cycle such as RPE65, retinol dehydrogenases (RDH5 & 12) and cellular retinaldehyde binding protein (CRALBP).
Mutations in the RPE65 gene have pointed toward the importance of constitutively active opsin as a mechanism associated with retinal disease. RPE65 mutations block 11-cis retinal synthesis by inhibiting the isomerization and hydrolysis of all-trans retinyl esters into 11-cis retinol, which results in an excess of constitutively active opsin49 and a severe form of childhood retinal degeneration, known as Leber’s Congenital Amaurosis (LCA).75 The strong relationship between the retinoid deficiency and retinal degeneration has been demonstrated by the visual improvement in patients whose supply of 11-cis retinal has been replaced by either gene therapy76 or by oral replacement of 9-cis retinyl acetate, a prodrug that is converted into 9-cis retinal by the RPE cell.77
Less severe disease causing delayed dark adaptation in the setting of 11-cis retinal deficiency is seen in patients with congenital stationary night blindness, a condition caused by mutations in retinol dehydrogenase 5 (RDH5). These patients have pronounced defects in dark adaptation due to a roadblock in the conversion of 11-cis retinol to 11-cis retinal in the RPE.78 In these cases the photoreceptors are preserved, and there is no significant retinal degeneration. It has been suggested that other enzymes in the RPE will compensate for the loss of RDH5, although at a much slower rate. Meanwhile, patients with RDH12 mutations accumulate excess all-trans retinal in the photoreceptor as a result of decreased reduction of all-trans retinal to all-trans retinol (vitamin A). This defect slows down the visual cycle, reduces recycling of 11-cis retinal and leads to severe retinal degenerations at an early age.79
Another condition causing night blindness from decreased 11-cis retinal stores is found in patients with retinitis punctate albescens. This condition is caused by mutations in cellular retinaldehyde binding protein (CRALBP),80,81 which delivers 11-cis retinol in the RPE to retinal dehydrogenase for oxidation to 11-cis retinal.82,83 These patients have minimal rod function but are able to dark-adapt following prolonged time periods.
HYPOTHESIS
In this thesis, we test the hypothesis that an excess of apo-opsin is present within the rod outer segments of patients with macular degeneration as a result of a deficiency of 11-cis retinal. The hypothesis explains many of the visual symptoms that are described by our patients, including prolonged dark adaptation and transient scotomas on awakening. We reason that the reduced macular sensitivity84 is consistent with a moderate level of “background noise”, which could be attributed to the constitutive activity of apo-opsin in the photoreceptor cells, as originally proposed for cases of vitamin A deficiency and certain forms of retinitis pigmentosa.6,37–39,41,42 We also predict that the rod photoreceptors in macular degeneration patients might follow a similar outcome as the photoreceptors in Vitamin A deficient animals and the RPE65 (−/−) mouse in which the deficiency of 11-cis retinal leads to photoreceptor degeneration.49
To pursue this hypothesis, we (1) developed methods of dark adapting normal human autopsy eyes, (2) refined methods to isolate rod outer segments from a single human retina and (3) measured the levels of apo-opsin in human retinal extracts by regenerating rhodopsin with exogenous 11-cis retinal.
MATERIALS AND METHODS
REAGENTS
Frozen bovine retinas were obtained from W.L. Lawson LLC (Omaha, NE). 11-cis retinal was a kind gift from Dr. Rosalie Crouch (Medical University of South Carolina, Charleston, SC) and the National Eye Institute. Hydroxylamine hydrochloride, fatty-acid free bovine serum albumin and dithiothreital (DTT) was purchased from Sigma-Aldrich, Inc. (Saint Louis, MO). n-dodecyl-β-D-maltopyranoside, (DM) Anagrade, was purchased from Affymetrix Inc. (Maumee, OH). Protease inhibitor cocktail tablets were purchased from Roche.
COLLECTION OF HUMAN AUTOPSY EYES
A waiver from full IRB review was obtained from the Scripps Office for the Protection of Research Subjects for the collection of human autopsy eyes. The donor tissue was obtained from the San Diego Eye Bank with the consent of the patient’s family and was consistent with the tenets of the Declaration of Helsinki. The tissues were de-identified to maintain patient confidentiality and recorded by eye bank number. Eye bank technicians recorded past medical and ocular history from family members, specifically any details about macular degeneration, injections and smoking history.
BIOCHEMICAL ISOLATION AND PURIFICATION OF BOVINE ROS
Bovine rod outer segments containing rhodopsin were purified in milligram quantities from frozen bovine retinas and used as a positive control for the experiments and methodology outlined in this proposal. Bovine rod outer segment purity was consistently high with 280nm/498nm absorbance ratios <2.5, which is indicative of the bulk of the total protein being rhodopsin. The methods for purifying ROS, measuring rhodopsin concentration, bleaching and regeneration were similar to the standard techniques used for bovine rhodopsin.85
Purification of Dark-Adapted Bovine ROS
Bovine rhodopsin was obtained from purified bovine rod outer segments, isolated according to a modification of the sucrose flotation technique described by Papermaster.85
Frozen aliquots of 50 bovine retinas were thawed under dim indirect red light (GBX-2 Kodak safe light filter with 15 watt bulb) at 4°C. The outer segments were mechanically dislodged in 75 ml of cold 47% (w/w) sucrose in Buffer A (20mM Tris HCl (pH 7.4), 1 mM CaCl2, 2mM DTT), supplemented with protease inhibitors. The slurry was transferred into tubes and centrifuged at 17,500 rpm in a SS34 rotor for 20 minutes. The lipid rich rod outer segments were collected from the surface and diluted with 100 ml Buffer A. The sample was pelleted at 13,500 rpm in a SS34 rotor at 4°C. The pellet was resuspended in Buffer A and loaded over a discontinuous sucrose gradient (30% (w/w), 25% (w/w)), and centrifuged at 25,000 rpm for 30 minutes at 4°C in an SW28 rotor. The heavy ROS band was removed from the 25–30% sucrose interface and diluted 1:1 with 80 ml of Buffer A. The sample was spun at 17,500 rpm in an SS34 rotor for 10 min at 4°C and rinsed with 50 ml of 10mM Tris HCl, (pH7.4), 100 mM NaCl, 5 mM MgCl2, 2mM DTT. The pellets were rinsed twice in 50 ml 10mM Tris HCl (pH 7.4), 0.1 mM EDTA and centrifuged for 20 mins at 17,500 rpm in an SS34 rotor. The rod outer segments (ROS) were stored in 10mM Tris HCl (pH 7.4), 0.1 mM EDTA at −80°C.
Measurement of Rhodopsin Concentration
Rod outer segments were solubilized in the presence of 0.05% DM and the spectrum of rhodopsin was recorded using a PerkinElmer Lambda 18 UV-Visible scanning spectrometer. The difference in the absorbance spectrum at 498nm was measured in the presence of 20 mM NH2OH (pH 6.5) after illumination with a yellow light from a high intensity Halogen light source (300 watt) equipped with a 520nm cut-off filter. The calculation of rhodopsin concentration was based on Beer’s law with the rhodopsin extinction coefficient of 40,600 M−1 cm−1.86 The purity of the rhodopsin was calculated from the 280/498 ratio.
Preparation of Apo-opsin from Bovine ROS
Apo-opsin was prepared from native rhodopsin by bleaching with yellow light from a high intensity Halogen light source (300 watt) equipped with a 520nm cut-off filter for 15 min at 4°C in Hanks Balanced Salt Solution (HBSS) containing 20 mM NH2OH (pH 6.5). The outer segment membranes were washed three times in 2.5% fatty acid free bovine serum albumin (BSA) at 4°C in HBSS to remove retinal oximes (Beckman Optima TLX, 30,000 g, 20 min), followed by three washes in HBSS to remove the BSA. The final pellet was resuspended to the original volume in buffer. Opsin concentration was determined after incubation with excess 11-cis retinal to regenerate rhodopsin.
Regeneration of Rhodopsin and Measurement of Apo-opsin
Rhodopsin was regenerated from apo-opsin by adding an excess of 11-cis retinal from a 100% ethanol stock, maintaining the final ethanol concentration at or below 1.0%. Regeneration was monitored over 15–20 minutes using scanning spectrophotometry and the difference between the initial absorption of rhodopsin and the final absorption was used to calculate the concentration of apo-opsin in the sample using Beer’s law and the rhodopsin extinction coefficient of 40,600 M−1 cm−1.86
COLLECTION OF HUMAN AUTOPSY EYES AND EXTRACTION OF DARK-ADAPTED HUMAN ROS
Retinal Dissection
Whole globes were enucleated within an average of 8.6 hr (+/− 5.5 hr) after death, (median 7.0 hr, range 2–20 hrs, death to preservation) and placed into dark canisters. The whole globes were stored in light-proof stainless steel canisters in a cooler with wet ice during transportation to the laboratory. The globes were dissected at room temperature and the retinal extracts were kept at 4° C during processing. The globe dissection was performed under infrared lighting using infrared image converters to eliminate any chance of bleaching rhodopsin with the moderate-high intensity red light that was needed to visualize the retina well enough to dissect it from the RPE. The globe was oriented using the medial rectus muscle insertion as a landmark and the anterior segments were removed at the pars plana using a sharp surgical scissors. The posterior pole was oriented to localize the optic nerve head and the cortical vitreous was removed with a pipet tip. A small peripheral retinal detachment was created at the pars plana using suction and blunt forceps and extended circumferentially to create a complete retinal detachment and separate the retina from the retinal pigmented epithelium. The retina was excised over the optic nerve head with Wescott scissors and placed into a plastic 15 ml cup for extraction and/or purification of rod outer segments. The dissection was performed as carefully as possible to reduce artifacts on the surface of the underlying RPE. The freed retina was removed with suction directed over the optic nerve head.
Extraction and Purification of Human Rod Outer Segments
Several protocols were used to extract rod outer segments. All procedures were performed using infrared lighting and infrared image converters. Retinal extracts were prepared from single eyes.
The first protocol for isolating human rod outer segments was based on the same protocol used for bovine rod outer segment purification,85 previously described.
In other studies, rhodopsin was extracted using a simplified method of detergent extraction.87 In this protocol, the entire retina was placed into a plastic beaker with 5 ml H2O and stirred inside dark (light-proof) stainless steel canisters which were positioned over a magnetic stirrer in the cold room at 4°C. 10.6 nmoles of 11-cis retinal was added to the beaker containing the right retina in each pair of eyes. The left retina was extracted without the addition of 11-cis retinal. After 45 min, the retina was transferred to a centrifuge tube and spun at 15,000 rpm in an SS34 rotor at 4°C. The supernatant was removed and the pellet was extracted on ice with 1.5% DM for 60 min. The extract was spun at 15,000 rpm in an SS34 rotor at 4°C to pellet insoluble debris. The supernatants were collected and scanned to calculate the concentration of rhodopsin in the extract. The difference between the final yield in the right eye (+11-cis retinal) was compared with the left eye (minus 11-cis retinal) to determine whether the procurement protocol resulted in complete dark adaptation.
In other studies, rod outer segments were purified in suspension, without detergent, over discontinuous sucrose gradients. This protocol was a slightly different modification of Papermaster85 than the procedure used for bovine ROS and was based on a series of low speed spins at 4,000–6000 rpm in a Sorvall SS34 rotor using 30% sucrose as the initial extraction solution. Subsequent to collecting the supernatants from the spins, the suspension was placed over a discontinuous sucrose gradient and spun in an ultracentrifuge using the same procedure outlined for bovine ROS. The purification was optimized using several different ultracentrifuge tubes and rotors, before the optimal purity was obtained using the SW41 Ti, swinging bucket rotor.
Methods of Grading Autopsy Eyes
The stage of macular degeneration was evaluated after the retina had been removed in the dark. The remaining eye cup was transferred to the dissecting microscope in room light where the retinal pigment epithelium was analyzed for abnormalities, including depigmentation and hyperpigmentation; drusen, geographic atrophy, subretinal fibrosis, scarring and choroidal neovascularization. Hemorrhage associated with choroidal neovascularization was noted.
RESULTS
EXTRACTION AND PURIFICATION OF HUMAN ROD OUTER SEGMENTS
In spite of having optimized our techniques for purifying bovine rod outer segments in suspension with a high degree of success, the purification of human ROS from a single retina was challenging. Our initial protocol relied on homogenization techniques described in the literature for bovine eyes.85 However, the suspensions of human ROS were impure and turbid, which led to excessive light scattering that masked the absorption differences at 498nm before and after bleaching. In other cases, the small amount of visual pigment, which typically ranged from 4–7 nanomoles, was lost in the course of multiple spins and extractions that were used during the purification protocol. In some cases, the pigment was partially bleached during tissue harvesting. We found that even limited exposure to direct red light during dissection partially bleached the visual pigment extracted from a single retina.
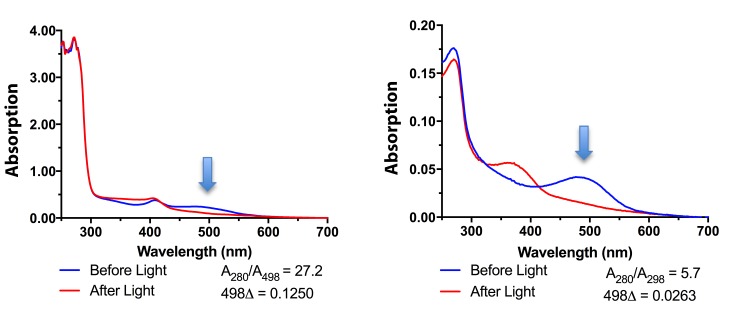
To address these challenges, we switched to infrared light and used infrared image converters to avoid all visible light. We also used the differential centrifugation and detergent extraction technique reported by Fulton87–89. This method eliminated the problems from light scattering as small contaminating lipid vesicles were reduced. Next, we eliminated the detergent extraction and combined the differential centrifugation spins with a modified sucrose gradient to achieve a high purity of human ROS in suspension. Figure 2 demonstrates a comparison of the absorption spectrums for human rhodopsin from suspensions of rod outer segments prepared using differential centrifugation without (left panel) and with a discontinuous sucrose gradient (right panel). The purity of rhodopsin in human rod outer segments improved by 500% as the A280/A498 ratios decreased to 5.7.
FIGURE 2.
Purification of human rod outer segments over discontinuous sucrose gradients. Left: Absorption spectra of human rhodopsin in rod outer segments before sucrose gradient purification. The presence of rhodopsin is indicated by the difference in the absorption reading at 498 nm before and after light exposure. The A280/A498 ratio indicates the sample is still impure. Right: Absorption spectra of human rhodopsin in rod outer segments after discontinuous sucrose gradient purification. The A280/A498 ratio indicates a higher degree of purity.
TECHNIQUES FOR DARK ADAPTING HUMAN AUTOPSY EYES
To accomplish the goals of this study, we first needed to establish methods to achieve complete dark-adaptation of human autopsy eyes from normal donors. This protocol was essential to conclude that any apo-opsin which we detected in the retinal extracts of macular degeneration eyes could be attributed to the pathophysiology of disease, as opposed to bleaching artifacts from light exposure during harvest.
We started with techniques that were published by previous investigators who showed that human retinas isolated from enucleated eyes after death were capable of dark-adaptation.87–90 These investigators demonstrated that human ROS were capable of complete rhodopsin regeneration by adding exogenous 11-cis retinal to extracts that had only partially dark-adapted. We started with the same conditions used by previous investigators, established baseline levels for rhodopsin and apo-opsin in our autopsy eye tissue and refined the protocol to achieve complete dark adaptation.
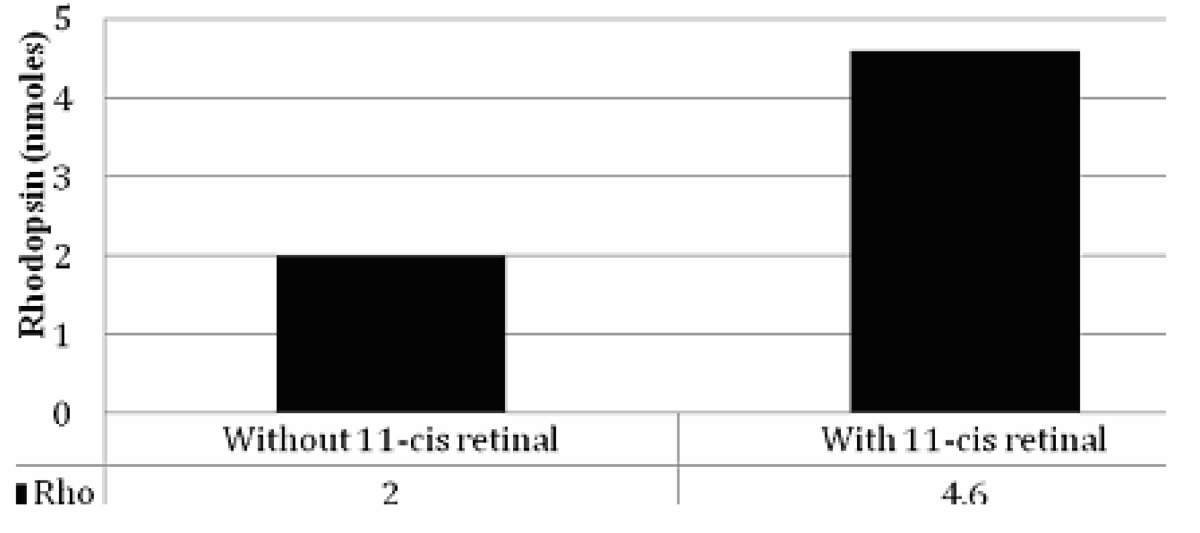
To establish an initial baseline, we obtained a pair of human autopsy eyes that were harvested in standard lighting conditions 5 hours after death and transported to the eye bank in a cooler on ice. We purified rod outer segments in the absence and presence of 11-cis retinal to determine how much dark-adapted pigment was present with the standard protocols used by previous investigators.87–90
Table 1 shows a comparison between the rhodopsin yields in the two eyes. We recovered 2 nmoles from the left eye, a relatively low yield, but one that was not unexpected since the autopsy eyes had been exposed to light during procurement and transportation. In comparison, we recovered 4.6 nmoles from the left eye, which was processed in the presence of exogenous 11-cis retinal to test for light exposure. The retina supplemented with 11-cis retinal contained 2.3 fold more rhodopsin, an increase that was likely due to the regeneration of rhodopsin from apo-opsin during the incubation with 11-cis retinal. This finding supported our initial expectations that the standard eye bank processing conditions were unlikely to provide human retinas that were fully dark-adapted.
TABLE 1.
RHODOPSIN RECOVERY FROM EYES HARVESTED IN LIGHT
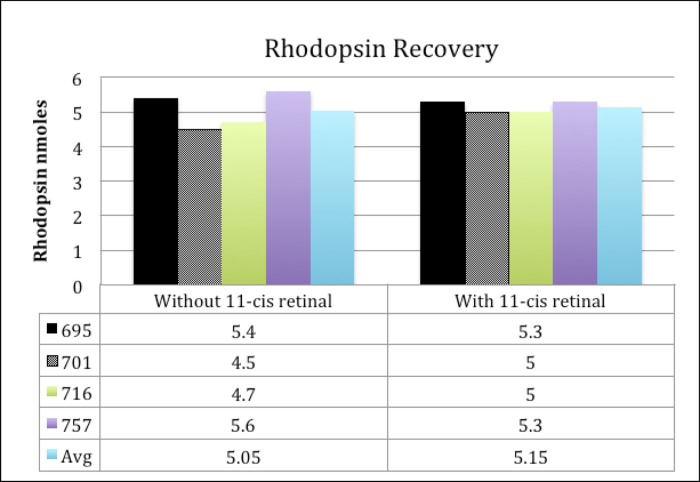
To address this problem, we requested that the eye bank personnel harvest the globes in low light conditions and immediately place the eyes into dark (light-proof) canisters. We expected the human globes would have sufficient metabolic activity to dark adapt within several hours of death, as shown for bovine retinas which dark adapt for up to four hours after enucleation.91 Eye bank technicians were advised not to shine a penlight on the eye to assess for the phakic status before enucleation and to transfer the globes directly into the dark canisters at the bedside instead of first transporting the globes back to the eye bank per their routine. This modified harvesting protocol was used with the next four pairs of eyes. Each pair of retinas was extracted with and without excess 11-cis retinal using infrared light to test for complete dark adaptation. Figure 3 shows a comparison between the rhodopsin yields in this series. In contrast to the yield with the standard harvesting conditions, the rhodopsin recovery in the modified technique was consistently high, averaging 5.05–5.15 nmoles/eye. In addition, there was no major difference in the recovery of rhodopsin between the right and left eyes, suggesting the visual pigment was fully dark-adapted.
FIGURE 3.
Rhodopsin recovery from eyes harvested using dark canisters. Four pairs of autopsy eyes (Donor # 695, 701, 716, 757) were harvested in low light conditions, immediately placed into dark canisters and extracted without (solid line) and with (dashed line) exogenous 11-cis retinal.
We concluded that dark adaptation was possible in postmortem human autopsy eyes using this harvesting method. We used this modified protocol for all the studies designed to measure apo-opsin in retinas from patients with and without macular degeneration
DETECTION OF APO-OPSIN IN HUMAN RETINA EXRACTS
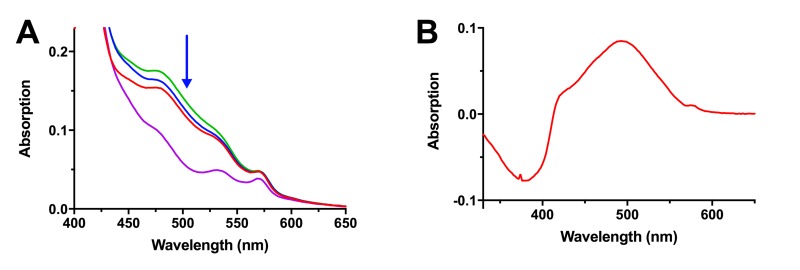
To further validate that we could detect apo-opsin in human rod outer segments, we prepared a retina extract from a dark-adapted eye that was exposed to a bright flash of light before dissection. This retina served as another positive control for apo-opsin. Figure 4 shows the spectral curve of rhodopsin in this sample before and during a 20 minute incubation with 11-cis retinal. The baseline spectrum is shown in red. Rhodopsin regeneration from apo-opsin is indicated by the progressive increase in absorption at 498 nm at 2 minutes (blue line) and 20 minutes (green line). The bleached spectrum, post-light exposure, was used to calculate the difference spectrum (purple line), which represents the spectral curve of rhodopsin. The presence of apo-opsin in this extract is demonstrated by the increase in absorption at 498 nm as opsin binds to 11-cis retinal to form rhodopsin.
FIGURE 4.
Detection of Apo-opsin in Human Retina Extracts. (A) The spectral scan of rhodopsin in the extract of an eye exposed to a bright flash of light was measured before and after the addition of 11-cis retinal, (before, red line; after 2 min, blue line; after 20 min, green line) and following bleaching with bright light (purple line). Regeneration of rhodopsin is seen as an increase in absorption at 498 nm (blue arrow). (B) The difference spectrum was taken by subtracting the scan taken 20 minutes after the addition of 11-cis retinal from the scan taken after light exposure (purple line), illustrating the typical rhodopsin peak at 498 nm. The conversion of 11-cis retinal to all-trans retinal after bleaching is detected as a negative deflection at 380 nm.
MEASUREMENTS OF APO-OPSIN IN NORMAL HUMAN EYES
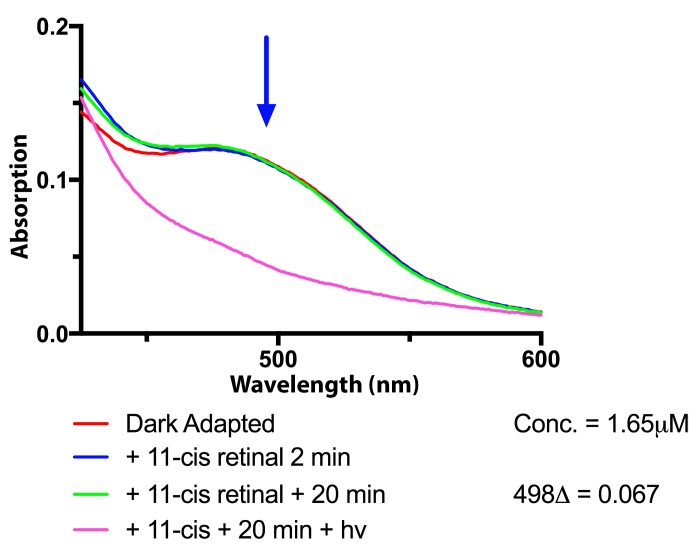
Once we had established that apo-opsin could be detected in human retinal extracts after light exposure, we began testing extracts from normal human eyes. Using the harvesting conditions established for dark adaptation, a series of 13 normal human eyes collected between 2 – 20 hours following death (median 7.5 hrs) were tested for apo-opsin. The initial scan was performed before the addition of 11-cis retinal, and repeat spectral scans were performed twice during a 20 minute period. The results showed no further rhodopsin regeneration in any extracts. A representative experiment is shown in Figure 5. The dark-adapted spectrum, shown in red, overlaps the spectra obtained two minutes (blue line) and 20 minutes (green line) following addition of 11-cis retinal. There is no evidence of a progressive increase in absorbance at 498 nm in this sample, nor in any of the retina extracts from normal eyes. In comparison with the results shown in Figure 3, where a progressive increase in absorbance at 498 nm indicates the presence of rhodopsin regeneration, these spectral scans did not change. These findings indicate that there is no apo-opsin in the human retina extracts obtained from normal autopsy eyes in this study.
FIGURE 5.
Measurements of apo-opsin in normal human eyes. Eyes from a normal donor was dark-adapted in black canisters and rhodopsin extracts were prepared for the measurement of apo-opsin. The dark-adapted spectrum of rhodopsin at baseline (red line) and the spectra obtained after incubation with 11-cis retinal show no difference in absorption at 498 nm (blue arrow) [2 min (blue line) and 20 min (green line)].
CHARACTERISTICS OF DONOR EYES WITH MACULAR DEGENERATION
Twelve eyes with macular degeneration were identified during gross microscopic examination of the posterior poles after retinal dissection. The pathologic findings in these eyes were categorized into three groups, corresponding to early (small drusen) (4 eyes), intermediate (drusen and macular pigmentary abnormalities) (8 eyes) and advanced macular degeneration (geographic atrophy involving the central macula and/or subretinal neovascular membranes and/or disciform scarring) (4 eyes). Table 2 describes the donor eye characteristics. The median death-preservation time of the eyes with early, intermediate and advanced disease was 2.5 hr (range 2–3 hr); 6.0 hr (range 5–17 hr); 9.5 hr (range 6–13 hr).
TABLE 2.
DONOR EYE CHARACTERISTICS
| MACULAR DEGENERATION STAGE | LEVEL | EYES # | AGE RANGE (Y) | DEATH TO PRESERVATION (HR) | MEDIAN (HR) | DISEASE CHARACTERISTIC |
|---|---|---|---|---|---|---|
| None | 1 | 16 | 56–85 | 2–20 | 7.5 | No disease |
| Early | 2 | 4 | 78–81 | 2–3 | 2.5 | Small drusen |
| Intermediate | 3 | 8 | 71–88 | 5–17 | 6 | Drusen and Pigmentary Abnormalities |
| Advanced | 4 | 4 | 85–97 | 6–13 | 9.5 | Geographic Atrophy, Neovascular membranes, Disciform Scars |
EXTRACTS FROM EYES WITH MACULAR DEGENERATION CONTAIN NO APO-OPSIN
Using the harvesting conditions established for dark adaptation, retina extracts were prepared and tested for apo-opsin. The initial scan was performed before the addition of 11-cis retinal, and repeat spectral scans were performed twice during a 20 minute period. Similar to the findings in the eyes of donors with normal retinas, the retinal extracts of the donors with macular degeneration showed no evidence of regeneration at 498 nm. These findings demonstrate there is no apo-opsin in the human retina extracts obtained from autopsy eyes of patients with early, intermediate or late stages of macular degeneration.
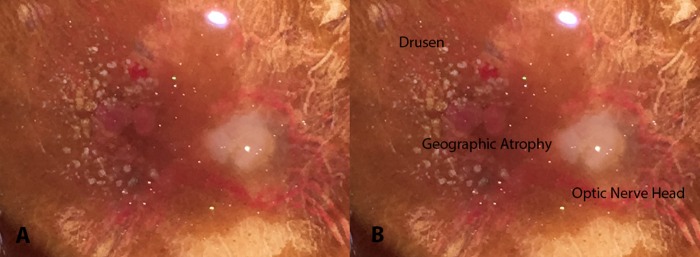
A representative example is shown in Figure 6. This pair of eyes was harvested within 6 hrs of death and had photographs of the retinal pigment epithelium after dissection of the retina to document the ocular pathology. The right-sided photograph is identical to the left and has been labeled. The photograph shows large macular drusen, retinal pigment epithelial abnormalities, subretinal blood and punched out areas of geographic atrophy. The fellow eye had identical findings.
FIGURE 6.
Fundus photograph taken after the dissection of the retina from 97 year-old right eye with advanced age-related macular degeneration. This photograph shows large drusen, retinal pigmented epithelial abnormalities, blood and areas of geographic atrophy. The right picture has been labeled.
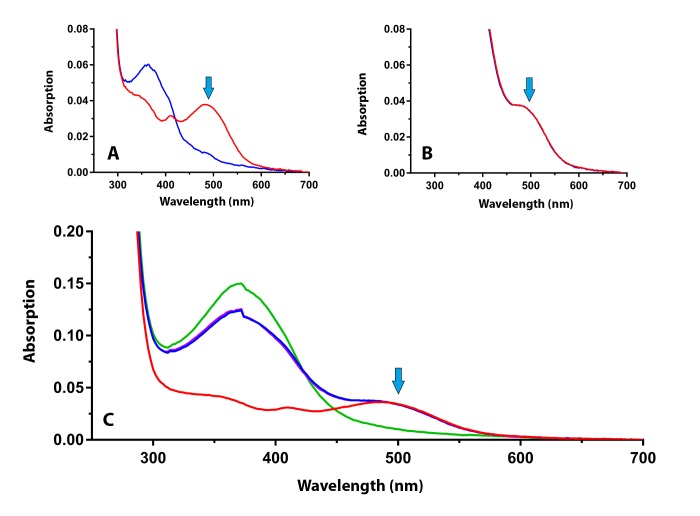
Figure 7 shows the results of the measurement of apo-opsin in this retinal extract. Figure 7A shows the dark-adapted spectral scan of rhodopsin before (red line) and after bleaching with light (blue line). Blue arrow indicates the maximum absorption of rhodopsin. Figure 7B shows the dark-adapted spectral scans before and after a 20 minute incubation with 11-cis retinal. No increase is seen in the absorption at 498 nm (blue arrow) during the 20 minute time period. Figure 7C shows the full spectrum from 700 – 250 nm before and after incubation with 11-cis retinal and following bleaching with light (11-cis peak at 380 nm; rhodopsin peak at 498 nm). From this result and the results of the other eyes, we conclude that no apo-opsin can be detected in the retinal extracts from donors with macular degeneration.
FIGURE 7.
Spectral scans of the retinal extracts from the donor eye with advanced macular degeneration shown in Figure 6. (A) The rhodopsin spectrum before (red line) and after (blue line) bleaching with bright light. (B) The dark-adapted spectrum after the addition of exogenous 11-cis retinal (2 min red line; 20 min blue line) to test for rhodopsin regeneration. (C) The full spectral curves before and after the addition of 11-cis retinal, and after bleaching in bright light. No increase in rhodopsin absorption is detected at 498 nm (blue arrows).
DISCUSSION
A deficiency in the availability of 11-cis retinal has been proposed to be a primary cause of many visual symptoms described by patients with macular degeneration, which include a constellation of dark adaptation abnormalities.8,84,92 These complaints are also described by patients with vitamin A deficiency, which creates a strong clinical correlation between the symptomatology and a potential pathophysiologic mechanism. Additional evidence for decreased transport of 11-cis retinal comes from the pathologic findings of increased thickening of Bruch’s membrane and the buildup of basal laminar and basal linear deposits,7,93–96 which potentially reduces the diffusion of vitamin A from the choriocapillaris to the RPE cells. Reticular drusen could create another barrier to the transport of 11-cis retinal from the RPE to photoreceptor outer segments.97,98 In addition, a reduction in the production of 11-cis retinal could also occur with RPE cell dysfunction and cell death.6,99
To address this hypothesis, we considered several approaches. Knowing that macular degeneration eyes contain a decrease in the density of photoreceptor cells,6,100 we anticipated that measurements of rhodopsin content alone would not be helpful because any decrease in rhodopsin content could be attributed solely to photoreceptor cell death. Electrophysiology approaches to measure apo-opsin in patients did not seem feasible and microspectrometric approaches in postmortem human eyes, similar to those used in in the salamander or mouse, would be challenging due to postmortem changes in photoreceptor viability. We concluded that measuring the presence of apo-opsin in dark-adapted macular degeneration eyes would be worth exploring.
MEASUREMENTS OF APO-OPSIN IN AUTOPSY EYES
The first outcome of this study is that normal human autopsy eyes can be collected in a dark-adapted state with modifications of standard harvesting conditions. Previous investigators had found substantial amounts of bleached pigment after harvesting human retinas in either normal or low light conditions.87,90,101 Transferring the whole globes into dark canisters immediately after enucleation and the dissection of the human globe and the removal of the retina in the dark using infrared lighting and image converters are key differences.
The second outcome of this study is that rod outer segments can be extracted and purified from a single human retina for use in biochemical studies of dark adaptation. Although the initial extraction protocols were performed in detergents, we were able to use modifications of sucrose gradient methods to recover and purify rod outer segments in suspension to a reasonably high degree of purity. This allowed us to avoid many problems associated with light scattering during spectroscopy.
The central finding in this study is that we did not identify apo-opsin in the retinal extracts of any of the dark-adapted eyes with macular degeneration. We predicted that normal eyes had the ability to fully dark-adapt after death but eyes from patients with macular degeneration would not have the same capacity to if there was a deficiency in 11-cis retinal. This prediction was based on studies showing normal human and bovine autopsy eyes maintain significant metabolic activity after death, at levels which are greatest at 4° C compared to 37° C for at least 4 hours.91,102,103 Postmortem bovine retinas will regenerate rhodopsin for at least 4 hours91 and postmortem human autopsy eyes will synthesize ATP, GTP and phosphorylate light-activated rhodopsin for at least 4 hours.102,103 While the metabolic activity of normal retinas is maintained at a high level for 1–2 hours, with a gradual decrease over 4 hours103, the metabolic activity of autopsy eyes with retinal disease, in this case retinitis pigmentosa, is significantly decreased at 1–2 hours, with 75% less GTP synthesized than in normal postmortem retinas.103 Based on these findings, we predicted the metabolic activity and synthesis of 11-cis retinal in RPE cells is likely to be significantly reduced in postmortem eyes with macular degeneration compared to the eyes from patients without disease.
There are several interpretations of our results. First, it is possible that there is no significant deficiency of 11-cis retinal in the autopsy eyes of patients with macular degeneration compared to normal eyes. In support of this interpretation, we had strong positive controls. We were able to detect apo-opsin in normal eyes harvested in standard conditions. In addition, we could detect apo-opsin in an eye that was transported in dark canisters and then exposed to a bright light flash prior to extraction. This second control shows that endogenous stores of 11-cis retinal are not sufficient to fully regenerate rhodopsin in autopsy eyes after bleaching. It also shows that a metabolically inactive retina is unable to fully dark-adapt after bright light.
However, we are cautious in accepting this interpretation. An important caveat to consider is that all of the autopsy eyes may have fully dark-adapted with the modified harvesting protocol if the metabolic activity in the retina remained sufficiently high that rhodopsin regenerated in vivo before enucleation and the dim light used for enucleation did not bleach detectable amounts of visual pigment. In this scenario, we would not detect apo-opsin in any of the macular degeneration eyes even if there was a relative deficiency at some point after death. The shortest death to enucleation time that we could achieve was two hours, which is relatively fast and within a time frame that might be expected to show a deficiency of 11-cis retinal in the setting of macular disease. Nonetheless, it is possible that there could have been enough regeneration within two hours in this eye and in the other pair with early macular degeneration that was recovered within three hours of death. If this was the case and the retina has enough metabolic activity that rhodopsin could be regenerated in vivo after death, it would be difficult to envision a means to study this hypothesis with autopsy tissues unless the eyes are removed so early after death that the viability of the tissue is maintained.
A second caveat to consider is that we recovered the whole retina, not just the macula. Since the number of rod photoreceptors within the central macula is a small percentage compared to the rods across the whole retina, a small amount of apo-opsin in the macula might not be detectable in these samples, especially if the amount of pigment bleached is a small percentage of the total. We chose to harvest the entire retina in this protocol to optimize our recovery of purified outer segments from a single retina. The concern was that purifying outer segments from the small area in the macula would make detection and measurement of small amounts of rhodopsin even more difficult. Rod outer segments might be extractable from the macula, but sample impurities could possibly mask the small levels of rhodopsin and light scattering could potentially interfere with spectrophotometric measurements. To address these challenges in future studies, we could use new spectrophotometers which incorporate small custom integrating spheres that would overcome the problems caused by extensive light scattering in small crude extracts.
Although this data must be cautiously interpreted with the caveats above, it is worth reconsidering our original hypothesis that a decrease in 11-cis retinal transport and an excess of apo-opsin explains the delays in dark adaptation and other visual function abnormalities seen in patients with age-related macular degeneration. Our data clearly does not support this hypothesis. Furthermore, other findings raise additional doubts as to its validity. In particular, if 11-cis retinal supplies are rate-limiting, one might expect high dose supplementation of vitamin A to lead to a rapid improvement in visual sensitivity, similar to the improvement seen in patients with vitamin A deficiency and Sorsby’s macular degeneration,104–106, who recover normal dark adaptation times within a week of initiating vitamin A treatment.107,108 Like macular degeneration eyes, Sorby’s macular degeneration is associated with an accumulation of sub-RPE deposits as a result of mutations in tissue inhibitor of metalloprotease III (TIMP3). Jacobson reported two patients with early and advanced stages of Sorby’s degeneration who showed rapid reversal of delayed dark adaptation and scotopic visual thresholds within a week after vitamin A supplementation.108 Yet, macular degeneration patients show only marginal improvement in dark adaptation after 30 days of high dose vitamin A supplementation, with a reduction in the time needed to achieve a log sensitivity of 3.0 from 38 minutes to 35 minutes, and an increase in the rod slope from 0.15 to 0.17. 109 These values achieved statistical significance but are considerably less impressive than what has been observed after treating vitamin A deficiency. Yet, macular degeneration is a complex and multifactorial condition and it is highly likely the pathophysiology involves a number of roadblocks, including complement mediated dysfunction of RPE cells, cell death, delayed transport across a thickened Bruch’s membrane-RPE interface, decreased recycling of 11-cis retinal in the visual cycle, and other mechanisms outlined below.
ALTERNATIVE HYPOTHESES
It is worth considering what other steps in the visual cycle might be involved in the delayed dark adaptation observed in patients with age-related macular degeneration. This is an important exercise because an understanding of other roadblocks in the visual cycle and/or the visual transduction cascade can direct the development of new treatments.
Alternative hypotheses all implicate a prolonged active photointermediate of bleaching40 and an energy-dependent process that may not be able to keep up with demand. Among the possibilities are (1) Delayed release and decay of all-trans retinal, (2) Delayed shutoff of active Meta-rhodopsin II and (3) Delayed or incomplete dephosphorylation of rhodopsin. Mouse models containing mutations in each of these pathways have shown abnormal dark adaptation with persistent activation of the phototransduction cascade and reduced rod photosensitivity.25,26,110,111 It is worth considering whether any of these energy-dependent pathways might be affected in the setting of macular degeneration.
Delayed release and decay of all-trans retinal
One alternative hypothesis for delayed dark adaptation implicates a delay in the release and/or decay of the active photoproduct all-trans retinal. This energy-dependent process occurs after the active photo-intermediate Meta II decays into apo-opsin and all-trans retinal. If stores of all-trans retinal are not removed, it is capable of diffusing back into the retinal binding pocket of apo-opsin and reinitiating visual transduction.112–115 This event will activate transducin at a much higher level than the constitutive signaling of apo-opsin and has been proposed to contribute to the reduction in photosensitivity seen after light exposure.45,116 To prevent this spontaneous activation, all-trans retinal must be reduced to all-trans retinol (vitamin A) by RDH12 in the outer segment in a rate limiting step in the visual cycle that is highly dependent on the metabolic stores of NADPH.54,117 Mutations in RDH12 interfere with this process and lead to severe photoreceptor degeneration79, suggesting this roadblock will have a significant impact on photoreceptor cell survival. Whether there is delayed release and/or decay of all-trans retinal in the setting of macular degeneration is not known.
Delayed shutoff of active Meta-rhodopsin II
A second hypothesis for delayed dark adaptation implicates another energy-dependent process, a delay in the phosphorylation of active metarhodopsin II by rhodopsin kinase and arrestin binding. Because complete quenching of Meta II rhodopsin requires both phosphorylation of serine and/or threonine residues and subsequent binding by arrestin, abnormalities in either of these energy-dependent processes will fail to quench active Meta II, leading to persistent activation of the transduction cascade, decreased rod photosensitivity and prolonged dark adaptation. 25,26,32 While the potential role of delayed shutoff of active Meta II has not been demonstrated in age-related macular degeneration eyes, a decrease in immunoreactive arrestin has been shown in human retinal extracts.118
Delayed or incomplete dephosphorylation of rhodopsin
A third hypothesis implicates a delay in the dephosphorylation of rhodopsin. Using ex vivo models of light-exposed retinas, dephosphorylation has been shown to be an energy dependent process which reduces rod photosensitivity but does not prevent rhodopsin regeneration.25 Mutations in this process have been proposed to prolong the last phase of dark adaptation.25 The role of this pathway in the setting of macular degeneration is also not known.
CONCLUSIONS
New approaches to improving vision in patients with macular degeneration would benefit from a more complete understanding of the specific chemical defects causing dark adaptation abnormalities and other visual symptoms. In the absence of a good animal model of age-related macular degeneration, investigators search for clues in human tissues.5,118–120 And, although there are significant limitations associated with using human autopsy tissue, many investigators attempt to correlate the pathology found in human autopsy eyes to molecular and biochemical outcomes,5,118,120 which in some cases have led to great insights.119
This study may not be viewed as a definitive test of the 11-cis retinal deficiency hypothesis in macular degeneration eyes. However, the finding that an excess of apo-opsin is not detected in the eyes of patients with macular degeneration challenges us to consider additional experimental approaches and alternatives for testing this theory. It also gives us pause to consider alternative hypotheses to explain the dark adaptation abnormalities in patients with macular degeneration, all within the context of what we know about the visual cycle and the visual transduction cascade.
ACKNOWLEDGMENTS/DISCLOSURES
Funding/Support: The authors acknowledge the support of the Money-Arenz Foundation; The Considine Family Foundation; The Fonseca Foundation; The Mericos Eye Institute; The Warren Family Foundation, Directed research funding through The Scripps Research Institute.
Financial Disclosures: None
Contributions of Authors: Design and conduct of the study (AH, TN, JJ, MK); Collection, management, analysis and interpretation of the data (AH, TN, JJ, MK); Preparation, review and approval of the manuscript (AH, TN, JJ, MK)
Other Acknowledgments: The authors thank Jennifer Nary and Jeff Penta at the San Diego Eye Bank for their assistance with tissue acquisition, and Drs. Rosalie Crouch and Carter Cornwall for their early discussions on study design and data collection.
REFERENCES
- 1.Zarbin MA. Age-related macular degeneration: review of pathogenesis. Eur J Ophthalmol. 1998;8(4):199–206. doi: 10.1177/112067219800800401. [DOI] [PubMed] [Google Scholar]
- 2.de Jong PT. Age-related macular degeneration. N Engl J Med. 2006;355(14):1474–1485. doi: 10.1056/NEJMra062326. [DOI] [PubMed] [Google Scholar]
- 3.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51(2):137–152. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards AO, Malek G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007;10(2):119–132. doi: 10.1007/s10456-007-9064-2. [DOI] [PubMed] [Google Scholar]
- 5.Sevilla MB, McGwin G, Jr, Lad EM, et al. Relating Retinal Morphology and Function in Aging and Early to Intermediate Age-related Macular Degeneration Subjects. Am J Ophthalmol. 2016;165:65–77. doi: 10.1016/j.ajo.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Curcio CA. Photoreceptor topography in ageing and age-related maculopathy. Eye (Lond) 2001;15(Pt 3):376–383. doi: 10.1038/eye.2001.140. [DOI] [PubMed] [Google Scholar]
- 7.Sarks S, Cherepanoff S, Killingsworth M, Sarks J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48(3):968–977. doi: 10.1167/iovs.06-0443. [DOI] [PubMed] [Google Scholar]
- 8.Dimitrov PN, Guymer RH, Zele AJ, Anderson AJ, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008;49(1):55–65. doi: 10.1167/iovs.06-1048. [DOI] [PubMed] [Google Scholar]
- 9.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81(1):117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 10.Soto F, Kerschensteiner D. Synaptic remodeling of neuronal circuits in early retinal degeneration. Front Cell Neurosci. 2015;9:395. doi: 10.3389/fncel.2015.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanneken AM, Babai N, Thoreson WB. Oral proton pump inhibitors disrupt horizontal cell-cone feedback and enhance visual hallucinations in macular degeneration patients. Invest Ophthalmol Vis Sci. 2013;54(2):1485–1489. doi: 10.1167/iovs.12-11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. The Journal of physiology. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- 13.Baylor DA, Matthews G, Yau KW. Two components of electrical dark noise in toad retinal rod outer segments. The Journal of physiology. 1980;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornwall MC, Jones GJ, Kefalov VJ, Fain GL, Matthews HR. Electrophysiological methods for measurement of activation of phototransduction by bleached visual pigment in salamander photoreceptors. Methods Enzymol. 2000;316:224–252. doi: 10.1016/s0076-6879(00)16726-6. [DOI] [PubMed] [Google Scholar]
- 15.Nymark S, Frederiksen R, Woodruff ML, Cornwall MC, Fain GL. Bleaching of mouse rods: microspectrophotometry and suction-electrode recording. The Journal of physiology. 2012;590(10):2353–2364. doi: 10.1113/jphysiol.2012.228627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owsley C, McGwin G, Jr, Clark ME, et al. Delayed Rod-Mediated Dark Adaptation Is a Functional Biomarker for Incident Early Age-Related Macular Degeneration. Ophthalmology. 2016;123(2):344–351. doi: 10.1016/j.ophtha.2015.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson GR, Scott IU, Kim IK, Quillen DA, Iannaccone A, Edwards JG. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55(3):1427–1431. doi: 10.1167/iovs.13-13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 A crystal structure. J Mol Biol. 2004;342(2):571–583. doi: 10.1016/j.jmb.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 19.Palczewski K, Kumasaka T, Hori T, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289(5480):739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 20.Zhou X, Sundholm D, Wesolowski TA, Kaila VR. Spectral tuning of rhodopsin and visual cone pigments. J Am Chem Soc. 2014;136(7):2723–2726. doi: 10.1021/ja411864m. [DOI] [PubMed] [Google Scholar]
- 21.Heck M, Schadel SA, Maretzki D, et al. Signaling states of rhodopsin. Formation of the storage form, metarhodopsin III, from active metarhodopsin II. J Biol Chem. 2003;278(5):3162–3169. doi: 10.1074/jbc.M209675200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arshavsky VY, Lamb TD, Pugh EN., Jr G proteins and phototransduction. Annu Rev Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 23.Arshavsky VY. Rhodopsin phosphorylation: from terminating single photon responses to photoreceptor dark adaptation. Trends Neurosci. 2002;25(3):124–126. doi: 10.1016/s0166-2236(00)02094-4. [DOI] [PubMed] [Google Scholar]
- 24.Lefkowitz RJ. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol Sci. 2004;25(8):413–422. doi: 10.1016/j.tips.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Berry J, Frederiksen R, Yao Y, Nymark S, Chen J, Cornwall C. Effect of Rhodopsin Phosphorylation on Dark Adaptation in Mouse Rods. J Neurosci. 2016;36(26):6973–6987. doi: 10.1523/JNEUROSCI.3544-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frederiksen R, Nymark S, Kolesnikov AV, et al. Rhodopsin kinase and arrestin binding control the decay of photoactivated rhodopsin and dark adaptation of mouse rods. J Gen Physiol. 2016;148(1):1–11. doi: 10.1085/jgp.201511538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuhn H. Light-dependent phosphorylation of rhodopsin in living frogs. Nature. 1974;250(467):588–590. doi: 10.1038/250588a0. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Makino CL, Peachey NS, Baylor DA, Simon MI. Mechanisms of rhodopsin inactivation in vivo as revealed by a COOH-terminal truncation mutant. Science. 1995;267(5196):374–377. doi: 10.1126/science.7824934. [DOI] [PubMed] [Google Scholar]
- 29.Hurley JB, Spencer M, Niemi GA. Rhodopsin phosphorylation and its role in photoreceptor function. Vision Res. 1998;38(10):1341–1352. doi: 10.1016/s0042-6989(97)00459-8. [DOI] [PubMed] [Google Scholar]
- 30.Mendez A, Burns ME, Roca A, et al. Rapid and reproducible deactivation of rhodopsin requires multiple phosphorylation sites. Neuron. 2000;28(1):153–164. doi: 10.1016/s0896-6273(00)00093-3. [DOI] [PubMed] [Google Scholar]
- 31.Wilden U, Hall SW, Kuhn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cideciyan AV, Zhao X, Nielsen L, Khani SC, Jacobson SG, Palczewski K. Null mutation in the rhodopsin kinase gene slows recovery kinetics of rod and cone phototransduction in man. Proc Natl Acad Sci U S A. 1998;95(1):328–333. doi: 10.1073/pnas.95.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stiles WS, Crawford BH.Equivalent adaptational levels in localised retinal areas Cambridge: Physical Society of London; 19321932 [Google Scholar]
- 34.Crawford BH. Visual adaptation in relation to brief conditioning stimuli. Proc R Soc Lond A Math Phys Sci. 1947;189(1017):284. doi: 10.1098/rspa.1947.0039. [DOI] [PubMed] [Google Scholar]
- 35.Barlow HB, Sparrock JM. The Role of Afterimages in Dark Adaptation. Science. 1964;144(3624):1309–1314. doi: 10.1126/science.144.3624.1309. [DOI] [PubMed] [Google Scholar]
- 36.Dowling JE. Neural and Photochemical Mechanisms of Visual Adaptation in the Rat. J Gen Physiol. 1963;46:1287–1301. doi: 10.1085/jgp.46.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fain GL, Lisman JE. Photoreceptor degeneration in vitamin A deprivation and retinitis pigmentosa: the equivalent light hypothesis. Exp Eye Res. 1993;57(3):335–340. doi: 10.1006/exer.1993.1132. [DOI] [PubMed] [Google Scholar]
- 38.Lisman J, Fain G. Support for the equivalent light hypothesis for RP. Nat Med. 1995;1(12):1254–1255. doi: 10.1038/nm1295-1254. [DOI] [PubMed] [Google Scholar]
- 39.Fain GL, Lisman JE. Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arrestin knockout mice. Invest Ophthalmol Vis Sci. 1999;40(12):2770–2772. [PubMed] [Google Scholar]
- 40.Leibrock CS, Reuter T, Lamb TD. Molecular basis of dark adaptation in rod photoreceptors. Eye (Lond) 1998;12(Pt 3b):511–520. doi: 10.1038/eye.1998.139. [DOI] [PubMed] [Google Scholar]
- 41.Lamb TD, Pugh EN., Jr Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004;23(3):307–380. doi: 10.1016/j.preteyeres.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Lamb TD, Pugh EN., Jr Phototransduction, dark adaptation, and rhodopsin regeneration the proctor lecture. Invest Ophthalmol Vis Sci. 2006;47(12):5137–5152. doi: 10.1167/iovs.06-0849. [DOI] [PubMed] [Google Scholar]
- 43.Cohen GB, Yang T, Robinson PR, Oprian DD. Constitutive activation of opsin: influence of charge at position 134 and size at position 296. Biochemistry. 1993;32(23):6111–6115. doi: 10.1021/bi00074a024. [DOI] [PubMed] [Google Scholar]
- 44.Melia TJ, Jr, Cowan CW, Angleson JK, Wensel TG. A comparison of the efficiency of G protein activation by ligand-free and light-activated forms of rhodopsin. Biophys J. 1997;73(6):3182–3191. doi: 10.1016/S0006-3495(97)78344-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surya A, Foster KW, Knox BE. Transducin activation by the bovine opsin apoprotein. J Biol Chem. 1995;270(10):5024–5031. doi: 10.1074/jbc.270.10.5024. [DOI] [PubMed] [Google Scholar]
- 46.Cornwall MC, Fain GL. Bleached pigment activates transduction in isolated rods of the salamander retina. The Journal of physiology. 1994;480(Pt 2):261–279. doi: 10.1113/jphysiol.1994.sp020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornwall MC, Matthews HR, Crouch RK, Fain GL. Bleached pigment activates transduction in salamander cones. J Gen Physiol. 1995;106(3):543–557. doi: 10.1085/jgp.106.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fan J, Woodruff ML, Cilluffo MC, Crouch RK, Fain GL. Opsin activation of transduction in the rods of dark-reared Rpe65 knockout mice. The Journal of physiology. 2005;568(Pt 1):83–95. doi: 10.1113/jphysiol.2005.091942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35(2):158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 50.Tang PH, Kono M, Koutalos Y, Ablonczy Z, Crouch RK. New insights into retinoid metabolism and cycling within the retina. Prog Retin Eye Res. 2013;32:48–63. doi: 10.1016/j.preteyeres.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wright CB, Redmond TM, Nickerson JM. A History of the Classical Visual Cycle. Prog Mol Biol Transl Sci. 2015;134:433–448. doi: 10.1016/bs.pmbts.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Illing M, Molday LL, Molday RS. The 220-kDa rim protein of retinal rod outer segments is a member of the ABC transporter superfamily. J Biol Chem. 1997;272(15):10303–10310. doi: 10.1074/jbc.272.15.10303. [DOI] [PubMed] [Google Scholar]
- 53.Liu J, Itagaki Y, Ben-Shabat S, Nakanishi K, Sparrow JR. The biosynthesis of A2E, a fluorophore of aging retina, involves the formation of the precursor, A2-PE, in the photoreceptor outer segment membrane. J Biol Chem. 2000;275(38):29354–29360. doi: 10.1074/jbc.M910191199. [DOI] [PubMed] [Google Scholar]
- 54.Haeseleer F, Huang J, Lebioda L, Saari JC, Palczewski K. Molecular characterization of a novel short-chain dehydrogenase/reductase that reduces all-trans-retinal. J Biol Chem. 1998;273(34):21790–21799. doi: 10.1074/jbc.273.34.21790. [DOI] [PubMed] [Google Scholar]
- 55.Okajima TI, Wiggert B, Chader GJ, Pepperberg DR. Retinoid processing in retinal pigment epithelium of toad (Bufo marinus) J Biol Chem. 1994;269(35):21983–21989. [PubMed] [Google Scholar]
- 56.Wu Q, Blakeley LR, Cornwall MC, Crouch RK, Wiggert BN, Koutalos Y. Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007;46(29):8669–8679. doi: 10.1021/bi7004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264(15):8636–8640. [PubMed] [Google Scholar]
- 58.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42(7):2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 59.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122(3):449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci U S A. 2005;102(35):12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci U S A. 2005;102(38):13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. J Biol Chem. 1993;268(21):15751–15757. [PubMed] [Google Scholar]
- 63.Travis GH, Golczak M, Moise AR, Palczewski K. Diseases caused by defects in the visual cycle: retinoids as potential therapeutic agents. Annu Rev Pharmacol Toxicol. 2007;47:469–512. doi: 10.1146/annurev.pharmtox.47.120505.105225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dowling JE, Gibbons IR. The Effect of Vitamin A Deficiency on the Fine Structure of the Retina. The Structure of the Eye. 1961:85–99. [Google Scholar]
- 65.Kemp CM, Jacobson SG, Borruat FX, Chaitin MH. Rhodopsin levels and retinal function in cats during recovery from vitamin A deficiency. Exp Eye Res. 1989;49(1):49–65. doi: 10.1016/0014-4835(89)90075-4. [DOI] [PubMed] [Google Scholar]
- 66.Dowling JE, Wald G. Vitamin a Deficiency and Night Blindness. Proc Natl Acad Sci U S A. 1958;44(7):648–661. doi: 10.1073/pnas.44.7.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Noell WK, Delmelle MC, Albrecht R. Vitamin A deficiency effect on retina: dependence on light. Science. 1971;172(978):72–75. doi: 10.1126/science.172.3978.72. [DOI] [PubMed] [Google Scholar]
- 68.Kemp CM, Jacobson SG, Faulkner DJ, Walt RW. Visual function and rhodopsin levels in humans with vitamin A deficiency. Exp Eye Res. 1988;46(2):185–197. doi: 10.1016/s0014-4835(88)80076-9. [DOI] [PubMed] [Google Scholar]
- 69.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20(4):344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 70.Ablonczy Z, Crouch RK, Goletz PW, et al. 11-cis-retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277(43):40491–40498. doi: 10.1074/jbc.M205507200. [DOI] [PubMed] [Google Scholar]
- 71.Robinson PR, Cohen GB, Zhukovsky EA, Oprian DD. Constitutively active mutants of rhodopsin. Neuron. 1992;9(4):719–725. doi: 10.1016/0896-6273(92)90034-b. [DOI] [PubMed] [Google Scholar]
- 72.Rao VR, Oprian DD. Activating mutations of rhodopsin and other G protein-coupled receptors. Annu Rev Biophys Biomol Struct. 1996;25:287–314. doi: 10.1146/annurev.bb.25.060196.001443. [DOI] [PubMed] [Google Scholar]
- 73.Chen J, Shi G, Concepcion FA, Xie G, Oprian D, Chen J. Stable rhodopsin/arrestin complex leads to retinal degeneration in a transgenic mouse model of autosomal dominant retinitis pigmentosa. J Neurosci. 2006;26(46):11929–11937. doi: 10.1523/JNEUROSCI.3212-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keen TJ, Inglehearn CF, Lester DH, et al. Autosomal dominant retinitis pigmentosa: four new mutations in rhodopsin, one of them in the retinal attachment site. Genomics. 1991;11(1):199–205. doi: 10.1016/0888-7543(91)90119-y. [DOI] [PubMed] [Google Scholar]
- 75.Thompson DA, Gyurus P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41(13):4293–4299. [PubMed] [Google Scholar]
- 76.Pierce EA, Bennett J. The Status of RPE65 Gene Therapy Trials: Safety and Efficacy. Cold Spring Harb Perspect Med. 2015;5(9):a017285. doi: 10.1101/cshperspect.a017285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scholl HP, Moore AT, Koenekoop RK, et al. Safety and Proof-of-Concept Study of Oral QLT091001 in Retinitis Pigmentosa Due to Inherited Deficiencies of Retinal Pigment Epithelial 65 Protein (RPE65) or Lecithin:Retinol Acyltransferase (LRAT) PLoS ONE. 2015;10(12):e0143846. doi: 10.1371/journal.pone.0143846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yamamoto H, Simon A, Eriksson U, Harris E, Berson EL, Dryja TP. Mutations in the gene encoding 11-cis retinol dehydrogenase cause delayed dark adaptation and fundus albipunctatus. Nat Genet. 1999;22(2):188–191. doi: 10.1038/9707. [DOI] [PubMed] [Google Scholar]
- 79.Janecke AR, Thompson DA, Utermann G, et al. Mutations in RDH12 encoding a photoreceptor cell retinol dehydrogenase cause childhood-onset severe retinal dystrophy. Nat Genet. 2004;36(8):850–854. doi: 10.1038/ng1394. [DOI] [PubMed] [Google Scholar]
- 80.Maw MA, Kennedy B, Knight A, et al. Mutation of the gene encoding cellular retinaldehyde-binding protein in autosomal recessive retinitis pigmentosa. Nat Genet. 1997;17(2):198–200. doi: 10.1038/ng1097-198. [DOI] [PubMed] [Google Scholar]
- 81.Burstedt MS, Forsman-Semb K, Golovleva I, Janunger T, Wachtmeister L, Sandgren O. Ocular phenotype of bothnia dystrophy, an autosomal recessive retinitis pigmentosa associated with an R234W mutation in the RLBP1 gene. Arch Ophthalmol. 2001;119(2):260–267. [PubMed] [Google Scholar]
- 82.Driessen CA, Janssen BP, Winkens HJ, van Vugt AH, de Leeuw TL, Janssen JJ. Cloning and expression of a cDNA encoding bovine retinal pigment epithelial 11-cis retinol dehydrogenase. Invest Ophthalmol Vis Sci. 1995;36(10):1988–1996. [PubMed] [Google Scholar]
- 83.Simon A, Hellman U, Wernstedt C, Eriksson U. The retinal pigment epithelial-specific 11-cis retinol dehydrogenase belongs to the family of short chain alcohol dehydrogenases. J Biol Chem. 1995;270(3):1107–1112. [PubMed] [Google Scholar]
- 84.Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015;133(4):442–448. doi: 10.1001/jamaophthalmol.2014.5963. [DOI] [PubMed] [Google Scholar]
- 85.Papermaster DS, Dreyer WJ. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- 86.Wald G, Brown PK. The molar extinction of rhodopsin. J Gen Physiol. 1953;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fulton AB, Dodge J, Hansen RM, Williams TP. The rhodopsin content of human eyes. Invest Ophthalmol Vis Sci. 1999;40(8):1878–1883. [PubMed] [Google Scholar]
- 88.Fulton AB, Dodge J, Schremser JL, et al. The quantity of rhodopsin in human eyes. Curr Eye Res. 1990;9(12):1211–1216. doi: 10.3109/02713689009003477. [DOI] [PubMed] [Google Scholar]
- 89.Fulton AB, Dodge J, Hansen RM, Schremser JL, Williams TP. The quantity of rhodopsin in young human eyes. Curr Eye Res. 1991;10(10):977–982. doi: 10.3109/02713689109020334. [DOI] [PubMed] [Google Scholar]
- 90.van Kuijk FJ, Lewis JW, Buck P, Parker KR, Kliger DS. Spectrophotometric quantitation of rhodopsin in the human retina. Invest Ophthalmol Vis Sci. 1991;32(7):1962–1967. [PubMed] [Google Scholar]
- 91.O’Brien PJ, Muellenberg CG, Bungenberg de Jong JJ. Incorporation of leucine into rhodopsin in isolated bovine retina. Biochemistry. 1972;11(1):64–70. doi: 10.1021/bi00751a012. [DOI] [PubMed] [Google Scholar]
- 92.Ying GS, Maguire MG, Liu C, Antoszyk AN Complications of Age-related Macular Degeneration Prevention Trial Research G. Night vision symptoms and progression of age-related macular degeneration in the Complications of Age-related Macular Degeneration Prevention Trial. Ophthalmology. 2008;115(11):1876–1882. doi: 10.1016/j.ophtha.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spraul CW, Grossniklaus HE. Characteristics of Drusen and Bruch’s membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115(2):267–273. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- 94.Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27. [PubMed] [Google Scholar]
- 95.Green WR, Enger C. Age-related macular degeneration histopathologic studies: the 1992 Lorenz E. Zimmerman Lecture. 1992. Retina. 2005;25(5 Suppl):1519–1535. doi: 10.1097/00006982-200507001-00015. [DOI] [PubMed] [Google Scholar]
- 96.Curcio CA, Millican CL. Basal linear deposit and large drusen are specific for early age-related maculopathy. Arch Ophthalmol. 1999;117(3):329–339. doi: 10.1001/archopht.117.3.329. [DOI] [PubMed] [Google Scholar]
- 97.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30(9):1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Curcio CA, Millican CL, Allen KA, Kalina RE. Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci. 1993;34(12):3278–3296. [PubMed] [Google Scholar]
- 100.Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37(7):1236–1249. [PubMed] [Google Scholar]
- 101.Plantner JJ, Barbour HL, Kean EL. The rhodopsin content of the human eye. Curr Eye Res. 1988;7(11):1125–1129. doi: 10.3109/02713688809001883. [DOI] [PubMed] [Google Scholar]
- 102.Schmidt SY, Berson EL. Postmortem metabolic capacity of photoreceptor cells in human and rat retinas. Invest Ophthalmol Vis Sci. 1980;19(11):1274–1280. [PubMed] [Google Scholar]
- 103.Schmidt SY, Berson EL. Opsin phosphorylation in retinas from autopsy eyes with retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1990;31(6):1023–1028. [PubMed] [Google Scholar]
- 104.Capon MR, Marshall J, Krafft JI, Alexander RA, Hiscott PS, Bird AC. Sorsby’s fundus dystrophy. A light and electron microscopic study. Ophthalmology. 1989;96(12):1769–1777. doi: 10.1016/s0161-6420(89)32664-9. [DOI] [PubMed] [Google Scholar]
- 105.Steinmetz RL, Polkinghorne PC, Fitzke FW, Kemp CM, Bird AC. Abnormal dark adaptation and rhodopsin kinetics in Sorsby’s fundus dystrophy. Invest Ophthalmol Vis Sci. 1992;33(5):1633–1636. [PubMed] [Google Scholar]
- 106.Chong NH, Alexander RA, Gin T, Bird AC, Luthert PJ. TIMP-3, collagen, and elastin immunohistochemistry and histopathology of Sorsby’s fundus dystrophy. Invest Ophthalmol Vis Sci. 2000;41(3):898–902. [PubMed] [Google Scholar]
- 107.McBain VA, Egan CA, Pieris SJ, et al. Functional observations in vitamin A deficiency: diagnosis and time course of recovery. Eye (Lond) 2007;21(3):367–376. doi: 10.1038/sj.eye.6702212. [DOI] [PubMed] [Google Scholar]
- 108.Jacobson SG, Cideciyan AV, Regunath G, et al. Night blindness in Sorsby’s fundus dystrophy reversed by vitamin A. Nat Genet. 1995;11(1):27–32. doi: 10.1038/ng0995-27. [DOI] [PubMed] [Google Scholar]
- 109.Owsley C, McGwin G, Jackson GR, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47(4):1310–1318. doi: 10.1167/iovs.05-1292. [DOI] [PubMed] [Google Scholar]
- 110.Xu J, Dodd RL, Makino CL, Simon MI, Baylor DA, Chen J. Prolonged photoresponses in transgenic mouse rods lacking arrestin. Nature. 1997;389(6650):505–509. doi: 10.1038/39068. [DOI] [PubMed] [Google Scholar]
- 111.Chen CK, Burns ME, Spencer M, et al. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc Natl Acad Sci U S A. 1999;96(7):3718–3722. doi: 10.1073/pnas.96.7.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fukada Y, Yoshizawa T. Activation of phosphodiesterase in frog rod outer segment by an intermediate of rhodopsin photolysis. II. Biochim Biophys Acta. 1981;675(2):195–200. doi: 10.1016/0304-4165(81)90226-9. [DOI] [PubMed] [Google Scholar]
- 113.Fukada Y, Kawamura S, Yoshizawa T, Miki N. Activation of phosphodiesterase in frog rod outer segment by an intermediate of rhodopsin photolysis I. Biochim Biophys Acta. 1981;675(2):188–194. doi: 10.1016/0304-4165(81)90225-7. [DOI] [PubMed] [Google Scholar]
- 114.Jager S, Palczewski K, Hofmann KP. Opsin/all-trans-retinal complex activates transducin by different mechanisms than photolyzed rhodopsin. Biochemistry. 1996;35(9):2901–2908. doi: 10.1021/bi9524068. [DOI] [PubMed] [Google Scholar]
- 115.Palczewski K, Jager S, Buczylko J, et al. Rod outer segment retinol dehydrogenase: substrate specificity and role in phototransduction. Biochemistry. 1994;33(46):13741–13750. doi: 10.1021/bi00250a027. [DOI] [PubMed] [Google Scholar]
- 116.Saari JC, Garwin GG, Van Hooser JP, Palczewski K. Reduction of all-trans-retinal limits regeneration of visual pigment in mice. Vision Res. 1998;38(10):1325–1333. doi: 10.1016/s0042-6989(97)00198-3. [DOI] [PubMed] [Google Scholar]
- 117.Rattner A, Smallwood PM, Nathans J. Identification and characterization of all-trans-retinol dehydrogenase from photoreceptor outer segments, the visual cycle enzyme that reduces all-trans-retinal to all-trans-retinol. J Biol Chem. 2000;275(15):11034–11043. doi: 10.1074/jbc.275.15.11034. [DOI] [PubMed] [Google Scholar]
- 118.Ethen CM, Feng X, Olsen TW, Ferrington DA. Declines in arrestin and rhodopsin in the macula with progression of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46(3):769–775. doi: 10.1167/iovs.04-0810. [DOI] [PubMed] [Google Scholar]
- 119.Crabb JW, Miyagi M, Gu X, et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. PNAS. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nordgaard CL, Berg KM, Kapphahn RJ, et al. Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(3):815–822. doi: 10.1167/iovs.05-0976. [DOI] [PubMed] [Google Scholar]