Abstract
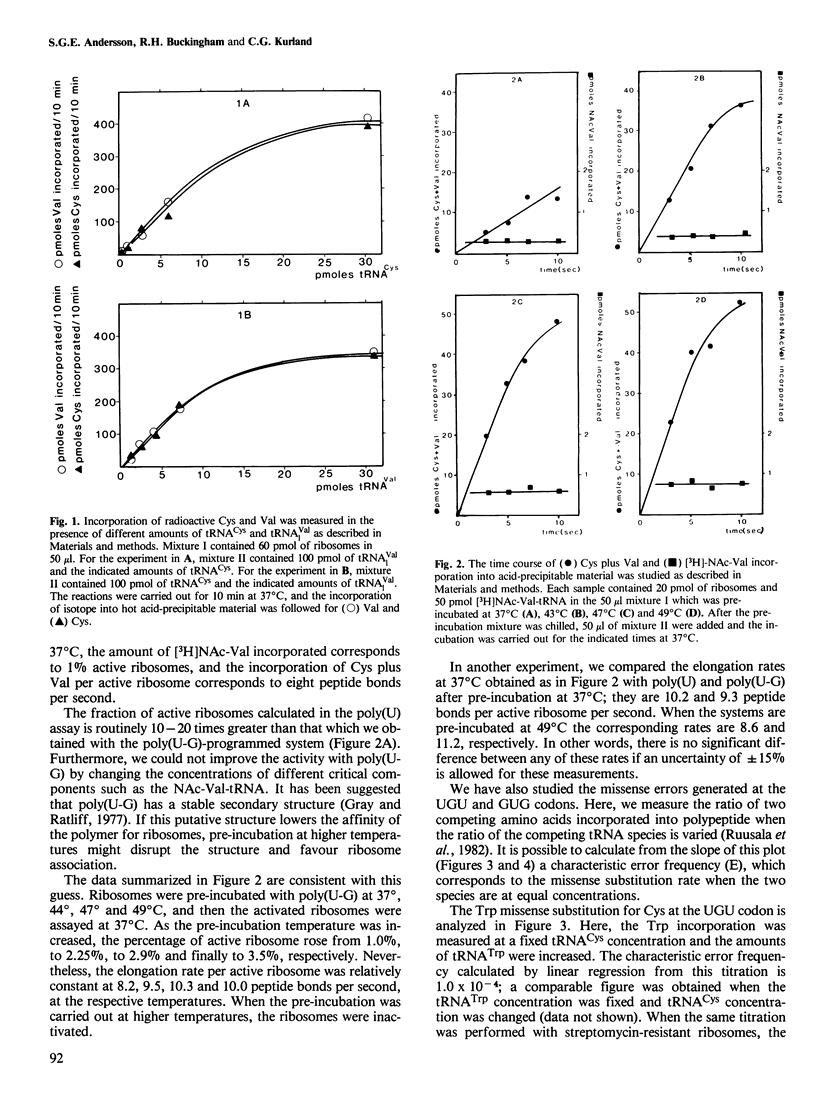
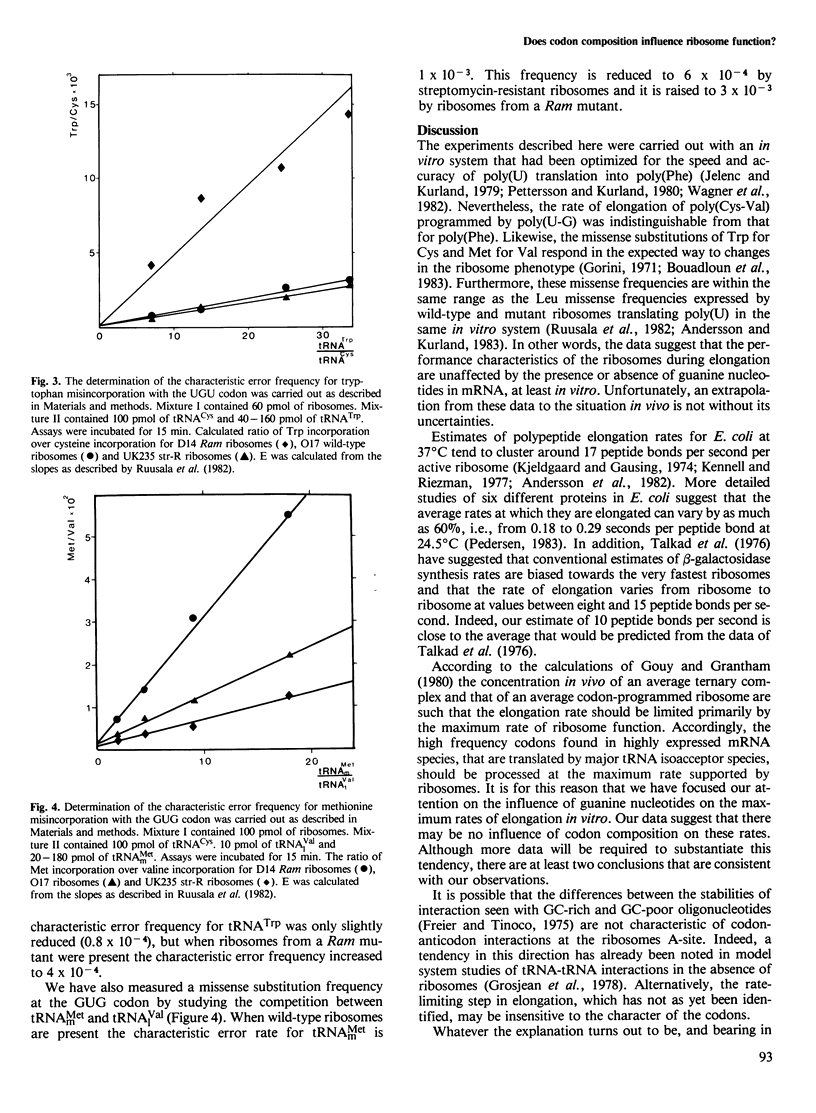
Escherichia coli ribosomes pre-initiated with N-acetyl-Val-tRNAVal elongate strictly alternating poly(U-G) at a rate between eight and 12 peptide bonds per second per ribosome in vitro. Comparisons with poly(U)-primed poly(Phe) synthesis show that these systems function with the same rates which are close to those of protein synthesis in vivo. This indicates that, at least in vitro, codon composition has no marked influence on the speed of elongation when the concentration of ternary complex is saturating. Furthermore, the missense frequencies for the two polymers are within the same range: the missense substitution of Trp for Cys is 10(-4) and that of Met for Val is 10(-3) in the poly(U-G)-primed system. These data argue against models that explain the codon preference of certain gene families by postulating effects of high or low GC content of codons on the performance characteristics of ribosomes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson D. I., Bohman K., Isaksson L. A., Kurland C. G. Translation rates and misreading characteristics of rpsD mutants in Escherichia coli. Mol Gen Genet. 1982;187(3):467–472. doi: 10.1007/BF00332630. [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Kurland C. G. Ram ribosomes are defective proofreaders. Mol Gen Genet. 1983;191(3):378–381. doi: 10.1007/BF00425749. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Bouadloun F., Donner D., Kurland C. G. Codon-specific missense errors in vivo. EMBO J. 1983;2(8):1351–1356. doi: 10.1002/j.1460-2075.1983.tb01591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Chavancy G., Garel J. P. Does quantitative tRNA adaptation to codon content in mRNA optimize the ribosomal translation efficiency? Proposal for a translation system model. Biochimie. 1981 Mar;63(3):187–195. doi: 10.1016/s0300-9084(81)80192-7. [DOI] [PubMed] [Google Scholar]
- Fiers W., Contreras R., De Wachter R., Haegeman G., Merregaert J., Jou W. M., Vandenberghe A. Recent progress in the sequence determination of bacteriophage MS2 RNA. Biochimie. 1971;53(4):495–506. doi: 10.1016/s0300-9084(71)80167-0. [DOI] [PubMed] [Google Scholar]
- Freier S. M., Tinoco I., Jr The binding of complementary oligoribonucleotides to yeast initiator Transfer RNA. Biochemistry. 1975 Jul 29;14(15):3310–3314. doi: 10.1021/bi00686a004. [DOI] [PubMed] [Google Scholar]
- Garel J. P. Functional adaptation of tRNA population. J Theor Biol. 1974 Jan;43(1):211–225. doi: 10.1016/s0022-5193(74)80054-8. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Gorini L. Ribosomal discrimination of tRNAs. Nat New Biol. 1971 Dec 29;234(52):261–264. doi: 10.1038/newbio234261a0. [DOI] [PubMed] [Google Scholar]
- Gouy M., Grantham R. Polypeptide elongation and tRNA cycling in Escherichia coli: a dynamic approach. FEBS Lett. 1980 Jun 30;115(2):151–155. doi: 10.1016/0014-5793(80)81155-0. [DOI] [PubMed] [Google Scholar]
- Grantham R., Gautier C. Genetic distances from mRNA sequences. Naturwissenschaften. 1980 Feb;67(2):93–94. doi: 10.1007/BF01054695. [DOI] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism evidence for G-U and G-T base pairing in poly[r(G-U)] and poly[d(G-T)]. Biopolymers. 1977 Jun;16(6):1331–1342. doi: 10.1002/bip.1977.360160613. [DOI] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes. J Mol Biol. 1981 Feb 15;146(1):1–21. doi: 10.1016/0022-2836(81)90363-6. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Joseph D. R., Muench K. H. Tryptophanyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification of the enzyme and of tryptrophan transfer ribonucleic acid. J Biol Chem. 1971 Dec 25;246(24):7602–7609. [PubMed] [Google Scholar]
- Kennell D., Riezman H. Transcription and translation initiation frequencies of the Escherichia coli lac operon. J Mol Biol. 1977 Jul;114(1):1–21. doi: 10.1016/0022-2836(77)90279-0. [DOI] [PubMed] [Google Scholar]
- Kern D., Lapointe J. The twenty aminoacyl-tRNA synthetases from Escherichia coli. General separation procedure, and comparison of the influence of pH and divalent cations on their catalytic activities. Biochimie. 1979;61(11-12):1257–1272. doi: 10.1016/s0300-9084(80)80285-9. [DOI] [PubMed] [Google Scholar]
- Leberman R., Antonsson B., Giovanelli R., Guariguata R., Schumann R., Wittinghofer A. A simplified procedure for the isolation of bacterial polypeptide elongation factor EF-Tu. Anal Biochem. 1980 May 1;104(1):29–36. doi: 10.1016/0003-2697(80)90272-9. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Jones D. S., Khorana H. G. Studies on polynucleotides. 48. The in vitro synthesis of a co-polypeptide containing two amino acids in alternating sequence dependent upon a DNA-like polymer containing two nucleotides in alternating sequence. J Mol Biol. 1965 Aug;13(1):302–324. doi: 10.1016/s0022-2836(65)80098-5. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. I. Comparison of mutants with various alterations in ribosomal protein S4. Mol Gen Genet. 1979 Feb 1;169(3):251–257. doi: 10.1007/BF00382271. [DOI] [PubMed] [Google Scholar]
- Ruusala T., Ehrenberg M., Kurland C. G. Is there proofreading during polypeptide synthesis? EMBO J. 1982;1(6):741–745. doi: 10.1002/j.1460-2075.1982.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno T., Kobayashi M., Nishimura S. Purification of Excherichia coli methionine tRNAF and methionine tRNAM and studies on their biophysical and biochemical properties. Biochim Biophys Acta. 1968 Nov 20;169(1):80–94. doi: 10.1016/0005-2787(68)90010-5. [DOI] [PubMed] [Google Scholar]
- Talkad V., Schneider E., Kennell D. Evidence for variable rates of ribosome movement in Escherichia coli. J Mol Biol. 1976 Jun 14;104(1):299–303. doi: 10.1016/0022-2836(76)90015-2. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Jelenc P. C., Ehrenberg M., Kurland C. G. Rate of elongation of polyphenylalanine in vitro. Eur J Biochem. 1982 Feb;122(1):193–197. doi: 10.1111/j.1432-1033.1982.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Wagner E. G., Kurland C. G. Escherichia coli elongation factor G blocks stringent factor. Biochemistry. 1980 Mar 18;19(6):1234–1240. doi: 10.1021/bi00547a030. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Nussinov R., Brown R. J., Sussman J. L. Preferential codon usage in genes. Gene. 1981 May;13(4):355–364. doi: 10.1016/0378-1119(81)90015-9. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Ohtsuka E., Khorana H. G. Studies on polynucleotides. L. Synthetic deoxyribopolynucleotides as templates for the DNA polymerase of Escherichia coli: a new double-stranded DNA-like polymer containing repeating dinucleotide sequences. J Mol Biol. 1965 Nov;14(1):221–237. doi: 10.1016/s0022-2836(65)80242-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Blomberg C. The concentration dependence of the error frequencies and some related quantities in protein synthesis. J Theor Biol. 1979 May 7;78(1):113–120. doi: 10.1016/0022-5193(79)90329-1. [DOI] [PubMed] [Google Scholar]



