Abstract
Purpose
Aging is a major risk factor in dry eye disease (DED), and understanding sexual differences is very important in biomedical research. However, there is little information about sex differences in the effect of aging on DED. We investigated sex differences in the effect of aging and other risk factors for DED.
Methods
This study included data of 16,824 adults from the Korea National Health and Nutrition Examination Survey (2010–2012), which is a population-based cross-sectional survey. DED was defined as the presence of frequent ocular dryness or a previous diagnosis by an ophthalmologist. Basic sociodemographic factors and previously known risk factors for DED were included in the analyses. Linear regression modeling and multivariate logistic regression modeling were used to compare the sex differences in the effect of risk factors for DED; we additionally performed tests for interactions between sex and other risk factors for DED in logistic regression models.
Results
In our linear regression models, the prevalence of DED symptoms in men increased with age (R=0.311, P=0.012); however, there was no association between aging and DED in women (P>0.05). Multivariate logistic regression analyses showed that aging in men was not associated with DED (DED symptoms/diagnosis: odds ratio [OR] =1.01/1.04, each P>0.05), while aging in women was protectively associated with DED (DED symptoms/diagnosis: OR =0.94/0.91, P=0.011/0.003). Previous ocular surgery was significantly associated with DED in both men and women (men/women: OR =2.45/1.77 [DED symptoms] and 3.17/2.05 [DED diagnosis], each P<0.001). Tests for interactions of sex revealed significantly different aging × sex and previous ocular surgery × sex interactions (P for interaction of sex: DED symptoms/diagnosis − 0.044/0.011 [age] and 0.012/0.006 [previous ocular surgery]).
Conclusion
There were distinct sex differences in the effect of aging on DED in the Korean population. DED following ocular surgery also showed sexually different patterns. Age matching and sex matching are strongly recommended in further studies about DED, especially DED following ocular surgery.
Keywords: dry eye disease, risk factors, sex differences, aging, previous ocular surgery
Introduction
Dry eye disease (DED) is a multifactorial disorder of the ocular surface and an important public health issue that causes ocular discomfort and visual disturbance.1 DED affects ~15% of the US population, and it is more common in older people.2 The Epidemiology Subcommittee of the 2007 Dry Eye WorkShop reported that major risk factors for DED included increasing age, female sex, and a history of previous ocular surgery.2 Men and women have differences in their basic physiology, their susceptibility to diseases, and their behavioral and psychosocial factors. Recently, consideration and characterization of sex-related differences are becoming increasingly important in biomedical research. Understanding sex differences in DED is essential for successful disease evaluation and management. There have been many epidemiologic studies that had evaluated several risk factors for DED.3–8 However, to the best of our knowledge, there is little information about how risk factors for DED, including aging and previous ocular surgery, are affected by sex. Therefore, we investigated sex-related differences in risk factors for DED using a well-stratified, large population-based dataset from the Korea National Health and Nutrition Examination Survey (KNHANES).
Methods
Study population and data collection
KNHANES is a nationwide, population-based, cross-sectional survey consisting of three parts: a health interview survey, a health examination survey, and a nutritional survey. A field survey team that included an ophthalmologist, as well as nurse examiners for health assessments, traveled with a mobile examination unit to perform interviews and physical examinations. Between January 1, 2010, and December 31, 2012, 11,400 households in 576 survey districts were enrolled in KNHANES V using the stratified, multistage, clustered sampling method that was based on 2009 National Resident Demographics; 31,596 individuals were sampled. The response rate for KNHANES V was 80.8% (25,533 subjects). After excluding possible subjects who did not provide information for both DED and our study covariates, 16,824 adult subjects were included in our statistical analysis (Figure 1). This study was approved by the institutional review board of the Korea Centers for Disease Control and Prevention and complied with the tenets of the Declaration of Helsinki. All participants provided written informed consent. All data were de-identified.
Figure 1.
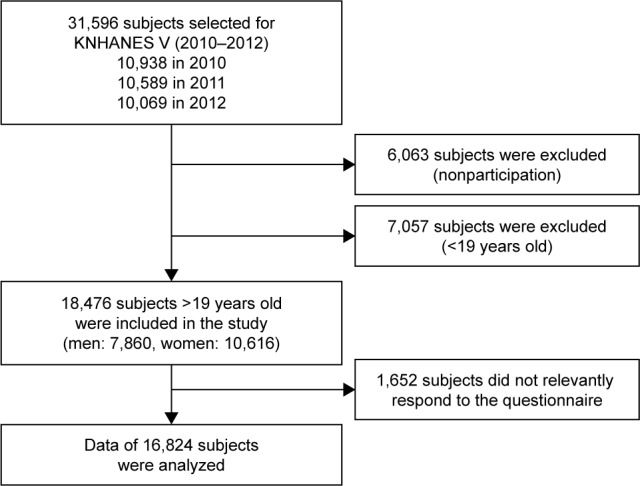
Flow diagram presenting the selection of study participants.
Abbreviation: KNHANES, Korea National Health and Nutrition Examination Survey.
Outcome measures in DED
We identified DED using the following two questions: 1) Do your eyes frequently feel dry or irritated? and 2) Have you ever been diagnosed by an ophthalmologist as having dry eye syndrome? Possible answers to both questions were “yes” or “no”. Based on these questions, we defined two outcome measures for DED: 1) DED symptoms or 2) DED diagnosis.
Potential risk factors of DED
Since DED is a multifactorial disease, we considered sociodemographic and previously known clinical factors as potential risk factors for the onset of DED. Sociodemographic factors included age, sex, region of residence (urban vs rural), education level (university or higher vs high school or lower), and income level (high [first or second quartile] vs low [third or fourth quartile]). Previous study and our pilot analysis (data not shown) using KNHANES data showed that DED symptoms and diagnosis were both significantly associated with the presence of thyroid disease, dyslipidemia, subjective health awareness, and previous ocular surgery history.9 Therefore, we considered the presence of thyroid disease (±), dyslipidemia (±), subjective health awareness (poor/good), and previous ocular surgery history (±) as potential risk factors for DED. After consideration of previous studies, we included the presence of additional diseases as potential risk factors: hypertension (±), diabetes mellitus (±), rheumatoid arthritis (±), depression (±), and atopic dermatitis (±).2,10 “Previous ocular surgery” included cataract surgery, pterygium surgery, corneal refractive surgery, and vitrectomy.
Statistical analysis
To sufficiently accommodate the complex survey design of stratification, random sampling, and cluster sampling, all statistical analyses were performed using SPSS Complex Samples procedures (PASW Statistics for Windows, Version 18.0. Chicago, IL, USA) in accordance with the SPSS manual from the Korea Centers for Disease Control and Prevention and the Korean Ophthalmological Society.11,12 Since increasing age is the most well-known risk factor for DED, linear regression models were used to evaluate associations between aging and the prevalence of DED in Korean men and women. Additionally, multivariate logistic regression models were constructed to compare sex-related differences in the risk factors for DED. In all logistic models, sociodemographic and clinical factors (age, region of residence, education level, income level, dyslipidemia status, thyroid disease status, subjective health awareness, hypertension status, diabetes mellitus status, rheumatoid arthritis status, depression status, atopic dermatitis status, and previous ocular surgery history) were included. The values of odds ratio (OR) in men and women were statistically compared using P for the interaction test. P-value <0.05 was assumed as significant.
Subgroup analysis in subjects >40 years old
A previous study using KNHANES data showed a double top pattern in the prevalence of DED according to age (high in individuals in their 20s and 60s). The authors of that study suggested that the main reason for this pattern could be contact lens usage by the younger generation.9 In Korea, the mean age of contact lens users is 22.9±6.8 years; most users are women (89.0%).13 Contact lens usage is a widely known risk factor for DED.2 Since KNHANES did not record data about contact lens usage, we performed the aforementioned statistical analyses separately on subjects >40 years old to control for confounding effects from contact lens usage.
Results
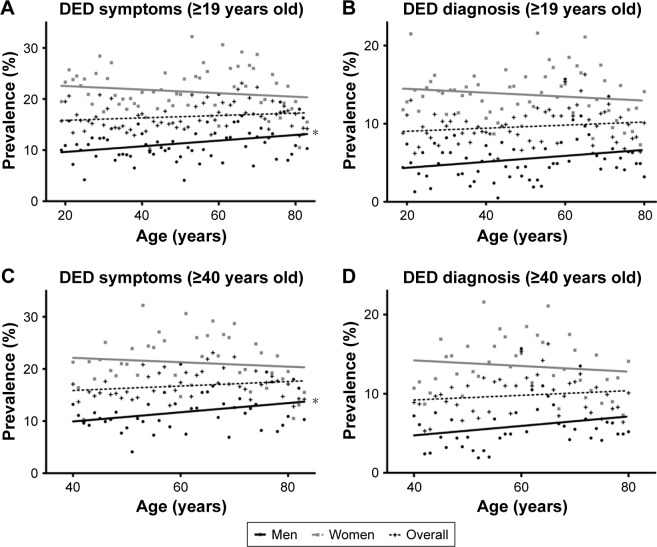
The baseline characteristics of the study subjects are shown in Table 1. The mean age of all subjects was 50.9±16.7 years. There were 7,104 men and 9,720 women. The estimated prevalences of DED symptoms and diagnosis were 10.8% (95% CI: 9.7–11.9) and 4.9% (95% CI: 4.3–5.5), respectively, in men; these prevalences were estimated at 21.6% (95% CI: 20.2–23.0) and 13.6% (95% CI: 12.7–14.7), respectively, in women. In a linear regression model, the prevalence of DED symptoms in men slightly increased with age (Figure 2A: R=0.311, P=0.012 [subjects ≥19 years old]; Figure 2C: R=0.320, P=0.034 [subjects ≥40 years old]). However, aging in women was not associated with the presence of DED symptoms or diagnosis in a linear regression model (each P>0.05; Figure 2A–D). When men and women were combined into a single group, aging was not associated with the presence of DED symptoms or diagnosis in a linear regression model (each P>0.05; Figure 2A–D). Multicollinearity between all baseline demographic variables was checked by ensuring that variance inflation factors did not exceed 10.
Table 1.
Characteristics of the study population
| Demographics | DED symptoms, +/− (%)* | DED diagnosis, +/− (%)* |
|---|---|---|
| Overall | 3,001/13,823 (16.3) | 1,778/15,046 (9.3) |
| Sex | ||
| Men | 821/6,283 (10.8) | 404/6,700 (4.9) |
| Women | 2,180/7,540 (21.6) | 1,374/8,346 (13.6) |
| Age (years) | ||
| 19–29 | 350/1,557 (16.6) | 200/1,707 (9.1) |
| 30–39 | 505/2,545 (15.3) | 288/2,762 (9.3) |
| 40–49 | 462/2,470 (14.9) | 275/2,657 (8.3) |
| 50–59 | 590/2,653 (16.8) | 367/2,876 (9.7) |
| 60–69 | 576/2,324 (18.7) | 374/2,526 (12.1) |
| ≥70 | 518/2,274 (16.8) | 274/2,518 (8.6) |
| Region of residence | ||
| Urban | 1,448/6,263 (17.7) | 900/6,811 (10.4) |
| Rural | 1,553/7,560 (15.0) | 878/8,235 (8.4) |
| Education level** | ||
| High school graduation or less | 2,075/9,388 (16.1) | 1,222/10,251 (9.3) |
| University or higher | 844/4,007 (16.6) | 505/4,346 (9.5) |
| Income level** | ||
| High (first or second quartile group) | 1,576/7,415 (16.1) | 977/8,014 (9.7) |
| Low (third or fourth quartile group) | 1,380/6,217 (16.5) | 775/6,822 (9.0) |
| Dyslipidemia** | ||
| (+) | 441/1,447 (21.8) | 314/1,464 (14.6) |
| (−) | 2,560/12,376 (15.7) | 1,574/13,472 (8.8) |
| Thyroid disease** | ||
| (+) | 189/489 (28.7) | 136/542 (21.1) |
| (−) | 2,812/13,334 (15.9) | 1,642/14,504 (9.0) |
| Subjective health awareness | ||
| Poor | 2,271/9,165 (18.1) | 1,333/10,103 (10.4) |
| Good | 730/4,658 (12.5) | 445/4,943 (7.2) |
| Hypertension** | ||
| (+) | 753/3,139 (17.4) | 439/3,453 (9.8) |
| (−) | 2,248/10,684 (16.0) | 1,339/11,593 (9.2) |
| Diabetes mellitus** | ||
| (+) | 266/1,165 (16.7) | 159/1,272 (9.5) |
| (−) | 2,735/12,658 (16.2) | 1,619/13,774 (9.3) |
| Rheumatoid arthritis** | ||
| (+) | 90/327 (20.4) | 66/351 (15.2) |
| (−) | 2,911/13,496 (16.2) | 1,712/14,695 (9.2) |
| Depression** | ||
| (+) | 620/1,874 (24.0) | 376/2,118 (14.0) |
| (−) | 2,381/11,949 (15.1) | 1,402/12,928 (8.6) |
| Atopic dermatitis** | ||
| (+) | 148/597 (16.7) | 83/662 (9.1) |
| (−) | 2,853/13,226 (16.2) | 1,695/14,384 (9.4) |
| Previous ocular surgery** | ||
| (+) | 685/1,824 (26.7) | 457/2,052 (17.8) |
| (−) | 2,316/11,999 (14.9) | 1,321/12,994 (8.2) |
Notes:
Calculated after applying weights.
Except for nonrespondents.
Abbreviations: OR, odds ratio; DED, dry eye disease; CI, confidence interval.
Figure 2.
Sex difference in the prevalence of DED symptoms and diagnosis according to age.
Note: (A and B) ≥19 years old and (C and D) ≥40 years old.
Abbreviation: DED, dry eye disease.
In multivariate logistic regression modeling, dyslipidemia, subjective health awareness, and previous ocular surgery were significantly associated with the presence of DED symptoms and diagnosis when men and women were combined in a single group (each P<0.05; Tables 2 and 3). In this multivariate logistic regression modeling, aging in men was not associated with the presence of DED (each P>0.05; Tables 2 and 3), while aging in women was protectively associated with the presence of DED (DED symptoms: OR =0.94, P=0.008 and DED diagnosis: OR =0.91, P=0.003 [subjects ≥19 years old]; DED symptoms: OR =0.92, P=0.031 and DED diagnosis: OR =0.90, P=0.018 [subjects ≥40 years old]; Tables 2 and 3). Higher education level was associated with the presence of DED symptoms and diagnosis (DED symptoms: OR =1.50, P=0.002 and DED diagnosis: OR =1.64, P=0.005; Table 3), and diagnosis with diabetes mellitus was protectively associated with the presence of DED symptoms in men who were >40 years old (OR =0.70, P=0.021; Table 3). Urban residence, diagnosis of thyroid disease, and diagnosis of depression were only associated with the presence of DED in women (each P<0.05; Tables 2 and 3). The associations of aging with DED were significantly different between men and women (“aging × sex” interaction – DED symptoms: P=0.044 and DED diagnosis: P=0.011 [subjects ≥19 years old]; DED symptoms: P=0.026 and DED diagnosis: P=0.009 [subjects ≥40 years old]; Tables 2 and 3). The effects of previous ocular surgery on DED symptoms and diagnosis in men were significantly greater than those in women (“previous ocular surgery × sex” interaction – DED symptoms: OR =2.45 [men], 1.77 [women], P=0.012; DED diagnosis: OR =3.17 [men], 2.05 [women], P=0.006; Table 2). In subjects ≥40 years old, the effects of previous ocular surgery on DED symptoms in men were also significantly greater than those in women (“previous ocular surgery × sex” interaction – DED symptoms: OR =2.16 [men], 1.67 [women], P=0.038; Table 3). The associations of higher education level with DED symptoms were also different between men and women in subjects who were ≥40 years old (“higher education level × sex” interaction: OR =1.50 [men], 0.84 [women], P=0.025; Table 3). Interactions between sex and the other risk factor variables (region of residence, dyslipidemia status, thyroid disease status, subjective health awareness, hypertension status, diabetes status, depression status, and atopic dermatitis status) were not significant (each P>0.05; Tables 2 and 3).
Table 2.
Multivariate logistic regression model for comparison of ORs between men and women (subjects who were >19 years old)
| Variables | DED symptoms
|
DED diagnosis
|
||||
|---|---|---|---|---|---|---|
| Men, OR (95% CI, P-value) | Women, OR (95% CI, P-value) | P for interaction of sex | Men, OR (95% CI, P-value) | Women, OR (95% CI, P-value) | P for interaction of sex | |
| Age (10-year increase)* | 1.01 (0.94–1.09, 0.737) | 0.94 (0.90–0.98, 0.008) | 0.044 | 1.04 (0.94–1.14, 0.424) | 0.91 (0.86–0.97, 0.003) | 0.011 |
| Region of residence (urban/rural) | 1.13 (0.89–1.42, 0.321) | 1.27 (1.07–1.51, 0.007) | 0.322 | 1.05 (0.80–1.39, 0.708) | 1.31 (1.11–1.55, 0.002) | 0.179 |
| Education level (university or higher/high school or less) | 1.28 (1.03–1.58, 0.023) | 1.02 (0.87–1.19, 0.855) | 0.271 | 1.30 (0.97–1.75, 0.075) | 0.95 (0.79–1.15, 0.621) | 0.149 |
| Income level (high/low) | 0.96 (0.78–1.17, 0.663) | 1.08 (0.95–1.23, 0.253) | 0.272 | 1.40 (1.08–1.83, 0.012) | 1.10 (0.94–1.29, 0.239) | 0.196 |
| Dyslipidemia (±) | 1.43 (1.07–1.92, 0.016) | 1.38 (1.14–1.67, <0.001) | 0.912 | 1.66 (1.15–2.37, 0.006) | 1.79 (1.46–2.20, <0.001) | 0.936 |
| Thyroid disease (±) | 1.59 (0.78–3.21, 0.198) | 1.52 (1.21–1.93, <0.001) | 0.840 | 1.70 (0.76–4.36, 0.266) | 1.84 (1.42–2.37, <0.001) | 0.992 |
| Subjective health awareness (poor/good) | 1.31 (1.07–1.61, 0.008) | 1.48 (1.27–1.72, <0.001) | 0.417 | 1.23 (0.93–1.63, 0.141) | 1.36 (1.13–1.63, <0.001) | 0.585 |
| Hypertension (±) | 0.92 (0.72–1.17, 0.495) | 0.99 (0.83–1.19, 0.928) | 0.824 | 0.86 (0.61–1.21, 0.394) | 0.90 (0.72–1.13, 0.358) | 0.515 |
| Diabetes mellitus (±) | 0.77 (0.55–1.06, 0.103) | 1.03 (0.81–1.31, 0.795) | 0.336 | 0.88 (0.58–1.31, 0.522) | 0.89 (0.67–1.18, 0.422) | 0.687 |
| Rheumatoid arthritis (±) | 0.72 (0.30–1.75, 0.476) | 1.08 (0.77–1.53, 0.653) | 0.480 | 0.84 (0.30–2.32, 0.730) | 1.44 (0.98–2.10, 0.064) | 0.412 |
| Depression (±) | 1.45 (1.07–1.96, 0.016) | 1.37 (1.18–1.61, <0.001) | 0.770 | 1.28 (0.82–2.00, 0.270) | 1.30 (1.10–1.52, 0.002) | 0.960 |
| Atopic dermatitis (±) | 0.92 (0.59–1.45, 0.721) | 1.00 (0.77–1.31, 0.996) | 0.609 | 0.60 (0.29–1.24, 0.169) | 1.02 (0.73–1.42, 0.907) | 0.124 |
| Previous ocular surgery (±)* | 2.45 (1.89–3.17, <0.001) | 1.77 (1.51–2.57, <0.001) | 0.012 | 3.17 (2.29–4.39, <0.001) | 2.05 (1.70–2.46, <0.001) | 0.006 |
Note:
Significant OR difference between men and women.
Abbreviations: OR, odds ratio; DED, dry eye disease; CI, confidence interval.
Table 3.
Multivariate logistic regression model for comparison of ORs in the risk factors of DED between men and women (subjects who were >40 years old)
| Variables | DED symptoms
|
DED diagnosis
|
||||
|---|---|---|---|---|---|---|
| Men, OR (95% CI, P-value) | Women, OR (95% CI, P-value) | P for interaction of sex | Men, OR (95% CI, P-value) | Women, OR (95% CI, P-value) | P for interaction of sex | |
| Age (10-year increase)* | 1.01 (0.95–1.09, 0.508) | 0.92 (0.85–0.98, 0.031) | 0.026 | 1.01 (0.91–1.13, 0.090) | 0.90 (0.82–0.97, 0.018) | 0.009 |
| Region of residence (urban/rural) | 1.16 (0.89–1.50, 0.267) | 1.26 (1.05–1.53, 0.015) | 0.551 | 1.05 (0.76–1.45, 0.770) | 1.36 (1.11–1.65, 0.002) | 0.174 |
| Education level (university or higher/high school or less) | 1.50 (1.15–1.94, 0.002) | 0.84 (0.65–1.07, 0.153) | 0.025 | 1.64 (1.16–2.33, 0.005) | 0.91 (0.69–1.20, 0.487) | 0.081 |
| Income level (high/low) | 0.82 (0.63–1.06, 0.123) | 1.13 (0.96–1.33, 0.142) | 0.038 | 1.25 (0.92–1.70, 0.161) | 1.23 (1.03–1.48, 0.026) | 0.760 |
| Dyslipidemia (±) | 1.39 (1.02–1.90, 0.035) | 1.37 (1.13–1.66, <0.001) | 0.897 | 1.61 (1.10–2.37, 0.014) | 1.79 (1.45–2.20, <0.001) | 0.839 |
| Thyroid disease (±) | 1.30 (0.98–1.95, 0.489) | 1.45 (1.13–1.86, 0.003) | 0.858 | 1.78 (0.66–4.85, 0.256) | 1.96 (1.49–2.48, <0.001) | 0.935 |
| Subjective health awareness (poor/good) | 1.35 (1.05–1.74, 0.020) | 1.67 (1.39–2.02, <0.001) | 0.142 | 1.39 (0.98–1.97, 0.065) | 1.55 (1.23–1.95, <0.001) | 0.496 |
| Hypertension (±) | 1.04 (0.80–1.34, 0.797) | 0.98 (0.82–1.17, 0.822) | 0.506 | 0.96 (0.67–1.38, 0.843) | 0.91 (0.73–1.14, 0.398) | 0.424 |
| Diabetes mellitus (±) | 0.70 (0.51–0.95, 0.021) | 1.04 (0.82–1.33, 0.731) | 0.142 | 0.90 (0.60–1.37, 0.628) | 0.94 (0.70–1.25, 0.648) | 0.795 |
| Rheumatoid arthritis (±) | 0.86 (0.35–2.10, 0.735) | 1.07 (0.75–1.51, 0.721) | 0.643 | 1.00 (0.36–2.75, 1.000) | 1.51 (1.02–2.23, 0.036) | 0.465 |
| Depression (±) | 1.38 (0.98–1.95, 0.066) | 1.39 (1.16–1.67, <0.001) | 0.928 | 1.23 (0.78–1.93, 0.379) | 1.30 (1.06–1.60, 0.013) | 0.723 |
| Atopic dermatitis (±) | 1.36 (0.75–2.46, 0.304) | 1.36 (0.91–2.03, 0.130) | 0.996 | 0.83 (0.28–2.42, 0.730) | 1.21 (0.73–1.99, 0.462) | 0.491 |
| Previous ocular surgery (±)* | 2.16 (1.59–2.93, <0.001) | 1.67 (1.39–2.02, <0.001) | 0.038 | 2.00 (1.42–2.82, <0.001) | 1.76 (1.38–2.24, <0.001) | 0.064 |
Note:
Significant OR difference between men and women.
Abbreviations: OR, odds ratio; DED, dry eye disease; CI, confidence interval.
Discussion
This study demonstrated obvious differences between men and women in risk factors for DED. In our linear and logistic regression models, distinct sex differences in the effect of aging on DED were revealed. In the logistic regression models, previous ocular surgery was significantly associated with DED for both men and women; however, its effects on DED risk were much greater for men than for women. Higher education level was more strongly related to DED symptoms in men than in women. Although dyslipidemia, thyroid disease, subjective health awareness, and depression showed different sex-related ORs, these interactions were not statistically significant.
In multivariate logistic regression models, men presented no significant associations between age and DED, while women showed negative associations between increasing age and DED. In addition, linear regression models also revealed different sex-related patterns in the prevalence of DED. Sex hormones seem to be responsible for these sex-related associations between aging and DED.14,15 Although the effects of sex hormones on the ocular surface are not fully understood, it is known that androgens enhance the function of the lacrimal and meibomian glands; estrogen may antagonize this action.16 In addition, it has been suggested that estrogen stimulates immune responses, whereas androgens suppress inflammatory reactions.17 The androgen/estrogen ratio is usually higher in men than in women, and this may contribute to the sex-related differences in the prevalence of DED.18 Androgen levels generally decrease linearly with age in both men and women.16 However, the estrogen level remains almost steady in men regardless of age; it decreases abruptly in women after menopause.16,19 Therefore, the androgen/estrogen ratio decreases in men, but increases in women, with increasing age.18,19 These changes may explain the sex-related different associations between DED and increasing age in our study.
Increasing age is a well-known risk factor in DED, and most ophthalmologists think that aging is positively associated with DED.20 Decreased tear production from lacrimal gland dysfunction, combined with increased tear evaporation rate, can induce more DED with increasing age.20 In addition, elderly patients usually undergo ocular surgery and use several drugs that can induce DED (such as antidepressants, diuretics, dopaminergic drugs, and antimetabolites).20 However, the prior study using KNHANES data did not show certain positive association between increased age and DED. In fact, some epidemiologic studies have suggested that aging was a major associated factor for DED;3,21–23 however, other studies have indicated that aging was not significantly associated with DED.4–6,8,24 Decreased corneal sensitivity and decreased corneal nerve density, which occur in elderly patients, may be involved in these inconsistent findings about DED.25,26 Further large-scale clinical and epidemiological studies are needed that consider the relationships between possible DED risk factors and corneal sensitivity.
Postoperative pain is usually more severe among women than men, which implicates sex hormones as factors that influence pain sensitivity.27 Contrary to our expectations, we found that DED following previous ocular surgery was more prevalent in men than in women. This is a previously unknown and interesting observation regarding the risk factors for DED. Most ocular surgeries are performed in elderly patients. Because the androgen/estrogen ratio is relatively decreased in elderly men as mentioned earlier, postoperative inflammation may be more exaggerated or sustained in men than in women, which may influence the onset of DED. Sex-related differences in compliance with postoperative medication regimens may also affect the prevalence of DED. Further clinical and experimental studies about sex difference in DED after ocular surgery are required.
Previous studies showed that psychological factors can affect DED and that correlations between mental state and the onset of DED were more prominent in women.28–31 This study also demonstrated that poor subjective health awareness and depression were deeply associated with DED; importantly, OR values were greater in women than in men. However, there was no significant difference between men and women for “subjective health awareness × sex” or “depression × sex” interactions (each P>0.05). Although psychological factors are important in DED, sex-related differences in the impacts of psychological factors on DED may not be as prominent as expected. Higher education level was also associated with the onset of DED, especially in men who were ≥40 years old. Decreased blinking during reading or video display terminal (VDT) use can be related.32,33 There has been no study to suggest that education level influences DED in a sex-related manner. Therefore, further detailed studies are needed to understand the sex-related differences in the onset of DED after reading or VDT work.
There are a few limitations in this study. First, DED was not identified from physical examination but from self-reported questionnaires. Therefore, our results could be different than if we had strictly used criteria for clinically diagnosed DED. We think that two questionnaires in this study were insufficient to reflect overall DED. Second, this study has a cross-sectional design; thus, the results do not definitively prove a causal relationship. Third, age-related changes in the meibomian gland were not considered. Fourth, hormonal status or information about drug use was not included in the analyses. Despite these limitations, this large-scale population-based study is noteworthy because it utilizes a well-defined dataset and several statistical methods to show consistent differences between men and women with DED. Furthermore, the subgroup analyses, designed to exclude the confounding effects of contact lens usage, presented similar results.
Conclusion
Aging and previous ocular surgery affected DED differently between Korean men and Korean women. Age- and sex-matching controls need to be more strongly considered in future studies about DED, especially in studies about DED after ocular surgery.
Acknowledgments
This work was supported by the Gachon University Gil Medical Center (grant number: GCU-2016-5202).
We thank the Epidemiologic Survey Committee of the Korean Ophthalmologic Society. The Epidemiologic Survey Committee of the Korean Ophthalmologic Society mainly participated in making and processing KNHANES data about ophthalmologic questionnaire and examinations and helped us to access KNHANES data.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Miljanovic B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. doi: 10.1016/j.ajo.2006.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The epidemiology of dry eye disease: report of the Epidemiology Subcommittee of the International Dry Eye WorkShop (2007) Ocul Surf. 2007;5(2):93–107. doi: 10.1016/s1542-0124(12)70082-4. [DOI] [PubMed] [Google Scholar]
- 3.Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. doi: 10.1001/archopht.118.9.1264. [DOI] [PubMed] [Google Scholar]
- 4.Chia EM, Mitchell P, Rochtchina E, Lee AJ, Maroun R, Wang JJ. Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol. 2003;31(3):229–232. doi: 10.1046/j.1442-9071.2003.00634.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin PY, Tsai SY, Cheng CY, Liu JH, Chou P, Hsu WM. Prevalence of dry eye among an elderly Chinese population in Taiwan: the Shihpai Eye Study. Ophthalmology. 2003;110(6):1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 6.Lekhanont K, Rojanaporn D, Chuck RS, Vongthongsri A. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25(10):1162–1167. doi: 10.1097/01.ico.0000244875.92879.1a. [DOI] [PubMed] [Google Scholar]
- 7.Viso E, Rodriguez-Ares MT, Gude F. Prevalence of and associated factors for dry eye in a Spanish adult population (the Salnes Eye Study) Ophthalmic Epidemiol. 2009;16(1):15–21. doi: 10.1080/09286580802228509. [DOI] [PubMed] [Google Scholar]
- 8.Uchino M, Nishiwaki Y, Michikawa T, et al. Prevalence and risk factors of dry eye disease in Japan: Koumi study. Ophthalmology. 2011;118(12):2361–2367. doi: 10.1016/j.ophtha.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 9.Ahn JM, Lee SH, Rim TH, et al. Prevalence and risk factors associated with dry eye: the Korea National Health and Nutrition Examination Survey 2010–2011. Am J Ophthalmol. 2014;158(6):1205–1214. doi: 10.1016/j.ajo.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Lemp MA. Advances in understanding and managing dry eye disease. Am J Ophthalmol. 2008;146(3):350–356. doi: 10.1016/j.ajo.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Yoon KC, Choi W, Lee HS, et al. An overview of Ophthalmologic Survey Methodology in the 2008–2015 Korean National Health and Nutrition Examination Surveys. Korean J Ophthalmol. 2015;29(6):359–367. doi: 10.3341/kjo.2015.29.6.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korea Centers for Disease Control and Prevention [webpage on the Internet] Korean National Health and Nutrition Examination Survey. 2013. [Accessed December 20, 2013]. Available from: http://knhanes.cdc.go.kr/
- 13.Kim JH, Song JS, Hyon JY, Chung SK, Kim TJ. A survey of contact lens-related complications in Korea: the Korean Contact Lens Study Society. J Korean Ophthalmol Soc. 2014;55(1):20–31. [Google Scholar]
- 14.Sullivan BD, Evans JE, Krenzer KL, Reza Dana M, Sullivan DA. Impact of antiandrogen treatment on the fatty acid profile of neutral lipids in human meibomian gland secretions. J Clin Endocrinol Metab. 2000;85(12):4866–4873. doi: 10.1210/jcem.85.12.7066. [DOI] [PubMed] [Google Scholar]
- 15.Gurwood AS, Gurwood I, Gubman DT, Brzezicki LJ. Idiosyncratic ocular symptoms associated with the estradiol transdermal estrogen replacement patch system. Optom Vis Sci. 1995;72(1):29–33. doi: 10.1097/00006324-199501000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Truong S, Cole N, Stapleton F, Golebiowski B. Sex hormones and the dry eye. Clin Exp Optom. 2014;97(4):324–336. doi: 10.1111/cxo.12147. [DOI] [PubMed] [Google Scholar]
- 17.Cutolo M, Wilder RL. Different roles for androgens and estrogens in the susceptibility to autoimmune rheumatic diseases. Rheum Dis Clin North Am. 2000;26(4):825–839. doi: 10.1016/s0889-857x(05)70171-x. [DOI] [PubMed] [Google Scholar]
- 18.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 19.Davison S, Bell R, Donath S, Montalto J, Davis S. Androgen levels in adult females: changes with age, menopause, and oophorectomy. J Clin Endocrinol Metab. 2005;90(7):3847–3853. doi: 10.1210/jc.2005-0212. [DOI] [PubMed] [Google Scholar]
- 20.Sharma A, Hindman HB. Aging: a predisposition to dry eyes. J Ophthalmol. 2014;2014:781683. doi: 10.1155/2014/781683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. doi: 10.1016/s0002-9394(03)00218-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–1351. doi: 10.1136/bjo.86.12.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaumberg DA, Dana R, Buring JE, Sullivan DA. Prevalence of dry eye disease among US men: estimates from the Physicians’ Health Studies. Arch Ophthalmol. 2009;127(6):763–768. doi: 10.1001/archophthalmol.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schein OD, Munoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124(6):723–728. doi: 10.1016/s0002-9394(14)71688-5. [DOI] [PubMed] [Google Scholar]
- 25.Bourcier T, Acosta MC, Borderie V, et al. Decreased corneal sensitivity in patients with dry eye. Invest Ophthalmol Vis Sci. 2005;46(7):2341–2345. doi: 10.1167/iovs.04-1426. [DOI] [PubMed] [Google Scholar]
- 26.Labbe A, Liang Q, Wang Z, et al. Corneal nerve structure and function in patients with non-Sjögren dry eye: clinical correlations. Invest Ophthalmol Vis Sci. 2013;54(8):5144–5150. doi: 10.1167/iovs.13-12370. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10(5):447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Na KS, Han K, Park YG, Na C, Joo CK. Depression, stress, quality of life, and dry eye disease in Korean women: a population-based study. Cornea. 2015;34(7):733–738. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 29.Kawashima M, Uchino M, Yokoi N, et al. Associations between subjective happiness and dry eye disease: a new perspective from the Osaka study. PLoS One. 2015;10(4):e0123299. doi: 10.1371/journal.pone.0123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799–806. doi: 10.1016/j.ajo.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schaumberg DA, Uchino M, Christen WG, Semba RD, Buring JE, Li JZ. Patient reported differences in dry eye disease between men and women: impact, management, and patient satisfaction. PLoS One. 2013;8(9):e76121. doi: 10.1371/journal.pone.0076121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a Consensus Report by the Asia Dry Eye Society. Ocul Surf. 2017;15(1):65–76. doi: 10.1016/j.jtos.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 33.Schlote T, Kadner G, Freudenthaler N. Marked reduction and distinct patterns of eye blinking in patients with moderately dry eyes during video display terminal use. Graefes Arch Clin Exp Ophthalmol. 2004;242(4):306–312. doi: 10.1007/s00417-003-0845-z. [DOI] [PubMed] [Google Scholar]