Abstract
Background: To our knowledge, the effect of magnesium supplementation on blood pressure (BP) in individuals with preclinical or noncommunicable diseases has not been previously investigated in a meta-analysis, and the findings from randomized controlled trials (RCTs) have been inconsistent.
Objective: We sought to determine the pooled effect of magnesium supplementation on BP in participants with preclinical or noncommunicable diseases.
Design: We identified RCTs that were published in English before May 2017 that examined the effect of magnesium supplementation on BP in individuals with preclinical or noncommunicable diseases through PubMed, ScienceDirect, Cochrane, clinicaltrials.gov, SpringerLink, and Google Scholar databases as well as the reference lists from identified relevant articles. Random- and fixed-effects models were used to estimate the pooled standardized mean differences (SMDs) with 95% CIs in changes in BP from baseline to the end of the trial in both systolic blood pressure (SBP) and diastolic blood pressure (DBP) between the magnesium-supplementation group and the control group.
Results: Eleven RCTs that included 543 participants with follow-up periods that ranged from 1 to 6 mo (mean: 3.6 mo) were included in this meta-analysis. The dose of elemental magnesium that was used in the trials ranged from 365 to 450 mg/d. All studies reported BP at baseline and the end of the trial. The weighted overall effects indicated that the magnesium-supplementation group had a significantly greater reduction in both SBP (SMD: −0.20; 95% CI: −0.37, −0.03) and DBP (SMD: −0.27; 95% CI: −0.52, −0.03) than did the control group. Magnesium supplementation resulted in a mean reduction of 4.18 mm Hg in SBP and 2.27 mm Hg in DBP.
Conclusion: The pooled results suggest that magnesium supplementation significantly lowers BP in individuals with insulin resistance, prediabetes, or other noncommunicable chronic diseases.
Keywords: blood pressure, cardiovascular diseases, insulin resistance, magnesium, magnesium supplementation, meta-analysis, noncommunicable chronic diseases, prediabetes, supplementation, type 2 diabetes
INTRODUCTION
Magnesium has been hypothesized to have a beneficial effect on hypertension by interacting with calcium (1, 2), reducing peripheral vascular resistance (1, 3, 4), increasing nitric oxide release (5) and endothelial prostaglandin I2 secretion (2, 6), and enhancing the effect of antihypertensive medications (7). A few meta-analyses and systematic reviews have summarized studies that were conducted mainly in the general populations and showed that magnesium supplementation provides a modest reduction in blood pressure (BP) (8–10). Studies have shown that even a modest reduction in BP is clinically relevant in reducing the risk of coronary heart diseases and stroke (11). In addition, a recent meta-analysis of 34 trials that was conducted in mixed participants showed an inverse association between magnesium supplementation and BP (12). Eight of the studies overlapped with the studies included in the present study (13–20). However, the authors of the meta-analysis did not conduct a stratified analysis by underlying metabolic conditions. In general, previous meta-analyses of randomized controlled trials (RCTs) have mixed studies in apparently healthy participants and participants with primary hypertension with studies in participants with preclinical health conditions or participants with chronic noncommunicable diseases. Note that individuals with or without preclinical or chronic disease may respond to magnesium supplementation differently. In the present study, we included only original studies in participants with underlying preclinical conditions or noncommunicable chronic diseases.
Some preclinical health conditions [e.g., insulin resistance (IR) and prediabetes] and noncommunicable chronic diseases (e.g., type 2 diabetes) are established risk factors for hypertension. Magnesium intake is inversely associated with these health conditions or diseases (21, 22). However, findings from RCTs (13–20, 23–25) on magnesium supplementation and BP in individuals with preclinical or chronic diseases have been inconsistent. To determine the weighted effect and provide additional information to the literature, we conducted this meta-analysis on the effect of magnesium supplementation on BP and hypertension in individuals with diagnosed IR, prediabetes, or other noncommunicable chronic diseases.
METHODS
Data sources and study selection
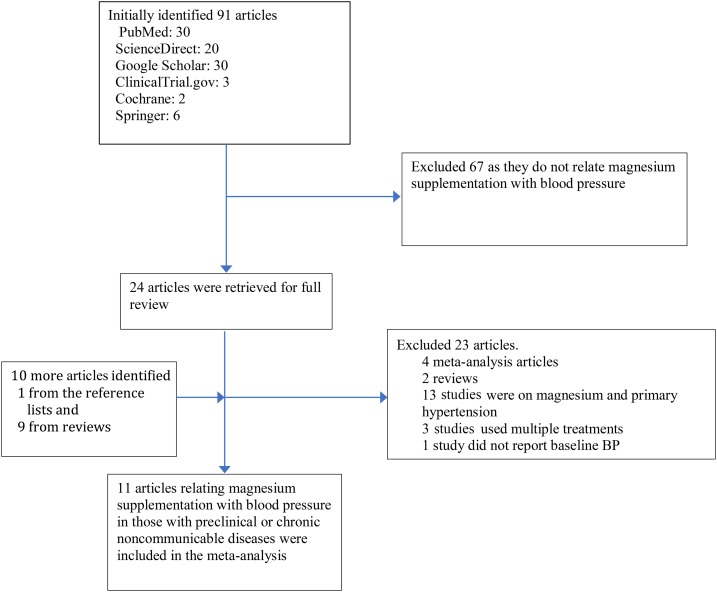
We searched for original trials that were published in English before May 2017 that related magnesium supplementation to BP or hypertension in individuals with IR, prediabetes, or noncommunicable chronic diseases including type 2 diabetes, cardiovascular diseases, renal diseases, or cancer. We searched for the journal articles in online databases including PubMed (www.ncbi.nlm.nih.gov/pubmed), ScienceDirect (www.sciencedirect.com), Cochrane, (www.cochrane.org) clinicaltrials.gov (www.clinicaltrials.gov), SpringerLink (www.link.springer.com), and Google Scholar (https://scholar.google.com). We used Medical Subject Headings terms including “magnesium,” OR “magnesium” AND “supplementation,” OR “mineral supplementation,” OR “dietary supplementation” combined with “secondary hypertension” OR “hypertension” OR “blood pressure” and “participants with insulin resistance” OR “participants with prediabetes” OR “participants with type 2 diabetes” OR “participants with cardiovascular diseases” OR “participants with renal disease” OR “participants with cancer.” We searched additional trials with the use of the reference lists of relevant articles. The study-selection process is presented in Figure 1.
FIGURE 1.
Study selection process. BP, blood pressure. Web addresses of searched online databases: PubMed (www.ncbi.nlm.nih.gov/pubmed), ScienceDirect (www.sciencedirect.com), Cochrane (Cochrane.org), clinicaltrials.gov, SpringerLink (Link.Springer.com/), and Google Scholar (scholar.google.com).
Inclusion and exclusion criteria
An original study was considered for inclusion if the design was an RCT that was conducted in humans. We included a trial in the meta-analysis if the exposure was magnesium supplementation; participants had IR, prediabetes, or any noncommunicable chronic diseases; and the main outcome was hypertension or BP. To be included, the trial should have reported differences in systolic blood pressure (SBP) and diastolic blood pressure (DBP) with SD, Hedges g, or Cohen’s d values or BP data with SDs that were reported at baseline and at the end of trial. A study was excluded if it was an observational study, an animal study, a review or meta-analysis, a trial in the general population, a trial without relevant effect measures, or a nonmagnesium-supplementation trial.
Data extraction
Two authors (DTD and PX) independently extracted the data and resolved discrepancies by a group discussion with a third author (KH). The extracted data included the first author’s name, year of publication, number of participants in each arm, type of magnesium supplementation, type of placebo, duration of intervention, baseline mean SBP and DBP, mean SBP and DBP at the end of the study, the SD in BP, and changes in BP.
Data synthesis
The pooled SD (Sp) was computed with the use of the following equation:
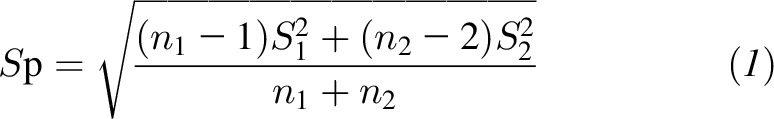 |
where S1 and S2 are the SDs, and n1 and n2 are sample sizes of the magnesium-supplementation group and control group, respectively (26). For trials that reported changes in BPs as mean differences and associated 95% CIs, the CIs were converted into SDs of changes as follows:
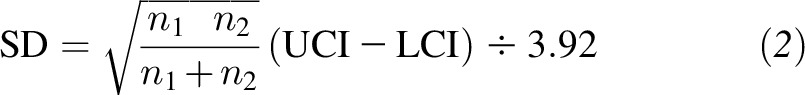 |
where the upper CI (UCI) and lower CI (LCI) are upper and lower confidence limits, respectively (26). For trials that did not report information on changes in BP, we estimated the SDs of changes in BP as follows:
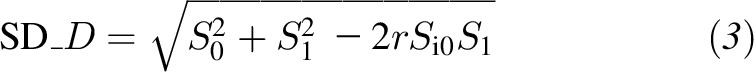 |
where S0 and S1 are the SDs of BP at baseline and at the end of the study, respectively, SD_D is the standard deviation of the changes in BP, and r is the assumed correlation between BP at baseline and the end of the study (27). We assumed r = 0.5 in our main analysis, and used r = 0.3 and 0.7 in the sensitivity analyses.
Statistical analysis
All of the included trials are RCTs that assessed the effect of magnesium supplementation on BP or hypertension in individuals with IR, prediabetes, type 2 diabetes, or cardiovascular diseases. We did not find a trial on the effect of magnesium supplementation on BP or hypertension in individuals with renal diseases or cancer.
We conducted a random-effects and fixed-effects meta-analysis to pool standardized mean differences (SMDs) between the magnesium supplementation group and the control group in the trials. The results from both methods are presented in forest plots, and we report the pooled summary from either method on the basis of heterogeneity. The SMD was calculated as follows:
 |
where μMg is the mean of the magnesium-supplementation group (mean change from baseline to the end of the trial), μP is the mean of the placebo arm or control group, and SP is the pooled SD.
We used Cochran’s chi-square test to examine the heterogeneity in the included trials and computed I2, which is the proportion of the total variation that was due to the heterogeneity between the trials, to quantify the degree of inconsistency across trials. We tested the null hypothesis of SMD = 0 with the use of the Z test. Egger’s test (28) and visual inspection of funnel plots were used to assess for the presence of publication bias. We conducted sensitivity analyses by eliminating one study at a time and replacing fixed-effects models with random-effects models. All analyses were conducted with the use of STATA statistical software (version 14; STATA Corp LP). P ≤ 0.05 was considered statistically significant.
RESULTS
Characteristics of included trials
Eleven trials (13–20, 23–25) with a total of 543 participants (278 subjects in the intervention groups) were identified. Eight trials (13, 16–20, 23, 25) used magnesium chloride for the magnesium-supplementation group and a similar-appearing placebo for the control group (Table 1). Two trials (15, 16) used magnesium aspartate hydrochloride with a similar-appearing placebo; 1 trial (14) used magnesium pidolate and a control with no placebo, and the other study (24) used Magnosolv-Granulat (a magnesium product by Meda that contains a total of 365 mg Mg) and an unspecified placebo. All trials (13–20, 23–25) used a parallel-group design.
TABLE 1.
Characteristics of trials included in the meta-analysis1
| SBP, mm Hg |
DBP, mm Hg |
||||||||||||
| Study (ref) | Year | Country | Preclinical or chronic condition | Duration | Group | Magnesium-supplementation regimen per day | n | Age at baseline, y | Sex, M/F, n | Baseline | End of the study | Baseline | End of the study |
| Rodríguez-Morán and Guerrero-Romero (18) | 2014 | Mexico | Insulin resistance | 4 mo | Treatment | 30 mL MgCl2 5% solution (equivalent to 383 mg elemental Mg) | 24 | 31.92 | NA | 111.3 ± 14.5 | 109.4 ± 12.4 | 71.5 ± 6.6 | 68.8 ± 7.4 |
| Placebo | 30 mL placebo | 23 | 39.52 | NA | 109.4 ± 12.4 | 116.6 ± 11.5 | 71.4 ± 9.3 | 76.8 ± 7.6 | |||||
| Simental-Mendía et al. (19) | 2014 | Mexico | Prediabetes | 12 wk | Treatment | 30 mL MgCl2 5% solution (equivalent to 383 mg elemental Mg) | 29 | 39.8 ± 163 | 16/13 | 114.8 ± 31.1 | 117.5 ± 18.6 | 76.9 ± 12.9 | 75 ± 14.5 |
| Placebo | 30 mL NaHCO3 0.1% solution | 28 | 41.1 ± 13.1 | 17/11 | 115.7 ± 21.4 | 123.4 ± 22.2 | 73.2 ± 10.5 | 76.9 ± 10.8 | |||||
| Simental-Mendía et al. (25) | 2012 | Mexico | Prediabetes | 12 wk | Treatment | 30 mL MgCl2 5% solution (equivalent to 383 mg elemental Mg) | 11 | 44.2 ± 10.8 | 4/7 | 116.9 ± 7.6 | 115.5 ± 18 | 66.7 ± 6.9 | 65.6 ± 10.4 |
| Placebo | 30 mL NaHCO3 0.1% solution | 11 | 43.2 ± 7.8 | 4/7 | 118.4 ± 8.2 | 114 ± 9.7 | 71.8 ± 7.5 | 66.6 ± 8.7 | |||||
| Mooren et al. (16) | 2011 | Germany | Insulin resistance | 6 mo | Treatment | Verum (magnesium aspartate hydrochloride), 365 mg Mg | 25 | NA | NA | 137.7 ± 14.9 | 131.4 ± 16.4 | 85.3 ± 9.4 | 81.6 ± 9.8 |
| Placebo | Not specified | 22 | NA | NA | 134.8 ± 15 | 133.1 ± 21.9 | 82.5 ± 9.6 | 83.2 ± 12.1 | |||||
| Barbagallo et al. (14) | 2010 | Italy | Type 2 diabetes | 1 mo | Treatment | 4.5 g Mg pidolate (equivalent to 368 mg Mg ion) | 30 | 71.0 ± 4.9 | 18/12 | 150 ± 7 | 148 ± 5 | 82 ± 5 | 79 ± 5 |
| Placebo | No placebo was used in the control | 30 | 71.2 ± 4.6 | 17/13 | 148 ± 8 | 147 ± 6.5 | 83 ± 5 | 82 ± 5 | |||||
| Guerrero-Romero and Rodríguez-Morán (13) | 2009 | Mexico | Type 2 diabetes | 4 mo | Treatment | 2.5 g MgCl2 (equivalent to 450 mg elemental Mg) | 40 | 58.9 ± 8.5 | 19/21 | 161.1 ± 26 | 140.7 ± 11.9 | 88.4 ± 14.5 | 79.7 ± 7.1 |
| Placebo | Inert placebo | 39 | 60.5 ± 9.4 | 19/20 | 154.5 ± 21.2 | 149.8 ± 20.6 | 84.9 ± 12.4 | 83.8 ± 9.7 | |||||
| Barragán-Rodríguez et al. (23) | 2008 | Mexico | Type 2 diabetes | 12 wk | Treatment | 50 mL MgCl2 5% solution (equivalent to 450 mg elemental Mg) | 12 | 69 ± 5.9 | NA | 134.1 ± 19.2 | 135.2 ± 20.5 | 77.2 ± 3.1 | 77 ± 3.6 |
| Placebo | Imipramine 50 mg | 9 | 66.4 ± 6.1 | NA | 141.0 ± 20.1 | 143.7 ± 19.9 | 84.7 ± 6.1 | 87.6 ± 6.4 | |||||
| Guerrero-Romero et al. (20) | 2004 | Mexico | Type 2 diabetes | 12 wk | Treatment | 2.5 g MgCl2 (equivalent to 450 mg elemental Mg) | 32 | 43.0 ± 7.9 | NA | 110 ± 8.4 | 108 ± 8.1 | 73 ± 7.5 | 72.3 ± 7.4 |
| Placebo | Not specified | 31 | 42.2 ± 6.8 | NA | 111 ± 12 | 110 ± 11 | 73 ± 9 | 72.4 ± 8.9 | |||||
| Rodríguez-Morán and Guerrero-Romero (17) | 2003 | Mexico | Insulin resistance | 16 wk | Treatment | 2.5 g MgCl2 (equivalent to 450 mg elemental Mg) | 32 | 59.7 ± 8.3 | NA | 148.2 ± 32.3 | 140.2 ± 28.1 | 86.3 ± 17 | 82.7 ± 16.4 |
| Placebo | Not specified | 31 | 54.1 ± 9.6 | NA | 138.1 ± 25.6 | 135 ± 19.6 | 80.5 ± 14.6 | 79.1 ± 13.5 | |||||
| Shechter et al. (24) | 2000 | United States | Coronary artery disease | 6 mo | Treatment | Magnesium product4 containing 365 mg total Mg (equivalent to 200 mg elemental Mg 2 times/d) | 25 | 68 ± 10 | 21/3 | 147 ± 21 | 143 ± 20 | 69 ± 10 | 69 ± 10 |
| Placebo | Not specified | 25 | 66 ± 12 | 19/6 | 145 ± 18 | 140 ± 19 | 69 ± 14 | 68 ± 7 | |||||
| de Valk et al. (15) | 1998 | Netherlands | Type 2 diabetes | 3 mo | Treatment | 15 mmol Mg aspartate HCl (365 mg Mg) | 18 | 63.0 ± 8.2 | 12/6 | 162.6 ± 23.3 | 158.7 ± 20 | 84 ± 11.5 | 82.9 ± 8.3 |
| Placebo | Not specified | 16 | 62.0 ± 7.3 | 8/8 | 157.4 ± 23.6 | 146.9 ± 21.8 | 83 ± 14.2 | 77.1 ± 8.4 | |||||
DBP, diastolic blood pressure; NA, not available; ref, reference; SBP, systolic blood pressure.
Mean.
Mean ± SD (all such values).
Magnosolv-Granulat (Meda).
The duration of the trials ranged from 1 to 6 mo (with an average of 3.6 mo) (Table 1). The mean age of the participants ranged from 31.9 to 71.0 y in the intervention groups and from 39.5 to 71.2 y in the control groups. Two trials (13, 18) reported a significant beneficial effect of magnesium supplementation on both SBP and DBP, whereas the rest of the trials (14–17, 19, 20, 23–25) did not achieve a significant reduction in SBP. One trial reported a significant reduction only in DBP (14).
Change in BP from baseline to the end of the trials
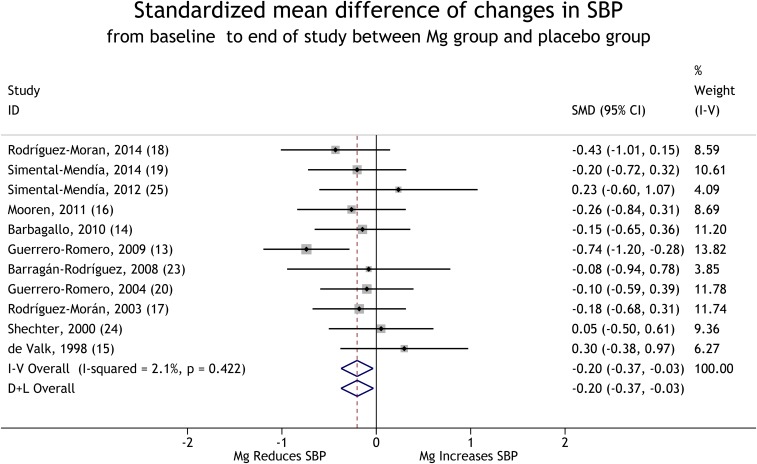
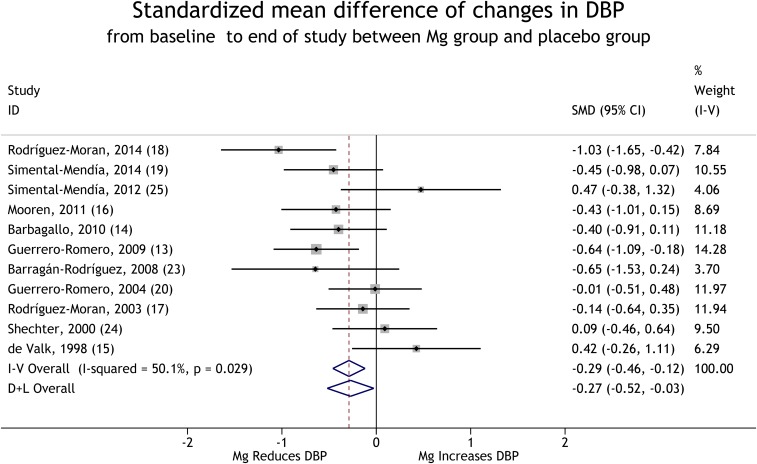
Eleven trials provided data that compared mean changes in BP from baseline to the end of the trials between the magnesium-supplementation group and the control group (13–20, 23–25). With the use of a random-effects model, the pooled results suggest that magnesium supplementation significantly reduces BP in the defined population (for SBP, see Figure 2), and for DBP, see Figure 3). These reductions corresponded to a weighted mean decrement in SBP by 2.22 mm Hg and in DBP by 2.54 mm Hg when comparing the supplementation group with the control group. The magnesium supplementation resulted in a mean SBP reduction of 4.18 mm Hg and a mean DBP reduction of 2.27 mm Hg for the BP at the end of trial compared with baseline.
FIGURE 2.
Forest plot of SMDs (95% CIs) of changes in SBP from baseline to the end of trials for the comparison of the Mg-supplementation group with the placebo group. The horizontal line next to each trial indicates the SMD and associated 95% CI. For each study, the size of the rectangular box indicates the relative weight of the trial and the diamond at the center indicates the point estimate. The dashed line indicates where the point estimate in each study lies compared to the pooled estimates, and the line passes through the pooled estimates. The solid line passes through SMD = 0, and 95% CIs that cross it are not significant. The diamonds at the bottom of the figure indicate pooled SMDs (95% CIs). D+L, DerSimonian and Laird for the random-effects method; ID, identifier; I-V, inverse variance for the fixed-effects method; SBP, systolic blood pressure; SMD, standardized mean difference.
FIGURE 3.
Forest plot of SMDs (95% CIs) of changes in DBP from baseline to the end of trials for the comparison of the Mg-supplementation with the placebo group. The horizontal line next to each trial indicates the SMD and associated 95% CI. For each study, the size of the rectangular box indicates the relative weight of the trial and the diamond at the center indicates the point estimate. The dashed line indicates where the point estimate in each study lies compared to the pooled estimates, and the line passes through the pooled estimates. The solid line passes through SMD = 0, and 95% CIs that cross it are not significant. The diamonds at the bottom of the figure indicate pooled SMDs (95% CIs) of all trials. DBP, diastolic blood pressure; D+L, DerSimonian and Laird for the random-effects method; I-V, inverse variance for the fixed-effects method; SMD, standardized mean difference.
Egger’s test suggested that there was no strong evidence of a publication bias for both SBP (P = 0.07) and DBP (P = 0.49). No significant heterogeneity was shown in the trials for SBP [χ2(10) = 10.21, P = 0.42], but for DBP, there was significant heterogeneity in the trials [χ2(10) = 20.03, P = 0.03].
A sensitivity analysis showed that the mean change in SBP was significantly different from zero in all analyses except in the analysis that excluded Guerrero-Romero and Rodríguez-Morán (13). In all the sensitivity analyses except those that excluded Simental-Mendía et al. (19), Mooren et al. (16), Barbagallo et al. (14), Guerrero-Romero and Rodríguez-Morán (13), or Barragán-Rodríguez et al. (23), the change in DBP was significantly different from zero (r was assumed to be 0.5) (Table 2). The result also remained the same when r = 0.7 was used but disappeared when r = 0.3 was used (Table 2).
TABLE 2.
Sensitivity analysis changes in blood pressure1
| Study omitted (ref) | ΔSBP | ΔDBP |
| Rodríguez-Morán and Guerrero-Romero (18) | −0.18 (−0.36, 0.00) | −0.23 (−0.40, −0.05)* |
| Simental-Mendía et al. (19) | −0.20 (−0.38, −0.02)* | −0.25 (−0.52, 0.02) |
| Simental-Mendía et al. (25) | −0.22 (−0.40, −0.05)* | −0.32 (−0.50, −0.15)* |
| Mooren et al. (16) | −0.20 (−0.38, −0.02) | −0.26 (−0.53, 0.02) |
| Barbagallo et al. (14) | −0.21 (−0.39, −0.03)* | −0.26 (−0.53, 0.02) |
| Guerrero-Romero and Rodríguez-Morán (13) | −0.12 (−0.30, 0.07) | −0.23 (−0.49, 0.04) |
| Barragán-Rodríguez et al. (23) | −0.21 (−0.38, −0.04)* | −0.25 (−0.51, 0.01) |
| Guerrero-Romero et al. (20) | −0.22 (−0.40, −0.04)* | −0.30 (−0.57, −0.03)* |
| Rodríguez-Morán and Guerrero-Romero (17) | −0.21 (−0.39, −0.03)* | −0.29 (−0.56, −0.01)* |
| Shechter et al. (24) | −0.23 (−0.41, −0.05)* | −0.31 (−0.57, −0.05)* |
| de Valk et al. (15) | −0.24 (−0.41, −0.06)* | −0.33 (−0.57, −0.10)* |
| None† | −0.20 (−0.37, −0.03)* | −0.27 (−0.52, −0.03)* |
| None‡ | −0.25 (−0.42, −0.08)* | −0.34 (−0.65, −0.03)* |
| None§ | −0.18 (−0.35, 0.01) | −0.25 (−0.42, 0.08) |
All values are standardized mean differences (95% CIs). In each row, results are from a fixed-effects model without the inclusion of the study listed. *Pooled results were significant when omitting the study listed in the row. †r = 0.5, ‡r = 0.7, §r = 0.3. DBP, diastolic blood pressure; ref, reference; SBP, systolic blood pressure; Δ, difference.
DISCUSSION
The results of this meta-analysis indicate that magnesium supplementation significantly reduces both SBP and DBP in individuals with IR, prediabetes, or other noncommunicable chronic diseases. The findings from this study are generally consistent with results from previous meta-analyses of RCTs in the general population or in participants with primary hypertension and provide additional evidences to the literature supporting the beneficial effect of magnesium supplementation on reducing BP (8–10, 12). In the previous meta-analyses, studies in individuals with or without underlying preclinical health conditions and chronic diseases were combined. This use of this method might partially explain the differences in the magnitude of the effect sizes in the present and previous meta-analyses because participants with underlying preclinical metabolic disease or noncommunicable chronic disease may respond to magnesium supplementation differently from participants without these conditions. In addition, the variations in the population, follow-up period, baseline BP, and dosage and form of magnesium supplementation in included studies may have contributed to the differences in the magnitude of the effect sizes.
The average reductions in blood pressure that were due to magnesium supplementation as observed in the present study (SBP : 4.18 mm Hg; DBP: 2.27 mm Hg) might have relevant clinical effects on cardiovascular health. A clinical trial examined the effects of antihypertensive medications on cardiac outcomes and showed that a reduction of 0.8–2 mm Hg SBP was clinically relevant in reducing the incidence of coronary heart disease, heart failure, and stroke. The trial suggested that a reduction of BP by 2–3 mm Hg might account for a difference of stroke rate by 6–12% between antihypertensive medications (11). This suggestion was also supported by other clinical trials (29) and observational studies (30). Thus, the magnitude of BP reduction in the present meta-analysis is of great clinical significance (31).
The mechanism of the beneficial effect of magnesium on hypertension may include the modulation of vascular tone (1, 3, 4) and the prevention of endothelial dysfunction, carotid intima thickness (32–35), atherosclerosis (36), IR, and hyperglycemia (4). The beneficial effect of magnesium on BP is 2-fold. In one way, magnesium supplementation may directly lower BP, whereas in contrast, it may also improve preclinical conditions and chronic diseases that commonly predispose individuals to hypertension. In addition, magnesium was suggested to have synergetic effects with antihypertensive medications (7). Moreover, accumulated evidences have suggested that magnesium intake is inversely associated with preclinical conditions such as IR (17) and prediabetes (21) and noncommunicable chronic diseases such as type 2 diabetes (37, 38), which are well-known risk factors for hypertension. A meta-analysis showed that magnesium supplementation improves glycemic control in type 2 diabetes patients (39). Furthermore, hypertension affects ∼70% of individuals with diabetes, which is twice the percentage of individuals without diabetes (40). The findings from previous studies have also linked hypertension, hyperinsulinemia, and cardiovascular diseases together (41, 42). A study suggested that hypertension and type 2 diabetes have a synergistic negative health impact on the development of other chronic diseases (43). Approximately 60–80% of individuals with diabetes die of cardiovascular complications and nearly 75% of the cardiovascular complications have been attributed to hypertension (44). Studies have also reported that magnesium has beneficial effects on cardiovascular health (22, 33, 45). In particular, one study suggested that the inverse association between magnesium intake and risk of fatal coronary heart disease may be mediated through hypertension (46). A trial documented that magnesium supplementation improved endothelial function, which resulted in a relatively more flow-mediated vasodilation compared with the placebo group and concluded that the potential mechanism through which magnesium intake is beneficial in coronary artery disease might be through an improvement of endothelial function (24). Another trial reported a nonlinear J-shaped relation of serum magnesium concentrations with all-cause mortality and cardiovascular mortality (47). Thus, magnesium supplementation may play role in breaking the cycle between the preclinical conditions and type 2 diabetes and cardiovascular diseases through the lowering of BP.
Our study has some strengths to highlight. To the best of our knowledge, this study is the first meta-analysis on the effect of magnesium supplementation on BP in participants with IR, prediabetes, type 2 diabetes, and cardiovascular diseases. In this study, the statistical method to calculate the SDs of the pooled changes in SBP and DBP was based on the assumed correlation between the baseline and trial-end BPs when the original trials did not report them. However, the robustness was checked using sensitivity analyses with varying correlation coefficients. The sensitivity analyses indicate that, except in the analysis that excluded Barbagallo et al. (14) (diastolic) or Guerrero-Romero and Rodríguez-Morán (13) or when the correlation coefficient between the baseline and the end of study was assumed to be 0.3, the association remained. In addition, the data indicate no evidence of publication bias. In addition, the studies are relatively more uniform than other previous studies because we included only studies in participants with underlying metabolic conditions. The absence of marked heterogeneity in SBP provided good evidence of the uniformity of the included studies.
However, our study also has some limitations that are in part due to inherent limitations in the original trials. The limitations of the study include relatively short trial durations, limited trials in participants with cardiovascular diseases, and a lack of trials in subjects with renal diseases or cancer, thereby possibly confounding the effect of dietary magnesium intake and the heterogeneity between some of the trials, particularly for DBP. The heterogeneity might partially be attributable to differences in dietary magnesium intake, the duration of study, the dose of supplemental magnesium, the type of supplementation used, unadjusted confounders such as antihypertension drugs, antigastritis drugs, and glucose-lowering drugs, differences in participants’ characteristics (e.g., diet and age), and study design.
In conclusion, the results of this study suggest that magnesium supplementation has a beneficial effect on BP or hypertension in participants with IR, prediabetes, or noncommunicable chronic diseases such as diabetes or cardiovascular disease. Because of the results of our study and the findings from previous studies, patients with hypertension and underlying preclinical metabolic conditions including IR and prediabetes and patients with type 2 diabetes or cardiovascular diseases may benefit from magnesium supplementation in the reduction of BP and the amelioration of the underlying health conditions. The findings from this study add complementary evidence to the literature on the effect of magnesium supplementation on BP. Because of the heterogeneity in the included trials on DBP and no study, to our knowledge, in individuals with renal disease or cancer, future large-scale, well-designed, double-blind, randomized, placebo-controlled clinical trials are warranted to provide more solid evidence of the benefit of magnesium supplementation on BP and possibly on disease outcomes in patients with IR, prediabetes, or noncommunicable chronic diseases.
Acknowledgments
The authors’ responsibilities were as follows—KH and DTD: conceptualized the study; DTD, AR, and MS: identified the included studies; DTD and PX: extracted the data; DTD: conducted the statistical analysis and drafted and revised the manuscript; PX: critically reviewed the statistical design and guided the analysis; KH: performed the critical review and revised the manuscript; YS, AR, and MS: read the manuscript and made critical reviews with substantial contributions; and all authors: read and approved the final manuscript as submitted. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BP, blood pressure; DBP, diastolic blood pressure; IR, insulin resistance; RCT, randomized controlled trial; SBP, systolic blood pressure; SMD, standardized mean difference.
REFERENCES
- 1.Teragawa H, Matsuura H, Chayama K, Oshima T. Mechanisms responsible for vasodilation upon magnesium infusion in vivo: clinical evidence. Magnes Res 2002;15:241–6. [PubMed] [Google Scholar]
- 2.Satake K, Lee JD, Shimizu H, Uzui H, Mitsuke Y, Yue H, Ueda T. Effects of magnesium on prostacyclin synthesis and intracellular free calcium concentration in vascular cells. Magnes Res 2004;17:20–7. [PubMed] [Google Scholar]
- 3.Feyh A, Bracero L, Lakhani HV, Santhanam P, Shapiro JI, Khitan Z, Sodhi K. Role of dietary components in modulating hypertension. J Clin Exp Cardiolog 2016;7;pii: 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, Pineo A, Busardo A, Paolisso G. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 2003;24:39–52. [DOI] [PubMed] [Google Scholar]
- 5.Cunha AR, Umbelino B, Correia ML, Neves MF. Magnesium and vascular changes in hypertension. Int J Hypertens 2012;2012:754250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiger H, Wanner C. Magnesium in disease. Clin Kidney J 2012;5 Suppl 1:i25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosanoff A. Magnesium supplements may enhance the effect of antihypertensive medications in stage 1 hypertensive subjects. Magnes Res 2010;23:27–40. [DOI] [PubMed] [Google Scholar]
- 8.Kass L, Weekes J, Carpenter L. Effect of magnesium supplementation on blood pressure: a meta-analysis. Eur J Clin Nutr 2012;66:411–8. [DOI] [PubMed] [Google Scholar]
- 9.Jee SH, Miller ER III, Guallar E, Singh VK, Appel LJ, Klag MJ. The effect of magnesium supplementation on blood pressure: a meta-analysis of randomized clinical trials. Am J Hypertens 2002;15:691–6. [DOI] [PubMed] [Google Scholar]
- 10.Rosanoff A, Plesset MR. Oral magnesium supplements decrease high blood pressure (SBP>155 mmHg) in hypertensive subjects on anti-hypertensive medications: a targeted meta-analysis. Magnes Res 2013;26:93–9. [DOI] [PubMed] [Google Scholar]
- 11.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA 2002;288:2981–97. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension 2016;68:324–33. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero-Romero F, Rodríguez-Morán M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, double-blind, placebo-controlled clinical trial. J Hum Hypertens 2009;23:245–51. [DOI] [PubMed] [Google Scholar]
- 14.Barbagallo M, Dominguez LJ, Galioto A, Pineo A, Belvedere M. Oral magnesium supplementation improves vascular function in elderly diabetic patients. Magnes Res 2010;23:131–7. [DOI] [PubMed] [Google Scholar]
- 15.de Valk HW, Verkaaik R, van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring type 2 diabetic patients. Diabet Med 1998;15:503–7. [DOI] [PubMed] [Google Scholar]
- 16.Mooren FC, Kruger K, Volker K, Golf SW, Wadepuhl M, Kraus A. Oral magnesium supplementation reduces insulin resistance in non-diabetic subjects - a double-blind, placebo-controlled, randomized trial. Diabetes Obes Metab 2011;13:281–4. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: a randomized double-blind controlled trial. Diabetes Care 2003;26:1147–52. [DOI] [PubMed] [Google Scholar]
- 18.Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation improves the metabolic profile of metabolically obese, normal-weight individuals: a randomized double-blind placebo-controlled trial. Arch Med Res 2014;45:388–93. [DOI] [PubMed] [Google Scholar]
- 19.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. Oral magnesium supplementation decreases C-reactive protein levels in subjects with prediabetes and hypomagnesemia: a clinical randomized double-blind placebo-controlled trial. Arch Med Res 2014;45:325–30. [DOI] [PubMed] [Google Scholar]
- 20.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-Martínez AM, Montes-Villarreal J, Treviño-Ortiz JH, Rodríguez-Morán M. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab 2004;30:253–8. [DOI] [PubMed] [Google Scholar]
- 21.Cahill F, Shahidi M, Shea J, Wadden D, Gulliver W, Randell E, Vasdev S, Sun G. High dietary magnesium intake is associated with low insulin resistance in the Newfoundland population. PLoS One 2013;8:e58278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hruby A, O’Donnell CJ, Jacques PF, Meigs JB, Hoffmann U, McKeown NM. Magnesium intake is inversely associated with coronary artery calcification: the Framingham Heart Study. JACC Cardiovasc Imaging 2014;7:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barragán-Rodríguez L, Rodríguez-Morán M, Guerrero-Romero F. Efficacy and safety of oral magnesium supplementation in the treatment of depression in the elderly with type 2 diabetes: a randomized, equivalent trial. Magnes Res 2008;21:218–23. [PubMed] [Google Scholar]
- 24.Shechter M, Sharir M, Labrador MJ, Forrester J, Silver B, Bairey Merz CN. Oral magnesium therapy improves endothelial function in patients with coronary artery disease. Circulation 2000;102:2353–8. [DOI] [PubMed] [Google Scholar]
- 25.Simental-Mendía LE, Rodríguez-Morán M, Reyes-Romero MA, Guerrero-Romero F. No positive effect of oral magnesium supplementation in the decreases of inflammation in subjects with prediabetes: a pilot study. Magnes Res 2012;25:140–6. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan LM. Essentials of biostatistics in public health.2nd ed. Riegelman R, editor. Burlington (MA): Jones & Bartlett Learning; 2012. [Google Scholar]
- 27.Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, Morton SC. Handling continuous outcomes in quantitative synthesis. In: Methods guide for effectiveness and comparative effectiveness reviews. Rockville (MD): Agency for Healthcare Research and Quality (US); 2008. [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000;355:253–9. [PubMed] [Google Scholar]
- 30.Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PP, Folsom AR, Heiss G, MacLehose RF, Matsushita K, Avery CL. Reducing the blood pressure–related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc 2015;4:e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sever PS, Poulter NR, Elliott WJ, Jonsson MC, Black HR, Sever PS, Poulter NR, Elliott WJ, Jonsson MC, Black HR. Blood pressure reduction is not the only determinant of outcome. Circulation 2006;113:2754–72, discussion 73–4. [DOI] [PubMed] [Google Scholar]
- 32.Kupetsky-Rincon EA, Li Q, Uitto J. Magnesium reduces carotid intima-media thickness in a mouse model of pseudoxanthoma elasticum: a novel treatment biomarker. Clin Transl Sci 2012;5:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turgut F, Kanbay M, Metin MR, Uz E, Akcay A, Covic A. Magnesium supplementation helps to improve carotid intima media thickness in patients on hemodialysis. Int Urol Nephrol 2008;40:1075–82. [DOI] [PubMed] [Google Scholar]
- 34.Mortazavi M, Moeinzadeh F, Saadatnia M, Shahidi S, McGee JC, Minagar A. Effect of magnesium supplementation on carotid intima-media thickness and flow-mediated dilatation among hemodialysis patients: a double-blind, randomized, placebo-controlled trial. Eur Neurol 2013;69:309–16. [DOI] [PubMed] [Google Scholar]
- 35.Dashti GR, Rezaei M, Adibi A, Golshan Iranpour F. Assessment of serum magnesium level and its relation with atherosclerotic carotid intima media thickness in post-menopausal women. Int J of Biomed & Adv Res 2015;3:203–11. [Google Scholar]
- 36.Altura BT, Brust M, Bloom S, Barbour RL, Stempak JG, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci USA 1990;87:1840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson SC, Wolk A. Magnesium intake and risk of type 2 diabetes: a meta-analysis. J Intern Med 2007;262:208–14. [DOI] [PubMed] [Google Scholar]
- 38.Dong JY, Xun P, He K, Qin LQ. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care 2011;34:2116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in type 2 diabetes: a meta-analysis of randomized double-blind controlled trials. Diabet Med 2006;23:1050–6. [DOI] [PubMed] [Google Scholar]
- 40.Lago RM, Singh PP, Nesto RW. Diabetes and hypertension. Nat Clin Pract End Met 2007;3:667. [DOI] [PubMed] [Google Scholar]
- 41.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest 2000;106:453–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reaven GM. Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med 2005;47:201–10. [PubMed] [Google Scholar]
- 43.Nosadini R. Hypertension and renal complications in type 2 diabetes. Semin Vasc Med 2002;2:109–19. [DOI] [PubMed] [Google Scholar]
- 44.Campbell NRC, Gilbert RE, Leiter LA, Larochelle P, Tobe S, Chockalingam A, Ward R, Morris D, Tsuyuki RT, Harris SB. Hypertension in people with type 2 diabetes: update on pharmacologic management. Can Fam Physician 2011;57:997–1002. [PMC free article] [PubMed] [Google Scholar]
- 45.Qu X, Jin F, Hao Y, Li H, Tang T, Wang H, Yan W, Dai K. Magnesium and the risk of cardiovascular events: a meta-analysis of prospective cohort studies. PLoS One 2013;8:e57720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chiuve SE, Sun Q, Curhan GC, Taylor EN, Spiegelman D, Willett WC, Manson JE, Rexrode KM, Albert CM. Dietary and plasma magnesium and risk of coronary heart disease among women. J Am Heart Assoc 2013;2:e000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int 2014;85:174–81. [DOI] [PubMed] [Google Scholar]