Abstract
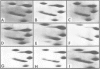
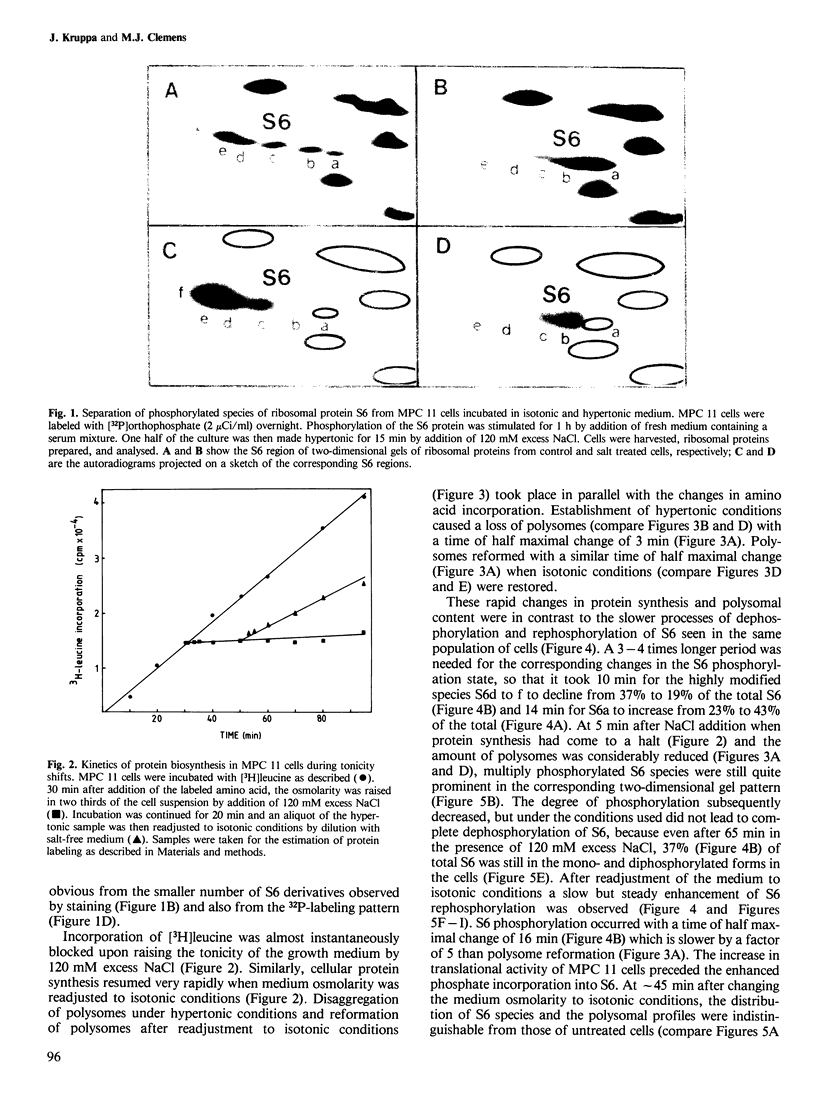
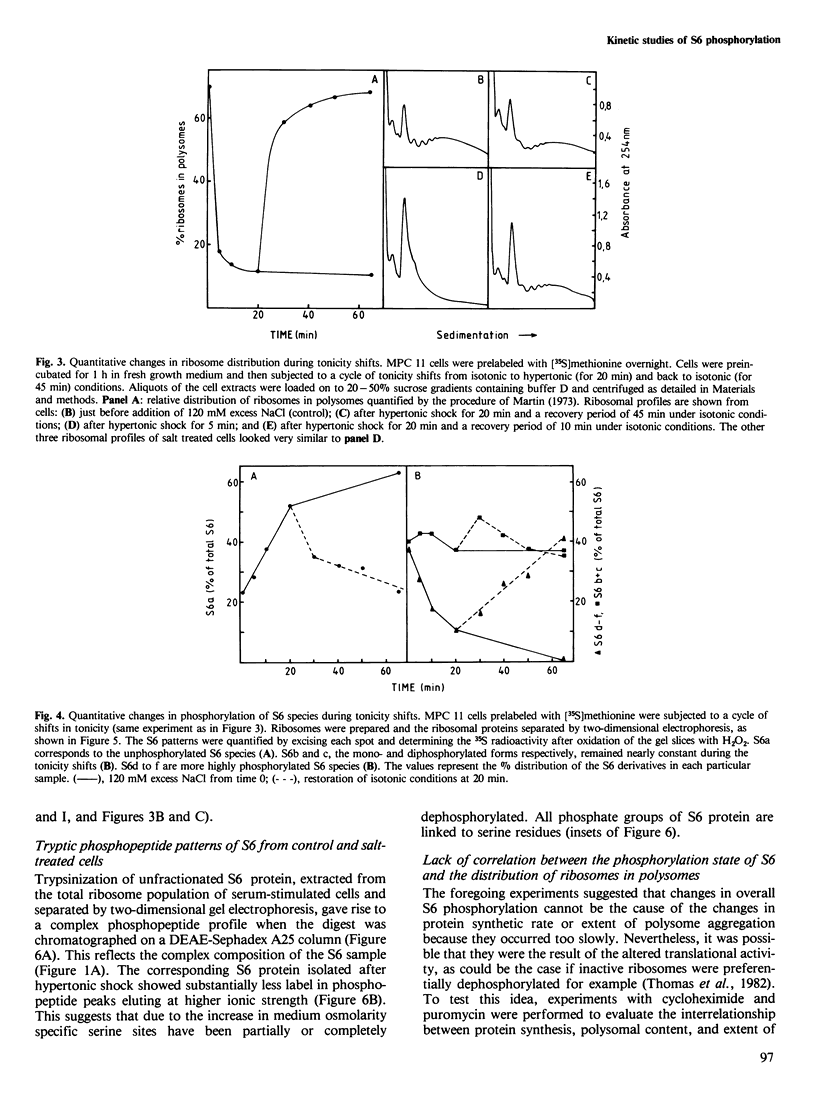
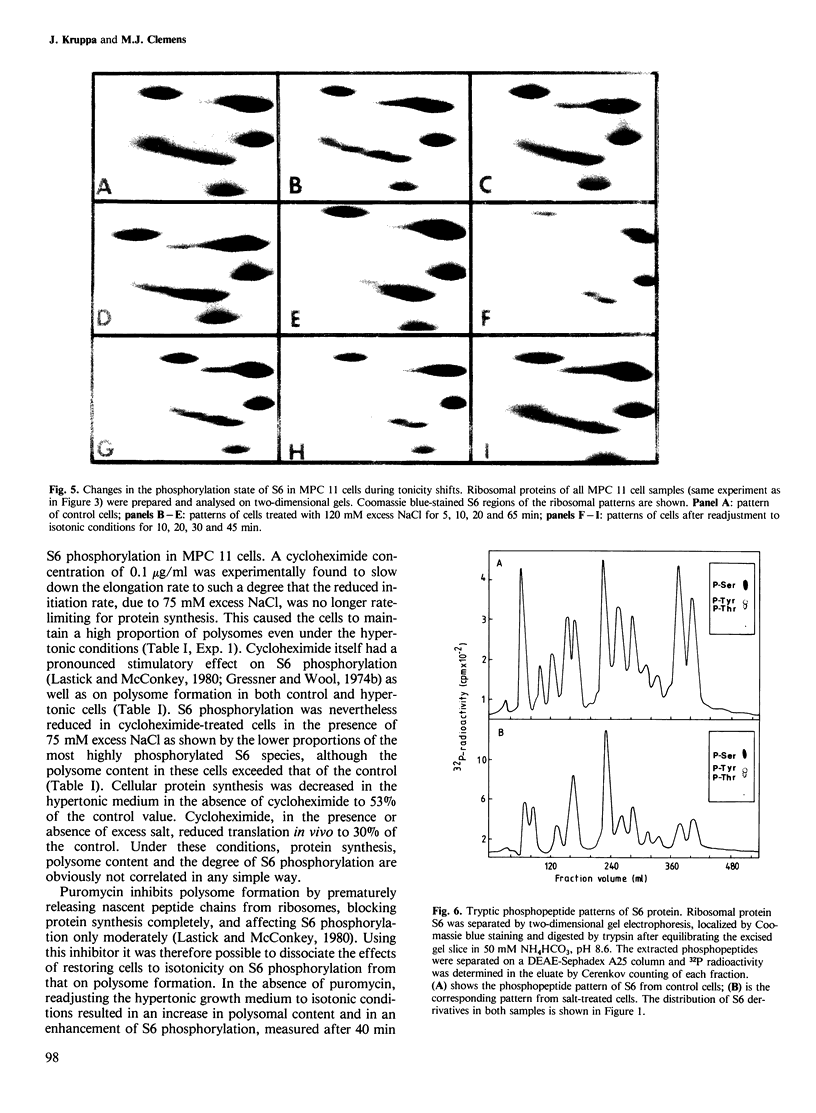
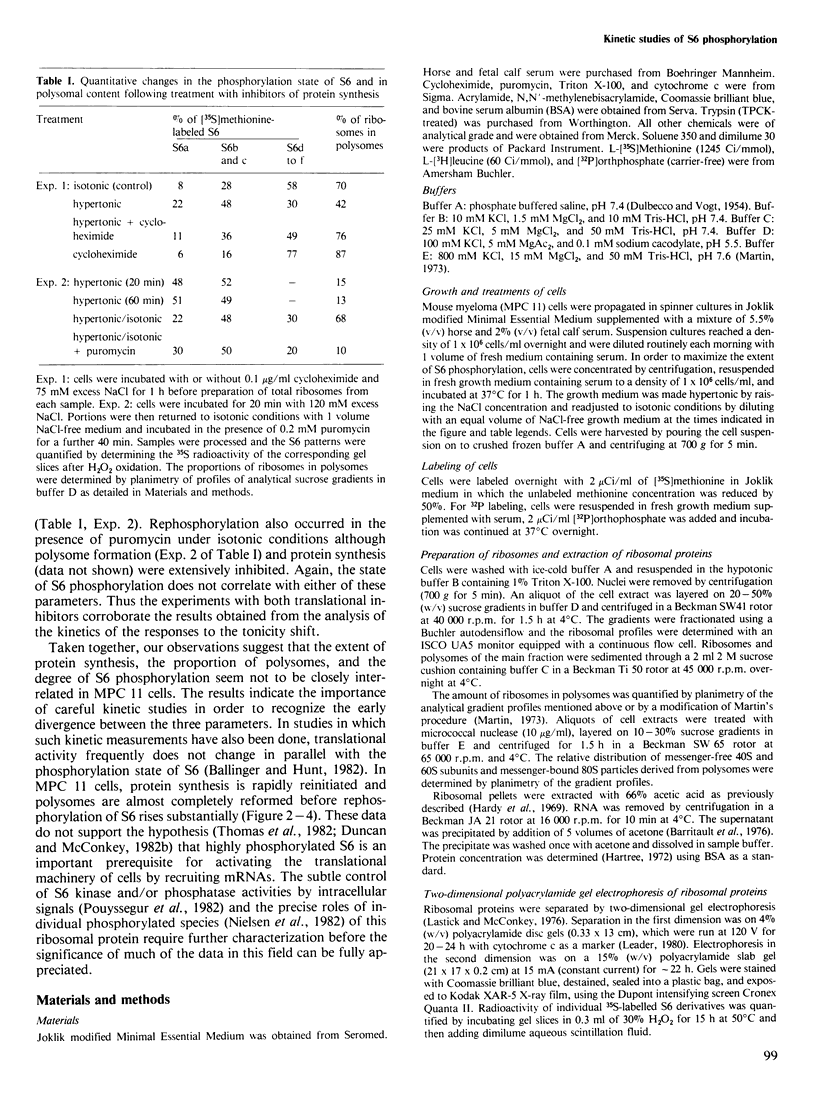
Mouse myeloma (MPC 11) cells respond rapidly to hypertonic conditions by shutting down protein synthesis at the level of polypeptide chain initiation. Translational activity recovers equally quickly upon a return to isotonicity. Disaggregation and reformation of polysomes occur in parallel to the changes in protein synthesis. Ribosomal protein S6 becomes dephosphorylated under hypertonic conditions and rephosphorylated when isotonic conditions are restored. The kinetics with which these changes occur are, however, too slow to account for the changes in protein synthesis. Treatment of the cells with a low concentration of cycloheximide allows reformation of polysomes under hypertonic conditions; conversely, puromycin prevents the restoration of polysomes which otherwise occurs on return to isotonicity. Neither inhibitor prevents the changes in S6 phosphorylation resulting from the tonicity shifts. We conclude that the overall extent of phosphorylation of S6 neither regulates nor is determined by the rate of protein synthesis and is not obligatorily related to the proportion of ribosomes in polysomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballinger D. G., Hunt T. Fertilization of sea urchin eggs is accompanied by 40 S ribosomal subunit phosphorylation. Dev Biol. 1981 Oct 30;87(2):277–285. doi: 10.1016/0012-1606(81)90151-2. [DOI] [PubMed] [Google Scholar]
- Barritault D., Expert-Bezancon A., Guérin M. F., Hayes D. The use of acetone precipitation in the isolation of ribosomal proteins. Eur J Biochem. 1976 Mar 16;63(1):131–135. doi: 10.1111/j.1432-1033.1976.tb10215.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Preferential utilization of phosphorylated 40-S ribosomal subunits during initiation complex formation. Eur J Biochem. 1982 Apr;123(3):535–538. doi: 10.1111/j.1432-1033.1982.tb06564.x. [DOI] [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Rapid alterations in initiation rate and recruitment of inactive RNA are temporally correlated with S6 phosphorylation. Eur J Biochem. 1982 Apr;123(3):539–544. doi: 10.1111/j.1432-1033.1982.tb06565.x. [DOI] [PubMed] [Google Scholar]
- Eil C., Wool I. G. Function of phosphorylated ribosomes. The activity of ribosomal subunits phosphorylated in vitro by protein kinase. J Biol Chem. 1973 Jul 25;248(14):5130–5136. [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974 Nov 10;249(21):6917–6925. [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The stimulation of the phosphorylation of ribosomal protein S6 by cycloheximide and puromycin. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1482–1490. doi: 10.1016/0006-291x(74)90365-9. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Kruppa J., Darmer D., Kalthoff H., Richter D. The phosphorylation of ribosomal protein S6 from progesterone-stimulated Xenopus laevis oocytes. Kinetic studies and phosphopeptide analysis. Eur J Biochem. 1983 Jan 1;129(3):537–542. doi: 10.1111/j.1432-1033.1983.tb07082.x. [DOI] [PubMed] [Google Scholar]
- Kruppa J., Martini O. H. Dephosphorylation of one 40S ribosomal protein in MPC 11 cells induced by hypertonic medium. Biochem Biophys Res Commun. 1978 Nov 14;85(1):428–435. doi: 10.1016/s0006-291x(78)80060-6. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Control of ribosomal protein phosphorylation in HeLa cells. Biochem Biophys Res Commun. 1980 Aug 14;95(3):917–923. doi: 10.1016/0006-291x(80)91560-0. [DOI] [PubMed] [Google Scholar]
- Lastick S. M., McConkey E. H. Exchange and stability of HeLa ribosomal proteins in vivo. J Biol Chem. 1976 May 25;251(10):2867–2875. [PubMed] [Google Scholar]
- Leader D. P. A tracking marker for the first dimension of the two-dimensional gel electrophoresis of ribosomal proteins. J Biochem Biophys Methods. 1980 Oct;3(4):247–248. doi: 10.1016/0165-022x(80)90064-0. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Thomas A., Voorma H. O. The protein synthetic activity in vitro of ribosomes differing in the extent of phosphorylation of their ribosomal proteins. Biochim Biophys Acta. 1981 Nov 27;656(1):69–75. doi: 10.1016/0005-2787(81)90028-9. [DOI] [PubMed] [Google Scholar]
- Martin T. E. A simple general method to determine the proportion of active ribosomes in eukaryotic cells. Exp Cell Res. 1973 Aug;80(2):496–498. doi: 10.1016/0014-4827(73)90333-9. [DOI] [PubMed] [Google Scholar]
- Martini O. H., Kruppa J. Ribosomal phosphoproteins of mouse myeloma cells. Changes in the degree of phosphorylation induced by hypertonic initiation block. Eur J Biochem. 1979 Apr 2;95(2):349–358. doi: 10.1111/j.1432-1033.1979.tb12972.x. [DOI] [PubMed] [Google Scholar]
- Meedel T. H., Levine E. M. Regulation of protein synthesis in human diploid fibroblasts: reduced initiation efficiency in resting cultures. J Cell Physiol. 1978 Feb;94(2):229–242. doi: 10.1002/jcp.1040940212. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Duncan R., McConkey E. H. Phosphorylation of ribosomal protein S6. Relationship to protein synthesis in HeLa cells. Eur J Biochem. 1981 Dec;120(3):523–527. doi: 10.1111/j.1432-1033.1981.tb05731.x. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Thomas G., Maller J. L. Increased phosphorylation of ribosomal protein S6 during meiotic maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 May;79(9):2937–2941. doi: 10.1073/pnas.79.9.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouysségur J., Chambard J. C., Franchi A., Paris S., Van Obberghen-Schilling E. Growth factor activation of an amiloride-sensitive Na+/H+ exchange system in quiescent fibroblasts: coupling to ribosomal protein S6 phosphorylation. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3935–3939. doi: 10.1073/pnas.79.13.3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saborio J. L., Pong S. S., Koch G. Selective and reversible inhibition of initiation of protein synthesis in mammalian cells. J Mol Biol. 1974 May 15;85(2):195–211. doi: 10.1016/0022-2836(74)90360-x. [DOI] [PubMed] [Google Scholar]
- Scharf K. D., Nover L. Heat-shock-induced alterations of ribosomal protein phosphorylation in plant cell cultures. Cell. 1982 Sep;30(2):427–437. doi: 10.1016/0092-8674(82)90240-9. [DOI] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Gordon J. Multiple phosphorylation of ribosomal protein S6 during transition of quiescent 3T3 cells into early G1, and cellular compartmentalization of the phosphate donor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3952–3956. doi: 10.1073/pnas.76.8.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettenhall R. E., Cohen P., Caudwell B., Holland R. Differential phosphorylation of ribosomal protein S6 in isolated rat hepatocytes after incubation with insulin and glucagon. FEBS Lett. 1982 Nov 8;148(2):207–213. doi: 10.1016/0014-5793(82)80809-0. [DOI] [PubMed] [Google Scholar]
- Wettenhall R. E., Howlett G. J. Phosphorylation of a specific ribosomal protein during stimulation of thymocytes by concanavalin A and prostaglandin E1. J Biol Chem. 1979 Sep 25;254(18):9317–9323. [PubMed] [Google Scholar]
- Wool I. G. The structure and function of eukaryotic ribosomes. Annu Rev Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]