Abstract
Ixazomib is the first oral proteasome inhibitor to be approved, in combination with lenalidomide and dexamethasone, for the treatment of patients with multiple myeloma who have received at least one prior therapy. Approval was on the basis of results from the phase 3, double-blind, placebo-controlled TOURMALINE-MM1 study, which demonstrated a 35% improvement in progression-free survival with the all-oral combination of ixazomib plus lenalidomide–dexamethasone versus lenalidomide–dexamethasone alone (median: 20.6 vs 14.7 months; hazard ratio: 0.74, p=0.012; median follow-up 14.7 months). The addition of ixazomib to the lenalidomide–dexamethasone regimen was associated with limited additional toxicity and had no adverse impact on patient-reported quality of life. Common grade ≥3 adverse events with ixazomib include gastrointestinal adverse events, rash, and thrombocytopenia. Here, we review the efficacy, safety, pharmacokinetics, and patient-reported quality of life data seen with ixazomib, and discuss the role of this oral agent in the treatment of patients with relapsed/refractory multiple myeloma, including in patients with high-risk cytogenetic abnormalities and those with multiple prior therapies.
Keywords: ixazomib, multiple myeloma, proteasome inhibitor, clinical, efficacy, tolerability, pharmacokinetics
Introduction
Multiple myeloma (MM) is a malignant plasma cell disorder characterized by the uncontrolled proliferation of monoclonal plasma cells in the bone marrow.1,2 It is the second most common hematologic malignancy and accounts for ~16.6% of all hematologic malignancies in the US,3 with an estimated worldwide 5-year prevalence in 2012 of 229,468 people.4 The risk of developing MM increases with age, and the median age at diagnosis is 69 years.1
The treatment of MM has advanced over the past 15 years following the introduction of the immunomodulatory drugs thalidomide, lenalidomide, and pomalidomide, and the proteasome inhibitors bortezomib and, more recently, carfilzomib5 and ixazomib.6 Median overall survival (OS) has improved from 4.6 years for patients diagnosed between 2001 and 2005 to 6.1 years for patients diagnosed between 2006 and 2010.7
Despite these advances, MM is a complex and incurable progressive disease characterized by multiple relapses, largely due to the persistence of residual disease, and the need for multiple lines of therapy.8–10 Based on real-world and claims analyses, ~48%–66% of patients are estimated to progress following first-line therapy and require subsequent lines of treatment, with other patients not reported as receiving subsequent therapy, possibly due to death or censoring prior to subsequent treatment, or loss to follow-up.11–13 Furthermore, similar analyses have indicated that ~21%–43% of patients are estimated to require third-line treatment and beyond.11–13 After each remission, MM typically recurs with a more aggressive disease course, resulting in shorter duration of disease response with each successive line of therapy and, eventually, treatment-refractory disease.14
Consequently, there has been a high unmet clinical need to expand the active treatment options, prolong therapy, and further improve outcomes for patients with relapsed/refractory MM (RRMM). There are also several “poor prognosis” groups of patients with MM for whom outcomes with current standards of care are poorer compared with those in the general MM patient population; these include patients with the high-risk cytogenetic abnormalities del(17p), t(4;14), and t(14;16)15–18; elderly patients (aged >75 years)19,20; patients with renal impairment21; and patients with high disease burden.5,22
Current and emerging treatment options in MM
Current treatment options for patients with MM
In the era of novel therapies, the immunomodulatory drugs lenalidomide and thalidomide, and the proteasome inhibitor bortezomib are the backbone of therapy for MM, often administered in two- or three-drug combinations with corticosteroids (such as dexamethasone or prednisone) and alkylating agents (such as melphalan or cyclophosphamide). These agents are used at all stages of the disease: as induction therapy prior to autologous stem cell transplant (ASCT), as initial therapy for newly diagnosed patients ineligible for ASCT, and as subsequent lines of therapy following relapse of the disease. Following the widespread use of immunomodulatory drugs and proteasome inhibitors, there is increasing evidence to support the use of the newer immunomodulatory drugs and proteasome inhibitors pomalidomide, carfilzomib, and ixazomib in patients with relapsed/refractory disease.6,14
While investigation into the optimal combinations and therapeutic strategies continues, results support the benefits of triplet versus doublet regimens.6,23–31 Several studies have also demonstrated that a triplet regimen including both an immunomodulatory drug and a proteasome inhibitor, such as bortezomib, carfilzomib, or ixazomib in combination with lenalidomide–dexamethasone (Rd), is particularly effective at inducing rapid and deep responses, leading to improved progression-free survival (PFS) and, in the case of bortezomib-Rd, OS.6,23–25,29,32
Long-term treatment is now emerging as a standard-of-care with the goals of continuous disease suppression, deepening responses, and prolonging survival. The benefits of continuous MM therapy have been demonstrated following ASCT or following an induction regimen, with sustainable, long-term maintenance treatment being associated with better OS versus fixed-term treatment.33–35 Long-term treatment appears particularly important for patients with high-risk cytogenetic abnormalities,36 for whom there are few effective treatment options. However, long-term treatment with current triplet regimens, particularly those including the proteasome inhibitors bortezomib or carfilzomib, is difficult to achieve as they have been associated with a substantial patient burden in terms of both treatment-related toxicities,14 such as peripheral neuropathy (PN),37 cardiovascular14 and renal38 toxicities, and the need for frequent clinic visits and repeat injections,39 all of which can have an adverse impact on duration of therapy. Real-world data from one retrospective cohort study showed median duration of second-line treatment to be just 6.9 months for an intravenously administered PI,40 which is in contrast to the ~20 months reported in a recent phase 3 trial in patients with RRMM,29 highlighting the need for new treatment strategies to enable patients to achieve sustainable and long-term benefit from their MM therapy.
The aim of achieving long-term treatment has also focused attention on improved patient quality of life (QoL), particularly with regard to the tolerability and convenience of the treatment regimen.41 Consequently, effective, sustainable therapies associated with manageable toxicities may have an important role in the treatment of MM, with the potential ability to enable long-term therapy.
Emerging therapeutic options for RRMM
Adding to the treatment armamentarium for patients with RRMM, several agents have recently been approved by the US Food and Drug Administration (FDA) for the treatment of RRMM. These include the histone deacetylase inhibitor panobinostat, the monoclonal antibodies elotuzumab and daratumumab, and the oral proteasome inhibitor ixazomib, many of which have been approved as components of triplet regimens including proteasome inhibitors or immunomodulatory drugs. For example, the histone deacetylase inhibitor panobinostat has been approved in combination with bortezomib and dexamethasone after showing longer PFS when compared with bortezomib plus dexamethasone.42 Similarly, the monoclonal antibodies elotuzumab and daratumumab have demonstrated encouraging PFS when administered in combination with the proteasome inhibitor bortezomib or immunomodulatory drug lenalidomide, plus dexamethasone,30,31,43 with daratumumab approved as a single agent and showing particular promise in triplet regimens.
The oral proteasome inhibitor ixazomib has been approved in more than 40 countries, including the United States and the European Union, for the treatment of MM patients, in combination with Rd, who have received at least one prior therapy. This approval was based on data from the phase 3 randomized, double-blind, placebo-controlled TOURMALINE-MM1 trial in 722 patients with relapsed/refractory MM, which demonstrated a 35% improvement in PFS, and a generally manageable toxicity profile.6 Here, we review the role of ixazomib in the management of relapsed/refractory MM, focusing on the pharmacokinetics (PK), efficacy, and safety of this oral proteasome inhibitor.
Clinical pharmacology of ixazomib
Ixazomib is a reversible proteasome inhibitor that preferentially binds and inhibits the 20S proteasome.44 Ixazomib is administered as the citrate ester prodrug (ixazomib citrate), which undergoes rapid and complete hydrolysis to the biologically active agent ixazomib under physiological conditions. Ixazomib is the first oral proteasome inhibitor to be approved for the treatment of MM.45 Prior to approval, the clinical development of ixazomib included a comprehensive clinical pharmacology characterization, based on phase 1 study data and other dedicated studies, which helped to understand the PK properties of ixazomib and inform its posology.
Pharmacokinetics
Early-phase studies investigated weekly and twice-weekly dosing schedules of single-agent ixazomib (weekly, days 1, 8, and 15 of 28-day cycles; twice-weekly, days 1, 4, 8, and 11 of 21-day cycles). Results demonstrated that, with both dosing schedules, ixazomib was rapidly absorbed (the median time to maximum plasma concentration was 1 hour) and had a long terminal half-life of 9.5 days,45,46 supporting the use of both ixazomib schedules. Data from these early-phase studies also demonstrated the dose-proportional nature of ixazomib plasma exposure.46–49
Ixazomib is highly plasma protein bound (99%)50,51 and distributes into red blood cells with a blood-to-plasma ratio of 10 (Merlini et al, unpublished data). Metabolism by multiple CYP enzymes and non-CYP proteins is expected to be the major clearance mechanism for ixazomib. Preclinical data have shown that, at clinically relevant ixazomib concentrations, no specific CYP isozyme predominantly contributes to ixazomib metabolism and non-CYP enzymes also contribute to overall metabolism. However, at concentrations exceeding those observed clinically (>90-fold), ixazomib was metabolized in vitro by multiple CYPs, with CYP3A contributing to the greatest extent (42%).45
Cardiac electrophysiology
As cardiac events have been associated with other proteasome inhibitors,14,38 part of the early characterization of ixazomib was to evaluate any effect of ixazomib on cardiac parameters. Within this cardiac effect characterization, an innovative concentration-QTc analysis integrating data from four phase 1 studies of single-agent ixazomib (N=245) demonstrated that ixazomib has no clinically meaningful effects on QTc interval or heart rate.52
Dose selection and posology
Phase 1 studies of single-agent ixazomib demonstrated the maximum tolerated dose (MTD) of ixazomib to be 2.97 mg/m2 for the weekly schedule and 2.0 mg/m2 for the twice-weekly schedule.48,49 Similarly, a MTD of 2.97 mg/m2 was also demonstrated for weekly ixazomib in combination with Rd, with a recommended phase 2 dose of 2.23 mg/m2.47
In contrast to the body surface area-based dosing used for bortezomib and carfilzomib, ixazomib administration involves a simple, fixed-dosing approach. The feasibility of this was demonstrated in a population PK analysis using pooled data from four phase 1 studies.53 This analysis indicated that ixazomib has high oral bioavailability and that body size does not impact ixazomib exposure, demonstrating that fixed rather than body surface area-based ixazomib dosing is appropriate. The MTD of 2.97 mg/m2 and recommended phase 2 dose of 2.23 mg/m2 ixazomib reported in the phase 1 study of weekly ixazomib plus Rd equate to fixed doses of 5.5 mg and 4.0 mg, respectively. Simplifying ixazomib administration, a fixed ixazomib dose of 4.0 mg, in combination with Rd, is used in current phase 3 studies.
As the PK and safety profiles of a drug can vary by ethnicity,54–56 following the phase 1 studies in Western patients, the PK of ixazomib was assessed in East Asian patients. Ixazomib exposures on day 1, cycle 1 were similar to those seen in Western patients. Although the exposure on day 15, after multiple dosing, was ~30% higher in East Asian patients, this increased exposure at the 4.0 mg dose was not anticipated to be greater than the in Western patients treated at the MTD. On the basis of these results, Asian patients have been enrolled in the ongoing phase 3 studies at the same starting dose of ixazomib (4.0 mg).57 Inclusion of these patients further enables the TOURMALINE phase 3 studies to better reflect the global MM population.
Several other dedicated PK studies have enabled the inclusion of simple dosing guidelines in relation to the ixazomib starting dose in the US prescribing information.45 Renal impairment is common in patients with MM.14 The population PK analysis outlined above indicated that mild-to-moderate renal impairment had no effect on ixazomib exposure.53 Such patients have subsequently been included in the phase 3 TOURMALINE program at the same ixazomib starting dose,6,58 better reflecting the global MM population and enhancing the relevance of the findings from the TOURMALINE studies to the real-world MM patient population. Subsequently, a dedicated PK study was conducted to assess ixazomib PK in patients with severe renal impairment or end-stage renal disease. Unbound and total systemic exposures of ixazomib were 38% and 39% higher, respectively, in patients with severe renal impairment or end-stage renal disease versus patients with normal renal function.50 These results support a lower ixazomib dose of 3 mg in patients with severe renal impairment or end-stage renal disease. Similarly, as metabolism appears to be the major mechanism of ixazomib clearance, a dedicated PK study assessed ixazomib PK in patients with hepatic impairment. Compared to patients with normal hepatic function, unbound and total systemic exposures of ixazomib were 27% and 20% higher, respectively, in patients with moderate or severe hepatic impairment, leading to a reduced dose of 3 mg being recommended for these patients.51
Administration of ixazomib after a high-fat meal was shown to decrease the rate and extent of oral absorption.59 As included in the US prescribing information, patients should therefore not take any food for 2 hours before and 1 hour after ixazomib dosing.45
Drug–drug interaction studies
When administered in combination with Rd, the PK profile of ixazomib was consistent for both weekly and twice-weekly schedules,46,47,60 suggesting no PK interaction between ixazomib and Rd. Similarly, there appears to be no interaction between ixazomib and cyclophosphamide–dexamethasone61 or melphalan–prednisone,62 suggesting the feasibility of including ixazomib in combination regimens. Coadministration of ixazomib with the strong CYP3A inducer rifampin decreased ixazomib Cmax by 54% and area under the curve by 74%; hence, systemic treatment with strong CYP3A inducers should be avoided in patients receiving ixazomib.45,63
Clinical efficacy in patients with MM
Single-agent ixazomib
The clinical efficacy of single-agent ixazomib in patients with relapsed/refractory MM was demonstrated in two phase 1 studies in heavily pretreated patients, including those with prior bortezomib and prior lenalidomide exposure (Table 1). Sixty patients were enrolled to each study and received single-agent ixazomib on a weekly (days 1, 8, and 15 of 28-day cycles) or twice-weekly (days 1, 4, 8, and 11 of 21-day cycles) schedule. Antimyeloma activity was demonstrated with both weekly and twice-weekly single-agent regimens (Table 1); of note, responses were seen in patients with relapsed and refractory MM who had previously received both bortezomib and lenalidomide. These preliminary data indicated that responses were rapid and durable, with time to first response of 1.6–4.4 months, and duration of disease control of >28 months reported.49
Table 1.
Clinical efficacy of ixazomib in patients with MM
| Study | Phase | Na | Regimen | Ixazomib dose schedule | Prior therapy | ORR | Outcomes |
|---|---|---|---|---|---|---|---|
| Single-agent ixazomib | |||||||
| C1600349 | 1 | 55/60 | Ixazomib twice-weekly (days 1, 4, 8, and 11 of 21-d cycles) | MTD 2.0 mg/m2 twice-weekly | Median 4 lines | 1 CR, 1 VGPR, 6 PR, 1 MR | NR |
| C1600448 | 2 | 50/60 | Ixazomib weekly (days 1, 8, and 15 of 28-d cycles) | MTD 2.97 mg/m2 weekly | Median 4 lines | 1 VGPR, 8 PR, 1 MR | NR |
| Mayo Clinic phase 264 | 2 | 32/33 | Ixazomib ± dex | 5.5 mg weekly | Median 2 therapies | ORR 34%; 2 sCR, 3 PR with ixazomib alone, + 6 PR with added Dex | EFS 12.4 mos 6-mo OS 96% |
| Mayo Clinic phase 265 | 2 | 71 | Ixazomib | 4 mg vs 5.5 mg weekly | Median 4 therapies | ORR 31% ORR 51% |
NR |
| Ixazomib–Rd | |||||||
| TOURMALINE-MM1 (C16010)6 | 3 | 360 | Ixazomib–Rd vs | 4 mg weekly | 62%/27%/11% | ORR 78%, ≥VGPR 48%, CR 12% | HR 0.74, p=0.01 |
| 362 | Placebo–Rd | 60%/31%/9% (1/2/3 prior therapies) | ORR 72%, ≥VGPR 39%, CR 7% | Median PFS 20.6 mos vs 14.7 mos | |||
| C1600547 | 1/2 | 65 | Ixazomib–Rd | 4 mg weekly | None | ORR 92%; ≥VGPR 58%; CR + nCR 34%; CR 27% | 1-year PFS: 88% |
| C1600860 | 1/2 | 65 | Ixazomib–Rd | 3 mg twice-weekly | None | ORR 94%; ≥VGPR 76%; CR + nCR 36%; CR/sCR 26% | NR |
| C1601357 | 1 | 43 | Ixazomib–Rd, Asian pts | 4 mg weekly | 47/23/29 (1/2/3 prior therapies) | ORR 65%; ≥VGPR 23%; CR 9% | NR |
| Other combinations | |||||||
| C16006 | 2 | 16 | Ixazomib twice-weekly + MP | 6/9 | None | 1 sCR, 4 PR | NR |
| 6/9 | Ixazomib weekly + MP | 7/7 | None | 4 PR | NR | ||
| C1602061 | 2 | 36 34 |
Ixazomib–Cd (C 300 mg vs C 400 mg) | 4 mg weekly | None | ORR 78%, ≥VGPR 28% ORR 65%, ≥VGPR 21% |
12-mo PFS 68% vs 91% |
| Case Comprehensive Cancer Center75 | 1 | 11 | Ixazomib + panobinostat + dex | 4 mg weekly | Median 5 therapies | 3 MR | NR |
| Alliance76 | 1 | 17 | Ixazomib + pomalidomide + dex | 3–4 mg weekly | All pts had received prior lenalidomide, bortezomib, and dex | In 13 pts receiving >1 cycle of therapy: ORR 62%, ≥VGPR 8% | NR |
| City of Hope Medical Center74 | 1 | 21 | Ixazomib + pomalidomide + dex | 3–4 mg weekly | Median 3 therapies | In 9 response-evaluable pts, ORR 33% | NR |
Notes:
Number of response-evaluable patients/total number of treated patients.
Abbreviations: Cd, cyclophosphamide-dexamethasone; CR, complete response; dex, dexamethasone; EFS, event-free survival; mo(s), month(s); HR, hazard ratio; MM, multiple myeloma; MP, melphalan–prednisone; MR, minimal response; MTD, maximum tolerated dose; nCR, near complete response; NR, not reported; ORR, overall response rate; OS, overall survival; PR, partial response; pts, patients; Rd, lenalidomide-dexamethasone; sCR, stringent CR; VGPR, very good partial response
The efficacy of single-agent ixazomib has also been demonstrated by the results from a phase 2 Mayo Clinic study (Table 1).64,65 In the first phase of this study, 33 patients with RRMM and who had received a median of 2 prior therapies (72% were bortezomib-naïve) received weekly ixazomib 5.5 mg, with additional dexamethasone for insufficient response.64 A second phase of this trial assessed the efficacy and tolerability of two doses of weekly ixazomib (4.0 mg and 5.5 mg) plus dexamethasone in heavily pretreated patients who had received a median of 4 prior therapies (range: 2–6); 90% of patients had received prior immunomodulatory drugs and 29% had received prior bortezomib. Overall response rates were 31% with ixazomib 4.0 mg and 51% with ixazomib 5.5 mg, indicating the efficacy in these heavily pretreated patients.65
Ixazomib in combination with Rd
Ixazomib is approved in combination with Rd in RRMM patients who have received at least one prior therapy, based on results from the phase 3 placebo-controlled, double-blind TOURMALINE-MM1 study.6 The rationale for this phase 3 study and the feasibility of the all-oral ixazomib–Rd triplet combination was demonstrated in two phase 1/2 studies in the newly diagnosed setting. These two studies assessed weekly ixazomib plus Rd (4 mg ixazomib on days 1, 8, and 15 of 28-day cycles, plus Rd) and twice-weekly ixazomib plus Rd (3 mg ixazomib on days 1, 4, 8, and 11 of 21-day cycles, plus Rd) in patients with newly diagnosed MM, including those eligible for ASCT.47,49,60 In both studies, patients received a fixed number of induction cycles of the ixazomib–Rd regimen followed by maintenance therapy with single-agent ixazomib on the same schedule. Encouraging efficacy was reported with both schedules (Table 1), with 62% and 76% of patients achieving ≥very good partial response (VGPR) with weekly and twice-weekly ixazomib plus Rd.47,49,60 These data indicate the quality of response attained and suggest there may be a clinical role for weekly and twice-weekly ixazomib dosing regimens. Importantly, in the context of long-term treatment, results from the phase 1/2 study of weekly ixazomib plus Rd demonstrated the feasibility of extended treatment, with patients remaining on therapy for >4 years and demonstrating deepening responses with maintenance therapy.7,47
On the basis of these encouraging early-phase results, ixazomib–Rd versus placebo–Rd was assessed in the global phase 3, randomized, double-blind, placebo-controlled TOURMALINE-MM1 study. Uniquely in phase 3 studies of MM triplet regimens, the all-oral administration of the ixazomib–Rd regimen enabled a placebo–controlled, double-blind study design, in which the independent review committee was blinded to both patient assignment and investigator response assessment, increasing the rigor and reliability of the study. The inclusion criteria were particularly broad to better represent the global MM patient population, including patients with mild-to-moderate renal impairment, primary refractory patients, patients with free light chain-only disease, and patients from East Asia.6
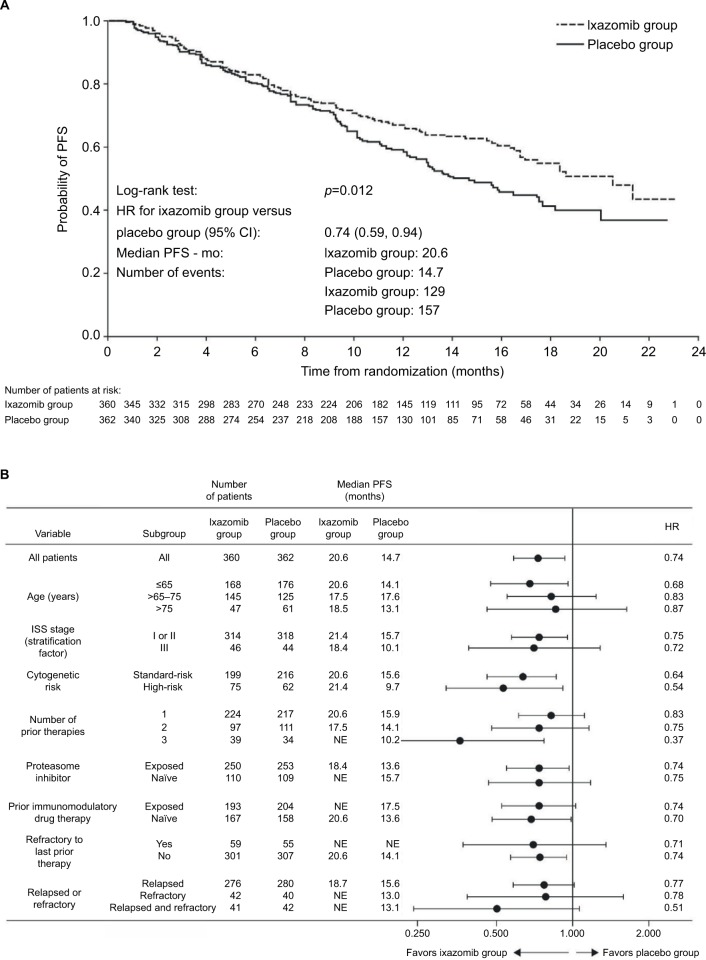
A total of 722 patients were randomized to receive ixazomib 4 mg weekly versus matching placebo plus lenalidomide and dexamethasone, until disease progression or unacceptable toxicity.6 After a median follow-up of ~15 months, there was a 35% improvement in the primary endpoint PFS with ixazomib–Rd vs placebo–Rd (hazard ratio [HR], 0.74, p=0.01), equating to a clinically meaningful ~6 month benefit in median PFS (median PFS 20.6 months vs 14.7 months; Table 1, Figure 1A).6 Differences in study designs, methodologies, and patient populations limit cross-trial comparisons; however, the relative benefit with ixazomib–Rd versus placebo–Rd appeared consistent with HRs reported versus Rd for other proteasome inhibitor–Rd combinations.29
Figure 1.
Kaplan–Meier analysis of PFS in the TOURMALINE-MM1 study on the intent-to-treat population (A) and by prespecified patient subgroups (B) (data from final statistical analysis for progression-free survival).
Notes: From New England Journal of Medicine, Moreau P, Masszi T, Grzasko N, et al., Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma, 374., 1621. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.6
Abbreviations: CI, confidence interval; HR, hazard ratio; mo, month; NE, not estimable; PFS, progression-free survival.
Overall response rates and ≥VGPR rates were 78.3% versus 71.5% (p=0.04) and 48% versus 39% (p=0.01) with ixazomib–Rd versus placebo–Rd (Table 1). Responses were rapid and durable, with a median time to response of 1.1 months versus 1.9 months and a median duration of 20.5 months versus 15.0 months in the ixazomib–Rd and placebo–Rd arms, respectively. Importantly in the context of long-term treatment, deepening responses were noted with increasing treatment duration,6 as seen in the earlier phase 1/2 study.47 OS data were not yet mature, and longer follow-up data are needed.
A separate regional expansion of the global TOURMALINE-MM1 study was conducted in China. The results of this China continuation study showed a consistent PFS benefit with ixazomib–Rd versus placebo–Rd (HR for PFS 0.6, p=0.035) and a consistent improvement in overall response rates (p=0.007), supporting the overall treatment benefit of the ixazomib–Rd regimen.66 Consistent with the global study, there was limited additional toxicity with ixazomib–Rd versus placebo–Rd.66
The ixazomib–Rd combination is also being assessed versus placebo–Rd in the newly diagnosed setting in the phase 3, double-blind, placebo-controlled TOURMALINE-MM2 study (NCT01850524). The study is ongoing, but recruitment is now complete.
Efficacy of ixazomib in specific patient populations
The clinically meaningful PFS benefit with ixazomib–Rd versus placebo–Rd in the TOURMALINE-MM1 study was seen across patient subgroups, including those with a poor prognosis such as those with high-risk cytogenetic abnormalities and multiple prior therapies (Figure 1B).
Patients with high-risk cytogenetic abnormalities
Patients with high-risk cytogenetic abnormalities have a particularly poor prognosis.15–18 The 2016 International Myeloma Working Group consensus paper recommends a triplet regimen including a proteasome inhibitor and an immunomodulatory drug for the treatment of patients with high-risk cytogenetic abnormalities.18,67 Further, patients with such poor prognosis should be treated until disease progression, recognizing the risk of rapid relapse in the absence of sustained exposure to chemotherapy.67 Although such regimens have shown improved outcomes vs previous standards of care in these patients, more needs to be done to close the gap vs patients with standard-risk cytogenetics.
In the 137 patients with the high-risk cytogenetic abnormalities del(17p), t(4;14), and/or t(14;16) in TOURMALINE-MM1, the HR for PFS was 0.543 (95% CI: 0.321, 0.918; p=0.021), and there was more than a doubling in median PFS with ixazomib–Rd versus placebo–Rd (median PFS 21.4 vs 9.5 months).68 Of note in this particularly poor prognosis patient population, the median PFS with ixazomib–Rd was similar to that seen in patients with standard-risk cytogenetics (median PFS with ixazomib–Rd, 21.4 months in high-risk patients vs 20.6 months in standard-risk patients).
The PFS benefit with ixazomib–Rd versus placebo–Rd was consistent according to the presence or absence of each cytogenetic abnormality, all of which have been shown to be independent poor prognostic markers in MM.18 Of note, the median PFS in the ixazomib group was 21.4 months in patients with del(17p), which appeared similar to the median of 20.6 months in the standard-risk patients.68
There is currently no agreed minimum percentage of plasma cells carrying del(17p) for defining the presence of this abnormality and for conferring poor prognosis;18 different studies have used different values, ranging from the presence of a single cell43 to a cut-off threshold of 60% of cells.29 In the absence of an agreed standard, the protocol-specified cut-off in TOURMALINE-MM1 was 5%,6 based on the false-positive rate of the fluorescence in situ hybridization probe used; however, a consistent PFS benefit was seen in post hoc analyses using cut-offs of 20% and 60% of cells, with HRs ranging from 0.490 to 0.611.68
Patients with prior exposure to proteasome inhibitors/immunomodulatory drugs
Given the widespread use of immunomodulatory drugs and proteasome inhibitors as front-line therapy, it is important for an RRMM regimen to demonstrate efficacy in patients with prior exposure to these agents. The TOURMALINE-MM1 study included patients with prior exposure to proteasome inhibitors and immunomodulatory drugs, although patients refractory to proteasome inhibitors or lenalidomide were not eligible (patients refractory to thalidomide were included; refractoriness was defined as disease progression on treatment or within 60 days after last dose of therapy). Of 722 patients, 69% had prior proteasome inhibitor therapy and 55% had prior thalidomide/lenalidomide therapy (23% were refractory to thalidomide). Results demonstrated a consistent efficacy benefit in terms of prolonged PFS with ixazomib–Rd versus placebo–Rd regardless of prior exposure to proteasome inhibitor or immunomodulatory drug therapy (HRs of 0.70–0.75 for proteasome inhibitor or immunomodulatory drug-exposed/-naïve patients; Figure 1B).69 This consistent benefit with ixazomib–Rd was also seen in patients who were refractory to their last prior therapy (HR 0.71, vs placebo-Rd).
Patients with multiple prior therapies
Long-term outcomes, including PFS and OS, are known to worsen with increasing numbers of prior therapies,8,70 possibly due to the development of treatment-resistant clones71 and also increased rates of comorbidities and complications.12 Effective therapies are therefore needed for this patient population. In the TOURMALINE-MM1 study, ixazomib–Rd was associated with a consistent PFS benefit versus placebo–Rd in patients with 1, 2, or 3 prior therapies, with a notable PFS benefit in patients with multiple prior therapies (Figure 1B).6 Of note, the benefit with ixazomib–Rd versus placebo–Rd extended to patients who were refractory to their last prior therapy and to those who were relapsed and refractory (Figure 1B).
Ixazomib in other combination regimens
Reflecting combinations also studied during the clinical development of the first-in-class proteasome inhibitor bortezomib,24,25,42,72,73 preliminary efficacy data have been reported for ixazomib in combination with cyclophosphamide–dexamethasone, melphalan–prednisone, panobinostat–dexamethasone, and pomalidomide–dexamethasone, supporting the suitability of ixazomib as a partner agent in combination regimens.61,74–76
Ixazomib is being investigated in combination with cyclophosphamide–dexamethasone, to give another all-oral triplet regimen. Weekly ixazomib (4 mg) plus cyclophosphamide (300 mg or 400 mg weekly) and dexamethasone is being investigated in patients with RRMM and in those with newly diagnosed MM, transplant-ineligible MM in a phase 2 study (NCT02046070). The first report from the study has shown preliminary efficacy in patients with newly diagnosed MM, with an overall response rate of 71% after a median of 9 treatment cycles (78% vs 65% in the ICd-300 and ICd-400 arms, respectively), including a ≥VGPR rate of 26% (28% vs 21% with ICd-300 and ICd-400, respectively; Table 1).61 As seen with the ixazomib–Rd regimen, responses appeared to deepen over time.61
Ixazomib is also under investigation in combination with panobinostat and dexamethasone. Although only including 11 patients, preliminary data from a phase 1 study of ixazomib plus panobinostat–dexamethasone (NCT02057640) indicated some activity in heavily pretreated RRMM patients who had received a median of 5 prior therapies (range, 2–10) (Table 1).75
Building on the results seen with ixazomib plus lenalidomide and dexamethasone, initial results from two phase 1/2 studies have indicated encouraging preliminary efficacy with ixazomib in combination with pomalidomide and dexamethasone, including in patients refractory to prior bortezomib or lenalidomide therapy.74,76 The phase 2 portions of both studies are ongoing, and updated data are expected.
Safety and tolerability
With the addition of the phase 3 TOURMALINE-MM1 data to the early-phase data reported previously, the generally manageable toxicity profile of ixazomib is becoming clear. Commonly reported adverse events (AEs) across studies of ixazomib, both as a single-agent and in combination regimens, included gastrointestinal AEs (diarrhea, constipation, nausea, vomiting), rash, and thrombocytopenia (Table 2),6,47–49,57,60,61,64,65,75 many of which appeared primarily to be low-grade. Encouragingly for the development of combination regimens, the addition of ixazomib to the Rd regimen was associated with limited additional toxicity, with similar rates of AEs (98% vs 99%), serious AEs (47% vs 49%) and deaths during the study period (4% vs 6%), and only a small increase in the rates of grade ≥3 AEs (74% vs 69%) with ixazomib-Rd vs placebo-Rd.6 Discontinuation of treatment due to AEs was similar on the two regimens, allowing for long-term use of this regimen.
Table 2.
Summary of safety with ixazomib, alone or in combination, in trials in RRMM and newly diagnosed MM
| Study | Phase | Na | Regimen | Ixazomib dose schedule | Hematologic AEs | Nonhematologic AEs |
|---|---|---|---|---|---|---|
| Single-agent ixazomib | ||||||
| C1600349 | 1 | 55/60 | Ixazomib | MTD 2 mg/m2 twice-weekly | Drug-related grade 3/4 AE (≥5%): thrombocytopenia 37%, neutropenia 17%, lymphopenia 5% | Drug-related grade 3/4 AE (≥5%): skin and SC tissue disorders 8%, fatigue 7% PN: 12% (no grade ≥3) |
| C1600448 | 2 | 50/60 | Ixazomib (weekly) | MTD 2.97 mg/m2 weekly | Drug-related grade 3/4 AE (≥5%): thrombocytopenia 33%, neutropenia 18%, lymphopenia 8%, anemia 7%, leukopenia 5% | Drug-related grade 3/4 AE (≥5%): diarrhea 17%, fatigue 8%, nausea 7%, decreased appetite 7%, vomiting 5% PN: 20% (2% grade 3) |
| Mayo Clinic phase 264 | 2 | 32/33 | Ixazomib ± dex | 5.5 mg weekly | NR | PN: 18% grade 1, 6% grade 2 (no grade ≥3) |
| Mayo Clinic phase 265 | 2 | 71 | Ixazomib | 4 mg vs 5.5 mg weekly | 15% vs 37% hematologic AEs | 6% vs 29% nonhematologic AEs; PN 55% (no grade 3) vs 43% (3% grade 3) |
| Ixazomib–Rd | ||||||
| TOURMALINE-MM1 (C16010)6 | 3 | 360 | Ixazomib–Rd vs | 4 mg weekly | Grade 3 AEs: neutropenia 18% vs 18%, thrombocytopenia 12% vs 5%, anemia 9% vs 13% | Grade 3 AEs: diarrhea 6% vs 3%, rash-related AEs 5% vs 2%, fatigue 4% vs 3%, PN 2% vs 2% |
| 362 | Placebo–Rd | Grade 4 AEs: neutropenia 5% vs 6%, thrombocytopenia 7% vs 4% | Any-grade second primary malignancies: 5% vs 4% Any-grade PN: 27% vs 22% |
|||
| C1600547 | 1/2 | 65 | Ixazomib–Rd | 4 mg weekly | Drug-related grade 3 AEs (≥5%): neutropenia 12%, thrombocytopenia 8%, lymphopenia 6%, leukopenia 5% | Drug-related grade 3 AEs (≥5%): rash 17%, fatigue 9%, diarrhea, hypokalemia, PN, vomiting each 6%, nausea, hypertension, hypophosphatemia each 5% |
| C1600860 | 1/2 | 65 | Ixazomib–Rd | 3 mg twice-weekly | Drug-related grade 3 AEs (≥5%): thrombocytopenia 6%, decreased lymphocyte count, hyponatremia, neutropenia, each 5% | Drug-related grade 3 AEs (≥5%): rash-related AEs 16%, hyperglycemia 8%, pneumonia 6%, peripheral neuropathies 5% |
| C1601357 | 1 | 43 | Ixazomib–Rd, Asian pts | 4 mg weekly | Drug-related grade 3 AEs: thrombocytopenia 14%, neutropenia 11%, anemia 6% Drug-related grade 4 AEs: thrombocytopenia 8% |
Drug-related grade 3 AEs: diarrhea 17%, fatigue 8% PN: 25% (all-grade, all cause; no grade 3/4) |
| Other combinations | ||||||
| C1600662 | 2 | 16 | Ixazomib twice-weekly + MP | 6/9 | Grade ≥3 AEs: neutropenia 33%, thrombocytopenia 22% | Grade ≥3 AEs: maculo-papular rash 22%, pruritic rash 22% |
| 6/9 | Ixazomib weekly + MP | 7/7 | Grade ≥3 AEs: neutropenia 29% | NR | ||
| C1602061 | 2 | 70 | Ixazomib–Cd (C 300 mg vs C 400 mg) | 4 mg weekly | Grade ≥3 AEs: anemia 11% vs 15%, neutropenia 14% vs 35%, thrombocytopenia 3% vs 10% | Grade ≥3 AEs: nausea 3% vs 0%, diarrhea 6% vs 0%, vomiting 3% vs 0%, constipation 3% vs 3% |
| Case Comprehensive Cancer Center75 | 1 | 11 | Ixazomib + panobinostat + dex | 4 mg weekly | Grade 3 AEs: neutropenia 2 pts, thrombocytopenia 1 pt | No grade ≥3 nonhematologic AEs |
| Alliance76 | 1 | 17 | Ixazomib + pomalidomide + dex | 3–4 mg weekly | Drug-related grade 3 AEs: neutropenia 29%, thrombocytopenia 12%, lymphopenia 29% Grade 4 AEs: neutropenia 6%, thrombocytopenia 6% |
No grade ≥3 drug-related nonhematologic AEs |
| City of Hope Medical Center | 1 | 21 | Ixazomib + pomalidomide + dex | 3–4 mg weekly | Grade ≥3 AEs: anemia 2 pts, neutropenia 6 pts, thrombocytopenia 3 pts | Grade 3 lung infection in 1 pt |
Notes:
Number of response-evaluable patients/total number of treated patients.
Abbreviations: AE, adverse event; CD, cyclophosphamide-dexamethasone; dex, dexamethasone; mo(s), month(s); MM, multiple myeloma; MP, melphalan–prednisone; MTD, maximum tolerated dose; NR, not reported; PN, peripheral neuropathy; pts, patients; Rd, lenalidomide-dexamethasone; RRMM, relapsed and/or refractory multiple myeloma.
Key AEs seen in ixazomib studies
Thrombocytopenia
Thrombocytopenia is an overlapping toxicity seen with ixazomib and lenalidomide, and has been seen in early-phase trials of single-agent ixazomib48,49 and ixazomib in combination with Rd (Table 2).47,60,61
Consistent with previous results with bortezomib, and results from the phase 1/2 study of ixazomib–Rd,47 there were transient and cyclical decreases in platelet count in both the ixazomib–Rd and placebo–Rd groups in TOURMALINE-MM1. Although the rate of grade 3/4 thrombocytopenia was higher with ixazomib–Rd versus placebo–Rd (12%/7% vs 5%/4%), most events appeared manageable with dose interruptions and reductions; there were few apparent clinical sequelae as the rates of serious AEs of thrombocytopenia (2% in each group), platelet transfusions (8% and 6%), study regimen discontinuation due to thrombocytopenia (1% in each group), and the occurrence of any-grade bleeding events (20% vs 19%) were similar in the two groups.6 Most thrombocytopenia events occurred within the first 3 cycles of therapy and there seemed to be no long-term cumulative toxicity.
In early-phase studies, the incidence of thrombocytopenia was similar with weekly and twice-weekly single-agent ixazomib (43% vs 42% overall, including 33% vs 37% grade ≥3),48,49 and with weekly and twice-weekly ixazomib in combination with Rd (grade ≥3 thrombocytopenia 8% vs 6%) (Table 2).
Gastrointestinal
AEs Consistent with results from early-phase studies of single-agent ixazomib and ixazomib–Rd, diarrhea was a common AE in the TOURMALINE-MM1 study (Table 2).6 However, also consistent with previous studies, most such events were of low grade and there were no apparent differences between the ixazomib–Rd and placebo–Rd groups in the incidence of potential complications of diarrhea such as hypokalemia, dehydration, weight loss, hyponatremia, and hypomagnesemia. The incidence of the first occurrence of diarrhea was highest during the first 3 months of treatment in both groups and generally declined over time. With ixazomib–Rd, the onset was predictable, primarily the day of or the day after ixazomib dosing. Antidiarrheal medications were used to manage diarrhea at the physician’s discretion, with loperamide being the most commonly prescribed medication.
Nausea and vomiting were also reported in early-phase studies of ixazomib.47–49 In TOURMALINE-MM1, nausea and vomiting were more common with ixazomib–Rd versus placebo–Rd, but were primarily seen within the first few months of treatment and were low-grade and manageable; 21% and 13% of patients in the ixazomib–Rd and placebo–Rd groups used antiemetics.6
Rash
Rash was identified as a common AE in phase 1 studies of single-agent ixazomib and of ixazomib–Rd, with incidences of skin and subcutaneous tissue disorders (MedDRA System organ class) ranging from 22% to 55%, including 3%–17% grade ≥3 events (Table 2).47–49,60 Using the higher level term rashes, eruptions, and exanthems not elsewhere classified, as reported in the US prescribing information, the incidences were 20% versus 13% for any-grade events, including 2% versus 2% for grade 3 events.6,45 Typical interventions included antihistamines or topical glucocorticoids, but the rash events tended to occur within the first few cycles and resolved without intervention in 21% of patients in the ixazomib–Rd group and 12% in the placebo–Rd group.
Peripheral neuropathy
PN is common with the first-in-class proteasome inhibitor bortezomib,37 particularly when administered intravenously rather than subcutaneously.77 Much lower incidences have been reported with carfilzomib.78 Low incidences of PN were similarly reported in early-phase studies of ixazomib–Rd and single-agent ixazomib,47–49,60 and supported by the phase 3 TOURMALINE-MM1 study results (Table 2). There was a 5% difference in the incidence of PN between treatment arms in the double-blind, placebo-controlled TOURMALINE-MM1 study: 27% versus 22% in the ixazomib–Rd versus placebo–Rd arms, with no difference in grade 3 PN (2% in each arm). In the ixazomib–Rd and placebo–Rd arms, the incidence of PN was similar in bortezomib-exposed and bortezomib-naive patients (25% vs 31% with ixazomib–Rd, and 21% vs 23% with placebo–Rd).69
Neutropenia
Neutropenia increases the risk of bacterial and fungal infections and is commonly reported with MM therapy, including bortezomib and lenalidomide. Phase 1 studies of single-agent ixazomib reported incidences of grade ≥3 neutropenia of 18% and 17% with weekly and twice-weekly ixazomib (Table 2), possibly reflecting patient populations with low absolute neutrophil counts at baseline.48,49 However, similar rates of grade ≥3 neutropenia were seen in the ixazomib–Rd and placebo–Rd groups in the TOURMALINE-MM1 study (22% vs 24%, respectively), together with similar rates of granulocyte colony-stimulating factor use (21% vs 20%),6 suggesting similar rates of neutropenia with the addition of ixazomib to the Rd regimen. Neutropenia was reported most frequently within the first 3 cycles, with no cumulative effect seen across the study.
Other AEs of clinical interest
Consistent with the findings of the PK concentration-QTc analysis,52 there have been no safety concerns with respect to cardiac toxicity for ixazomib. In TOURMALINE-MM1, there were similar incidences of cardiac arrhythmia, heart failure, and myocardial infarction in the ixazomib–Rd and placebo–Rd arms (16% vs 15%, 4% in each arm, 1% vs 2%, respectively).6
Similarly, there appear to be no safety concerns for ixazomib with respect to renal failure or thromboembolism. In TOURMALINE-MM1, the rates of any-grade renal failure (9% vs 11%) and any-grade thromboembolism (8% vs 11%) were similar with ixazomib–Rd and placebo–Rd.6
Patient-reported QoL
With the increased focus on long-term treatment, patient-reported QoL is an increasingly important endpoint in MM clinical trials. Patient-reported QoL was a secondary endpoint of TOURMALINE-MM1 and was assessed using the EORTC QLQ-C3079 and MY-2080 questionnaires. Over a median follow-up of 23 months, the addition of ixazomib to the Rd regimen appeared to have no adverse impact on patient-reported QoL, with similar mean EORTC QLQ-C30 global scores and MY-20 side effect scores maintained during treatment in both the ixazomib–Rd and placebo–Rd arms, with significantly higher mean scores seen in the ixazomib–Rd vs placebo–Rd arm for the physical, emotional, and social scales,81 supporting the feasibility of long-term administration of the ixazomib–Rd regimen. These QoL data are notable given that TOURMALINE-MM1 was a double-blinded, placebo-controlled study, and there can be a tendency to overestimate QoL and underestimate treatment burden in the active arms of open-label studies.82,83
The sustainability of the ixazomib–Rd regimen was also highlighted by the high median relative dose intensity for ixazomib (97.4% for ixazomib vs 98.8% for placebo); median relative dose intensities for lenalidomide and dexamethasone were also similar between the two arms (93.8% vs 96.6% for lenalidomide, 92.2% vs 94.9% for dexamethasone).6
Ongoing phase 3 trials in MM
In addition to the TOURMALINE-MM1 study, three other phase 3 studies of ixazomib in MM are ongoing (Table 3).6 In the TOURMALINE-MM2 study (NCT01850524), weekly ixazomib–Rd and placebo–Rd are being compared in newly diagnosed patients with MM who are not eligible for ASCT. As with TOURMALINE-MM1, the study incorporates a continuous therapy approach, with patients being treated until disease progression or unacceptable toxicity; after 18 cycles of initial treatment, dexamethasone will be discontinued and lenalidomide and ixazomib will be continued at a reduced dose until progression. The TOURMALINE-MM3 (NCT02181413) and TOURMALINE-MM4 (NCT02312258) studies are assessing ixazomib, versus placebo, as maintenance therapy. Patients with newly diagnosed MM who have had a response to induction therapy followed by ASCT are eligible for TOURMALINE-MM3, and NDMM patients who have had a response to induction therapy but have not undergone ASCT are eligible for TOURMALINE-MM4. In both studies, patients will receive ixazomib at a fixed dose of 3.0 mg for the first 4 cycles, which will then be increased to 4.0 mg if tolerability is acceptable, per the results of the exposure–safety–efficacy analyses.84
Table 3.
Phase 3 trials of ixazomib in MM
| Study | NCT number | Regimen(s) | Patients | 1° endpoint | Status |
|---|---|---|---|---|---|
| C160106 | NCT01564537 | Ixazomib-Rd vs placebo-Rd | Relapsed and/or refractory, 1–3 prior therapies | PFS | Ongoing |
| C16014 | NCT01850524 | Ixazomib-Rd vs placebo-Rd | Newly diagnosed ASCT-ineligible | PFS | Ongoing |
| C16019 | NCT02181413 | Ixazomib vs placebo | Newly diagnosed, with response to induction therapy followed by ASCT | PFS | Ongoing |
| C16021 | NCT02312258 | Ixazomib vs placebo | Newly diagnosed, with response to induction therapy but have not undergone ASCT | PFS | Recruiting |
Abbreviations: ASCT, autologous stem cell transplant; MM, multiple myeloma; PFS, progression-free survival; Rd, lenalidomide-dexamethasone.
This comprehensive phase 3 program is complemented by a large investigator-initiated clinical study program (>70 ongoing investigator-initiated clinical studies), with MM cooperative groups throughout the world now assessing ixazomib in a clinical trial setting. Further, the open-label observation INSIGHT-MM trial (NCT02761187) will collect ‘real-world’ clinical data on ixazomib outside of a clinical trial setting. This continued clinical research aims to further understand both the disease and the role of ixazomib in its treatment.
Conclusion
Proteasome inhibition is a backbone of MM treatment, and the oral proteasome inhibitor ixazomib is a promising MM treatment, demonstrating anti-myeloma activity and a generally well-tolerated and manageable toxicity profile, both as a single agent and in combination.
Data from the phase 3 TOURMALINE-MM1 study demonstrated that ixazomib adds another option to the RRMM treatment armamentarium, with a similar HR seen for ixazomib plus Rd versus Rd as seen in other studies of proteasome inhibitors plus Rd. Together with limited additional toxicity, and maintained patient-reported QoL, the oral administration of ixazomib may offer a simpler, less burdensome, and sustained proteasome inhibitor therapy. Particularly encouraging data have also been reported in patients with a poor prognosis, such as those with multiple prior lines of therapy and those with high-risk cytogenetic abnormalities. The efficacy in patients with high-risk cytogenetics is particularly notable, as these patients appear to need prolonged, sustained, active therapy with a proteasome inhibitor,36,67 all of which appears feasible with ixazomib.
Importantly in the era of triplet regimens, PK studies have demonstrated that ixazomib can be readily combined at full dose with other therapeutic agents. Of relevance to the global, real-world MM patient population, PK studies have also shown that ixazomib can be administered without any dose adjustment to Asian patients and, at a lower dose of 3 mg, to patients with severe renal impairment or end-stage renal disease and to patients with moderate or severe hepatic impairment.
Investigation of ixazomib is ongoing, in NDMM and as long-term maintenance therapy after ASCT or other induction regimens, and in combination with other agents in patients with RRMM. The results of these trials are awaited with interest and will be used to further define the role of ixazomib in the treatment of MM in terms of optimal combination regimens and therapeutic approaches in different patient populations.
In summary, ixazomib is an effective therapeutic option in the treatment of RRMM, which, due to its oral administration, limited additional toxicity, and the demonstrated feasibility of its administration in combination regimens, is likely to have an important role in the long-term treatment of patients with MM.
Acknowledgments
The authors acknowledge the writing support of Jane Saunders of FireKite, an Ashfield company, part of UDG Healthcare plc, in the development of this manuscript, which was funded by Millennium Pharmaceuticals, Inc., and complied with Good Publication Practice 3 ethical guidelines (Battisti et al, Ann Intern Med 2015;163:461-4).
Footnotes
Disclosure
PGR: Membership on an entity’s board of directors, speakers bureau, or its advisory committees: Takeda, Janssen. SK: Consultancy: Takeda, Celgene, Janssen, BMS, Noxxon, Kesios, Glycomimetics, Skyline; honoraria, Skyline Dx. JPL: Consultancy: Takeda and Novartis; research funding: Takeda, Novartis, Celgene. NG, DB, and HvdV: Employment: Takeda Pharmaceutical Company Limited. PM: Honoraria, BMS, Takeda, Janssen, Celgene, Novartis. The authors report no other conflicts of interest in this work.
References
- 1.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 2.Rollig C, Knop S, Bornhauser M. Multiple myeloma. Lancet. 2015;385:2197–2208. doi: 10.1016/S0140-6736(14)60493-1. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2012. [Accessed December 13, 2016]. Available from: http://globocan.iarc.fr/Default.aspx.
- 5.Anderson KC. Oncogenomics to target myeloma in the bone marrow microenvironment. Clin Cancer Res. 2011;17:1225–1233. doi: 10.1158/1078-0432.CCR-10-3366. [DOI] [PubMed] [Google Scholar]
- 6.Moreau P, Masszi T, Grzasko N, et al. Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;374:1621–1634. doi: 10.1056/NEJMoa1516282. [DOI] [PubMed] [Google Scholar]
- 7.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Lopez J, Lahuerta JJ, Pepin F, et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood. 2014;123:3073–3079. doi: 10.1182/blood-2014-01-550020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munshi NC, Anderson KC. Minimal residual disease in multiple myeloma. J Clin Oncol. 2013;31:2523–2526. doi: 10.1200/JCO.2013.49.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cid Ruzafa J, Merinopoulou E, Baggaley RF, et al. Patient population with multiple myeloma and transitions across different lines of therapy in the USA: an epidemiologic model. Pharmacoepidemiol Drug Saf. 2016;25(8):871–879. doi: 10.1002/pds.3927. [DOI] [PubMed] [Google Scholar]
- 12.Song X, Cong Z, Wilson K. Real-world treatment patterns, comorbidities, and disease-related complications in patients with multiple myeloma in the United States. Curr Med Res Opin. 2016;32:95–103. doi: 10.1185/03007995.2015.1105202. [DOI] [PubMed] [Google Scholar]
- 13.Willenbacher E, Weger R, Rochau U, Siebert U, Willenbacher W. Real-world use of 3rd line therapy for multiple myeloma in Austria: an Austrian Myeloma Registry (AMR) analysis of the therapeutic landscape and clinical outcomes prior to the use of next generation myeloma therapeutics. PLoS One. 2016;11:e0147381. doi: 10.1371/journal.pone.0147381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dimopoulos MA, Richardson PG, Moreau P, Anderson KC. Current treatment landscape for relapsed and/or refractory multiple myeloma. Nat Rev Clin Oncol. 2015;12:42–54. doi: 10.1038/nrclinonc.2014.200. [DOI] [PubMed] [Google Scholar]
- 15.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109:3489–3495. doi: 10.1182/blood-2006-08-040410. [DOI] [PubMed] [Google Scholar]
- 16.Avet-Loiseau H, Fonseca R, Siegel D, et al. Efficacy and safety of carfilzomib, lenalidomide, and dexamethasone vs lenalidomide and dexamethasone in patients with relapsed multiple myeloma based on cytogenetic risk status: subgroup analysis from the phase 3 Study Aspire ( NCT01080391) [abstract] Blood. 2015;126:731. [Google Scholar]
- 17.Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San Miguel JF. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple myeloma. Blood. 2013;121:884–892. doi: 10.1182/blood-2012-05-432203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sonneveld P, Avet-Loiseau H, Lonial S, et al. Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood. 2016;127(24):2955–2962. doi: 10.1182/blood-2016-01-631200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristinsson SY, Anderson WF, Landgren O. Improved long-term survival in multiple myeloma up to the age of 80 years. Leukemia. 2014;28:1346–1348. doi: 10.1038/leu.2014.23. [DOI] [PubMed] [Google Scholar]
- 20.Palumbo A, Bringhen S, Mateos MV, et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: an International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;25:195–200. doi: 10.1093/annonc/mdt483. [DOI] [PubMed] [Google Scholar]
- 22.Greipp PR, San MJ, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 23.Cavo M, Pantani L, Petrucci MT, et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 24.Durie B, Hoering A, Rajkumar SV, et al. Bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate Autologous Stem Cell Transplant (ASCT): results of the randomized phase III trial SWOG S0777. Blood. 2015;126:25. doi: 10.1038/s41408-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreau P, Hulin C, Macro M, et al. VTD is superior to VCD prior to intensive therapy in multiple myeloma: results of the prospective IFM2013-04 trial. Blood. 2016;127:2569–2574. doi: 10.1182/blood-2016-01-693580. [DOI] [PubMed] [Google Scholar]
- 26.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 27.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 28.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart AK, Rajkumar SV, Dimopoulos MA, et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 31.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375:754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 32.Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 33.Benboubker L, Dimopoulos MA, Dispenzieri A, et al. Lenalidomide and dexamethasone in transplant-ineligible patients with myeloma. N Engl J Med. 2014;371:906–917. doi: 10.1056/NEJMoa1402551. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo A, Hajek R, Delforge M, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366:1759–1769. doi: 10.1056/NEJMoa1112704. [DOI] [PubMed] [Google Scholar]
- 35.Palumbo A, Gay F, Cavallo F, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015;33:3459–3466. doi: 10.1200/JCO.2014.60.2466. [DOI] [PubMed] [Google Scholar]
- 36.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 37.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- 38.Amgen . KYPROLIS® (carfilzomib) for injection, for intravenous use [prescribing information] Thousand Oaks, CA: Onyx Pharmaceuticals, Inc; 2016. [Accessed December 13, 2016]. Available from: http://pi.amgen.com/united_states/kyprolis/kyprolis_pi.pdf. [Google Scholar]
- 39.Baz R, Lin HM, Hui AM, et al. Development of a conceptual model to illustrate the impact of multiple myeloma and its treatment on health-related quality of life. Support. Care Cancer. 2015;23:2789–2797. doi: 10.1007/s00520-015-2644-6. [DOI] [PubMed] [Google Scholar]
- 40.Romanus D, Raju A, Yong C. Duration of therapy in U.S. patients treated for relapsed/refractory multiple myeloma (RRMM) in the real-world [abstract] Haematologica. 2016;538 abstract E1301. [Google Scholar]
- 41.Richardson PG, Palumbo A, Mateos MV, et al. The current unmet medical needs in the treatment and management of multiple myeloma. Clin Lymphoma Myeloma Leuk. 2015;15:e244. [Google Scholar]
- 42.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 43.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 44.Kupperman E, Lee EC, Cao Y, et al. Evaluation of the proteasome inhibitor MLN9708 in preclinical models of human cancer. Cancer Res. 2010;70:1970–1980. doi: 10.1158/0008-5472.CAN-09-2766. [DOI] [PubMed] [Google Scholar]
- 45.Takeda Pharmaceuticals Limited NILARO® (ixazomib) capsules, for oral use [prescribing information] 2015. [Accessed December 13, 2016]. Available from: https://www.ninlarohcp.com/prescribing-information.pdf.
- 46.Gupta N, Diderichsen PM, Hanley MJ, et al. Population pharmacokinetic analysis of ixazomib, an oral proteasome inhibitor, including data from the phase III TOURMALINE-MM1 study to inform labelling. Clin Pharmacokinet. 2017 Mar 13; doi: 10.1007/s40262-017-0526-4. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar SK, Berdeja JG, Niesvizky R, et al. Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol. 2014;15:1503–1512. doi: 10.1016/S1470-2045(14)71125-8. [DOI] [PubMed] [Google Scholar]
- 48.Kumar SK, Bensinger WI, Zimmerman TM, et al. Phase 1 study of weekly dosing with the investigational oral proteasome inhibitor ixazomib in relapsed/refractory multiple myeloma. Blood. 2014;124:1047–1055. doi: 10.1182/blood-2014-01-548941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson PG, Baz R, Wang M, et al. Phase 1 study of twice-weekly ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma patients. Blood. 2014;124:1038–1046. doi: 10.1182/blood-2014-01-548826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta N, Hanley MJ, Harvey RD, et al. A pharmacokinetics and safety phase 1/1b study of oral ixazomib in patients with multiple myeloma and severe renal impairment or end-stage renal disease requiring haemodialysis. Br J Haematol. 2016;174(5):748–759. doi: 10.1111/bjh.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta N, Hanley MJ, Venkatakrishnan K, et al. Pharmacokinetics of ixazomib, an oral proteasome inhibitor, in solid tumour patients with moderate or severe hepatic impairment. Br J Clin Pharmacol. 2016;82(3):728–738. doi: 10.1111/bcp.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta N, Huh Y, Hutmacher MM, et al. Integrated nonclinical and clinical risk assessment of the investigational proteasome inhibitor ixazomib on the QTc interval in cancer patients. Cancer Chemother Pharmacol. 2015;76:507–516. doi: 10.1007/s00280-015-2815-7. [DOI] [PubMed] [Google Scholar]
- 53.Gupta N, Zhao Y, Hui AM, Esseltine DL, Venkatakrishnan K. Switching from body surface area-based to fixed dosing for the investigational proteasome inhibitor ixazomib: a population pharmacokinetic analysis. Br J Clin Pharmacol. 2015;79:789–800. doi: 10.1111/bcp.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson JA. Predictability of the effects of race or ethnicity on pharmacokinetics of drugs. Int J Clin Pharmacol Ther. 2000;38:53–60. doi: 10.5414/cpp38053. [DOI] [PubMed] [Google Scholar]
- 55.Ramamoorthy A, Pacanowski M, Bull J, Zhang L. Racial/ethnic differences in drug disposition and response: Review of recently approved drugs. Clin Pharmacol Ther. 2015;97:263–273. doi: 10.1002/cpt.61. [DOI] [PubMed] [Google Scholar]
- 56.Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84:417–423. doi: 10.1038/clpt.2008.141. [DOI] [PubMed] [Google Scholar]
- 57.Gupta N, Goh YT, Min CK, et al. Pharmacokinetics and safety of ixazomib plus lenalidomide-dexamethasone in Asian patients with relapsed/refractory myeloma: a phase 1 study. J Hematol Oncol. 2015;8:103. doi: 10.1186/s13045-015-0198-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Palumbo A, Morgan G, Rajkumar SV, et al. Two phase 3 studies of the oral proteasome inhibitor (PI) ixazomib for multiple myeloma (MM) in the maintenance setting: TOURMALINE-MM3, and -MM4 [abstract] J Clin Oncol. 2016;34 abstr TPS8068. [Google Scholar]
- 59.Gupta N, Hanley MJ, Venkatakrishnan K, et al. The effect of a high-fat meal on the pharmacokinetics of ixazomib, an oral proteasome inhibitor, in patients with advanced solid tumors or lymphoma. J Clin Pharmacol. 2016;56(10):1288–1295. doi: 10.1002/jcph.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson PG, Hofmeister CC, Rosenbaum CA, et al. Twice-weekly oral MLN9708 (ixazomib citrate), an investigational proteasome inhibitor, in combination with lenalidomide (len) and dexamethasone (dex) in patients (pts) with newly diagnosed multiple myeloma (MM): final phase 1 results and phase 2 data [abstract] Blood. 2013;122:535. [Google Scholar]
- 61.Dimopoulos MA, Grosicki S, Jedrzejczak WW, et al. Randomized phase 2 study of the all-oral combination of investigational proteasome inhibitor ixazomib plus cyclophosphamide and low-dose dexamethasone (ICd) in patients with newly diagnosed multiple myeloma who are transplant-ineligible ( NCT02046070) [abstract] Blood. 2015;126:26. [Google Scholar]
- 62.San Miguel JF, Hajek R, Spicka I, et al. Oral MLN9708, an investigational proteasome inhibitor, in combination with melphalan and prednisone in patients with previously untreated multiple myeloma: a phase 1 study [abstract] Haematologica. 2012;97:1. [Google Scholar]
- 63.Gupta N, Hanley MJ, Venkatakrishnan K, et al. A phase 1 drug-drug interaction study between ixazomib, an oral proteasome inhibitor, and rifampin in patients with advanced solid tumors [abstract] Mol Center Ther. 2015;14(12 Suppl 2) Abstract nr B147. [Google Scholar]
- 64.Kumar S, Roy V, Reeder CB, et al. Phase 2 trial of single agent MLN9708 in patients with relapsed multiple myeloma not refractory to bortezomib [abstract] Blood. 2013;122:1944. [Google Scholar]
- 65.Kumar S, Laplant B, Reeder CB, et al. Randomized phase 2 trial of two different doses of ixazomib in patients with relapsed multiple myeloma not refractory to bortezomib [abstract] Blood. 2015;126:3050. doi: 10.1038/bcj.2015.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou J, Jin J, Xu Y, et al. Ixazomib plus lenelidomide-dexamethasone (IRd) vs placebo-Rd in patients with relapsed/refractory multiple myeloma: China continuation of TOURMALINE-MM1 [abstract] Haematologica. 2016;101:540. [Google Scholar]
- 67.Laubach J, Garderet L, Mahindra A, et al. Management of relapsed multiple myeloma: recommendations of the International Myeloma Working Group. Leukemia. 2016;30:1005–1017. doi: 10.1038/leu.2015.356. [DOI] [PubMed] [Google Scholar]
- 68.Avet-Loiseau H, Bahlis N, Chng WJ, et al. Impact of cytogenetic risk status on efficacy and safety of ixazomib-lenalidomide-dexamethasone (IRd) vs placebo-Rd in relapsed/refractory multiple myeloma patients in the global TOURMALINE-MM1 study [abstract] Haematologica. 2016;101:80. [Google Scholar]
- 69.Mateos MV, Masszi T, Grzasko N, et al. Efficacy and safety of oral ixazomib-lenalidomide-dexamethasone (IRd) vs placebo-Rd in relapsed/refractory multiple myeloma patients: impact of prior therapy in the phase 3 TOURMALINE-MM1 study [abstract] Haematologica. 2016;101:527. doi: 10.3324/haematol.2017.170118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brioli A, Melchor L, Cavo M, Morgan GJ. The impact of intra-clonal heterogeneity on the treatment of multiple myeloma. Br J Haematol. 2014;165:441–454. doi: 10.1111/bjh.12805. [DOI] [PubMed] [Google Scholar]
- 72.Kumar S, Flinn I, Richardson PG, et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 73.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 74.Krishnan AY, Kapoor P, Palmer J, et al. A phase I/II study of ixazomib, pomalidomide, dexamethasone in relapsed refractory multiple myeloma: initial results [abstract] J Clin Oncol. 2016;34:8008. [Google Scholar]
- 75.Reu FJ, Valent J, Malek E, et al. A phase 1 study of ixazomib in combination with panobinostat and dexamethasone in patients with relapsed or refractory multiple myeloma [abstract] Blood. 2015;126:4221. [Google Scholar]
- 76.Voorhees P, Mulkey F, Hassoun H, et al. Alliance A061202. a phase I/II study of pomalidomide, dexamethasone and ixazomib versus pomalidomide and dexamethasone for patients with multiple myeloma refractory to lenalidomide and proteasome inhibitor based therapy: phase I results [abstract] Blood. 2015;126:375. [Google Scholar]
- 77.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous bortezomib in patients with relapsed multiple myeloma: sub-analysis of patients with renal impairment in the phase III MMY-3021 study. Haematologica. 2015;100:e207–e210. doi: 10.3324/haematol.2014.118182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harvey RD. Incidence and management of adverse events in patients with relapsed and/or refractory multiple myeloma receiving single-agent carfilzomib. Clin Pharmacol. 2014;6:87–96. doi: 10.2147/CPAA.S62512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 80.Cocks K, Cohen D, Wisloff F, et al. An international field study of the reliability and validity of a disease-specific questionnaire module (the QLQ-MY20) in assessing the quality of life of patients with multiple myeloma. Eur J Cancer. 2007;43:1670–1678. doi: 10.1016/j.ejca.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 81.Leleu X, Masszi T, Bahlis N, et al. Patient-reported quality of life with ixazomib-lenalidomide-dexamethasone (IRd) vs placebo-Rd in relapsed/refractory multiple myeloma patients in the global, placebo-controlled TOURMALINE-MM1 study [abstract] Haematologica. 2016;101:261. [Google Scholar]
- 82.Food and Drug Administration . Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. 2009. 27-10-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Razzouk BI, Hord JD, Hockenberry M, et al. Double-blind, placebo-controlled study of quality of life, hematologic end points, and safety of weekly epoetin alfa in children with cancer receiving myelosuppressive chemotherapy. J Clin Oncol. 2006;24:3583–3589. doi: 10.1200/JCO.2005.03.4371. [DOI] [PubMed] [Google Scholar]
- 84.Gupta N, Labotka R, Liu G, Hui AM, Venkatakrishnan K. Exposure-safety-efficacy analysis of single-agent ixazomib, an oral proteasome inhibitor, in relapsed/refractory multiple myeloma: dose selection for a phase 3 maintenance study. Invest New Drugs. 2016;34:338–346. doi: 10.1007/s10637-016-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]