ABSTRACT
Many two-component regulatory systems, including Escherichia coli PhoRB, are positively autoregulated, so stimuli result in an increase in the concentration of signaling proteins. When the quantity of signaling proteins depends on exposure history, how do past conditions affect future responses to stimuli? Hoffer et al. (J. Bacteriol. 183:4914–4917, 2001, https://doi.org/doi:10.1128/JB.183.16.4914-4917.2001) previously reported that E. coli bacteria “learn” from phosphate starvation and respond more rapidly to subsequent episodes of starvation. Gao et al. (J. Bacteriol. 199:e00390-17, 2017, https://doi.org/doi:10.1128/JB.00390-17) describe another aspect of hysteresis in the PhoRB regulon. Phosphate starvation also leads to a global decline in transcription, counteracting the effects of positive autoregulation and resulting in a similar net pho response (homeostasis), regardless of exposure history.
KEYWORDS: homeostasis, hysteresis, PhoB, PhoR, positive autoregulation, two-component systems
COMMENTARY
A striking feature of many bacterial species is the ability to thrive under diverse environmental conditions. For the sake of efficiency, these versatile capabilities are not constitutively expressed but are instead turned on in a “just in time” manner when actually needed. Thus, bacteria continuously monitor properties of interest in their environment (e.g., nutrients, pH, the presence of other organisms, etc.) and respond to the challenges and opportunities posed by changing conditions.
Positive autoregulation of two-component systems can lead to hysteresis.
Two-component regulatory systems are used by most bacteria to sense and respond to environmental change. In the basic scheme (1), transmembrane sensor kinases monitor specific environmental conditions and record that information in the form of phosphoryl groups covalently attached to a cytoplasmic domain. The phosphoryl groups are then transferred to partner response regulator proteins, which modulate appropriate adaptive responses (most commonly, changes in gene expression) based on their phosphorylation state. Loss of phosphoryl groups from the response regulators usually terminates activation of the response, although the products of the response may persist for some time.
The manner in which the basic elements of a two-component system are functionally connected contributes to the information-processing properties of the circuit (2). For example, many two-component systems positively autoregulate expression of the genes encoding their sensor kinase and response regulator (3). Basal levels of sensor proteins monitor the environment at minimal energetic cost. When conditions warrant a response, a positive-feedback loop substantially increases the population of sensor kinase and response regulator proteins available to detect and respond to the situation, which in turn affects the magnitude and kinetics of the response. A question concerning hysteresis then arises—how do the responses of bacteria to fluctuating conditions depend on their history of exposure? (I use the term “hysteresis” in the broad sense that the responses of a system to the same input differ depending on system history, rather than the commonly used but narrower sense that approaching a condition from opposite directions yields different responses [4], such as occurs in thermal hysteresis, where the melting and freezing points of a solution are different [5].)
Can Escherichia coli “remember” and “learn” from phosphate starvation?
As a case in point, the E. coli PhoRB two-component system governs assimilation of the essential nutrient phosphorus. As inorganic phosphate (Pi) sensed via PhoR becomes depleted, PhoB activates transcription of genes in the pho regulon in a hierarchical manner, sequentially implementing increasingly desperate measures to obtain phosphorus (6). In 2001, Hoffer et al. (7) reported an interesting consequence of PhoRB autoregulation. If cells initially starved of Pi are exposed to excess Pi and subsequently starved of Pi again, they respond to the second starvation event more rapidly than cells that had not previously experienced starvation. The increased levels of PhoRB generated by positive autoregulation during the initial starvation event allow bacteria to “learn” from their experience and respond more quickly when starved for a second time. However, the “memory” of starvation fades as the duration of the intervening period of Pi abundance increases. Rong Gao, Ann Stock, and colleagues have revisited the phenomenon of Pi starvation and hysteresis in more detail, with intriguing results (8). The present work is the latest in a series of studies by Gao and Stock (6, 9–11) that combine clever analyses of experimental data with computer modeling to explore the in vivo performance of PhoRB.
Starting with a yellow fluorescent protein reporter expressed from the alkaline phosphatase promoter, Gao et al. (8) used the time derivative of fluorescence to measure PhoRB-regulated promoter activity. The authors first provided a simple explanation for the results of Hoffer et al. (7) by demonstrating that the amounts of PhoRB in the cell could account for creation and loss of “memories” of Pi starvation as expected. (i) Changing the basal level of PhoRB via an inducible promoter altered the kinetics of response to Pi starvation (more PhoRB resulted in a faster response). (ii) When PhoRB was not being synthesized, the concentration of existing PhoB declined at the rate expected for dilution through cell growth and division.
The stress of phosphate starvation apparently leads to response homeostasis.
A thorough investigation of the cellular response to cycles of Pi starvation and abundance revealed surprising results. Although cells grown in abundant Pi for shorter times between Pi starvation events responded more rapidly to the second starvation (as expected from conditions that included a lower dilution of PhoRB), promoter activity declined after the initial response. The decline was greater for cells that experienced shorter recovery times between starvation events. Counterbalancing effects of positive autoregulation of PhoRB and negative regulation of promoter activity resulted in similar levels of reporter expression (reflecting the net response to a given level of Pi starvation) regardless of history (Fig. 1). This remarkable, but not perfect, homeostasis of Pho system output strongly suggests that the observed response was nearly optimal. The convergence of Pho responses from different starting points is particularly striking because Gao and Stock previously also demonstrated that the autoregulated amounts of PhoRB signaling proteins generated under conditions of various degrees of Pi starvation provide optimal fitness (9).
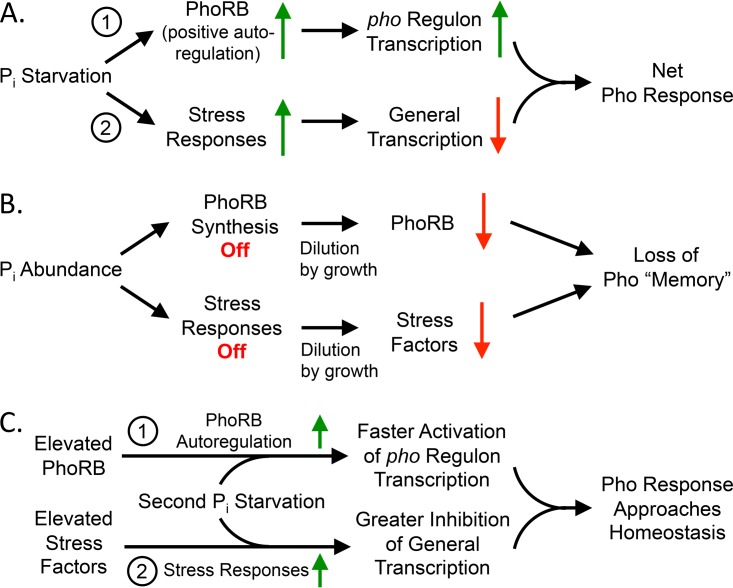
FIG 1.
Model of hysteresis and homeostasis in the E. coli PhoRB system. (A) Pi starvation activates PhoRB, resulting in more PhoRB and increased transcription of the pho regulon (path 1). Pi starvation also causes stress, which diminishes global transcription (path 2). The sum of these two effects determines the net Pho response. (B) When Pi is abundant, additional PhoRB and stress factors are not synthesized. The concentrations of PhoRB and stress factors remaining from previous episodes of Pi starvation decrease exponentially due to cell division, erasing the “memories” of past Pi starvation events. (C) History affects the kinetics of pho output (hysteresis). Cells that previously experienced Pi starvation consequently contain increased amounts of PhoRB. If subsequent growth in Pi abundance is too brief to eliminate the excess PhoRB, then such cells initially respond more rapidly to a second Pi starvation event (path 1), because they are starting from a higher baseline of PhoRB than naive cells. Similarly, previously starved cells also have accumulated stress factors and so more extensively diminish general transcription (path 2) when starved again. Together, these two effects result in similar final pho outputs (homeostasis) regardless of history.
Additional experiments provided important clues to the mechanism of reduced promoter activity. The history-dependent decline in promoter activity was not a consequence of PhoRB autoregulation. All tested promoters were affected similarly, including a promoter not regulated by PhoB. Because Pi starvation is known to induce stress responses (12, 13), a plausible explanation for the observed decline in promoter activity is simple competition for RNA polymerase between the housekeeping σ70 and the stationary-phase σS proteins. In this scheme, the negative “memory” of stress caused by Pi starvation dilutes during growth in excess Pi in a similar manner to the positive “memory” embodied in PhoRB. A mutant lacking σS did not support homeostasis, and a computer simulation incorporating the sigma factor competition hypothesis faithfully reproduced all features of the experimental data. Nevertheless, stress responses are complex and the mechanism(s) actually responsible for negative regulation of promoter activity remains to be determined.
What's next?
In addition to its use in deciphering the mechanism(s) of promoter inactivation, the PhoRB system appears to be rich with opportunities for future investigation. The existing computer model may provide a head start. In silico studies allow rapid and convenient predictions regarding system behavior(s) under many permutations of conditions such as the duration and magnitude of Pi starvation. Investigators can then choose to experimentally test the most informative predictions. (i) For example, what happens when more than two episodes of Pi starvation occur? Is the history completely erased during intervening periods of Pi abundance or does it continue to accumulate? (ii) The present work focused on activity of the phoA promoter. Does the near-homeostasis of the Pho response apply to all levels of the pho regulon hierarchy? (iii) What happens under conditions of intermediate rather than extreme levels of Pi limitation? In the absence of full starvation for Pi, stress responses might not be sufficient to cause the negative regulation of global transcription that led the Pho response to approach homeostasis. (iv) Positive autoregulation can lead to bistable circuit conditions and mixed populations of “on” and “off” cells, if conditions are such that some cells cross the threshold for autoamplification whereas others do not. The pho response exhibits just such heterogeneity in the artificial circumstance represented by cells that lack PhoR and activate PhoB by inefficient cross talk from a nonpartner sensor kinase (14). On the other hand, when E. coli bacteria with the wild-type PhoRB system are starved once for Pi, the population response is homogeneous and bistability is not seen (9). What happens with a more complex exposure history? The experiments reported by Gao et al. (8) reasonably examined populations rather than single cells. Could circumstances in which cycles of starvation and abundance lead to bistability have been overlooked, particularly if the positive and negative regulatory effects were not precisely balanced?
What is bacterial memory?
There is a fascinating literature on phenomena that may be interpreted as bacterial memory, learning, or anticipation (15–19). However, it is much easier to design experiments to determine the mechanism of a system than to deduce its purpose, and data are subject to multiple interpretations. For example, positive autoregulation has been alternately regarded as a mechanism for memory and learning (7) or as a means to smooth out responses to a fluctuating environment (20). Nevertheless, there are unambiguous examples of bacterial memory. The methylation status of chemoreceptors provides short-term memory of chemoeffector exposure that extends several seconds into the past (21, 22). By comparing current conditions to the immediate past, bacteria decide whether to continue on the same path or change direction, thus accomplishing chemotaxis. CRISPR (clustered regularly interspaced short palindromic repeat) provides a long-term memory of exposure to foreign genetic elements, written into the genetic material of the cell to ensure transmission to future generations (23). Regardless of whether the phenomena exhibited by PhoRB are widely accepted to constitute “memory,” the work of Gao et al. suggests that E. coli has learned the value of an optimal response.
ACKNOWLEDGMENTS
I thank Clay Foster, Emily Kennedy, and Ruth Silversmith for helpful comments on the manuscript.
Research in my laboratory is supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM050860.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
For the article discussed, see https://doi.org/10.1128/JB.00390-17.
REFERENCES
- 1.Zschiedrich CP, Keidel V, Szurmant H. 2016. Molecular mechanisms of two-component signal transduction. J Mol Biol 428:3752–3775. doi: 10.1016/j.jmb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goulian M. 2010. Two-component signaling circuit structure and properties. Curr Opin Microbiol 13:184–189. doi: 10.1016/j.mib.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Groisman EA. 2016. Feedback control of two-component regulatory systems. Annu Rev Microbiol 70:103–124. doi: 10.1146/annurev-micro-102215-095331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitrophanov AY, Groisman EA. 2008. Positive feedback in cellular control systems. Bioessays 30:542–555. doi: 10.1002/bies.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bar Dolev M, Braslavsky I, Davies PL. 2016. Ice-binding proteins and their function. Annu Rev Biochem 85:515–542. doi: 10.1146/annurev-biochem-060815-014546. [DOI] [PubMed] [Google Scholar]
- 6.Gao R, Stock AM. 2015. Temporal hierarchy of gene expression mediated by transcription factor binding affinity and activation dynamics. mBio 6:e00686-15. doi: 10.1128/mBio.00686-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffer SM, Westerhoff HV, Hellingwerf KJ, Postma PW, Tommassen J. 2001. Autoamplification of a two-component regulatory system results in “learning” behavior. J Bacteriol 183:4914–4917. doi: 10.1128/JB.183.16.4914-4917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao R, Godfrey KA, Sufian MA, Stock AM. 2017. Counterbalancing regulation in response memory of a positively autoregulated two-component system. J Bacteriol 199:e00390-17. doi: 10.1128/JB.00390-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao R, Stock AM. 2013. Evolutionary tuning of protein expression levels of a positively autoregulated two-component system. PLoS Genet 9:e1003927. doi: 10.1371/journal.pgen.1003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao R, Stock AM. 2013. Probing kinase and phosphatase activities of two-component systems in vivo with concentration-dependent phosphorylation profiling. Proc Natl Acad Sci U S A 110:672–677. doi: 10.1073/pnas.1214587110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao R, Stock AM. 2017. Quantitative kinetic analyses of shutting off a two-component system. mBio 8:e00412-17. doi: 10.1128/mBio.00412-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentry DR, Hernandez VJ, Nguyen LH, Jensen DB, Cashel M. 1993. Synthesis of the stationary-phase sigma factor sigma s is positively regulated by ppGpp. J Bacteriol 175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz N, Silhavy TJ. 2003. Constitutive activation of the Escherichia coli Pho regulon upregulates rpoS translation in an Hfq-dependent fashion. J Bacteriol 185:5984–5992. doi: 10.1128/JB.185.20.5984-5992.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Gregori G, Blackman JM, Robinson JP, Wanner BL. 2005. Stochastic activation of the response regulator PhoB by noncognate histidine kinases. J Integr Bioinform 2:11. doi: 10.2390/biecoll-jib-2005-11. [DOI] [Google Scholar]
- 15.Casadesús J, D'Ari R. 2002. Memory in bacteria and phage. Bioessays 24:512–518. doi: 10.1002/bies.10102. [DOI] [PubMed] [Google Scholar]
- 16.Lambert G, Kussell E. 2014. Memory and fitness optimization of bacteria under fluctuating environments. PLoS Genet 10:e1004556. doi: 10.1371/journal.pgen.1004556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyon P. 2015. The cognitive cell: bacterial behavior reconsidered. Front Microbiol 6:264. doi: 10.3389/fmicb.2015.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathis R, Ackermann M. 2016. Response of single bacterial cells to stress gives rise to complex history dependence at the population level. Proc Natl Acad Sci U S A 113:4224–4229. doi: 10.1073/pnas.1511509113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf DM, Fontaine-Bodin L, Bischofs I, Price G, Keasling J, Arkin AP. 2008. Memory in microbes: quantifying history-dependent behavior in a bacterium. PLoS One 3:e1700. doi: 10.1371/journal.pone.0001700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray JC, Tabor JJ, Igoshin OA. 2011. Non-transcriptional regulatory processes shape transcriptional network dynamics. Nat Rev Microbiol 9:817–828. doi: 10.1038/nrmicro2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macnab RM, Koshland DE Jr. 1972. The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A 69:2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block SM, Segall JE, Berg HC. 1982. Impulse responses in bacterial chemotaxis. Cell 31:215–226. doi: 10.1016/0092-8674(82)90421-4. [DOI] [PubMed] [Google Scholar]
- 23.Jackson SA, McKenzie RE, Fagerlund RD, Kieper SN, Fineran PC, Brouns SJ. 2017. CRISPR-Cas: adapting to change. Science 356:eaal5056. doi: 10.1126/science.aal5056. [DOI] [PubMed] [Google Scholar]