ABSTRACT
Many aspects of bacterial physiology and behavior, including motility, surface attachment, and the cell cycle, are controlled by cyclic di-GMP (c-di-GMP)-dependent signaling pathways on the scale of seconds to minutes. Interrogation of such processes in real time requires tools for introducing rapid and reversible changes in intracellular c-di-GMP levels. Inducing the expression of genes encoding c-di-GMP-synthetic (diguanylate cyclases) and -degrading (c-di-GMP phosphodiesterase) enzymes by chemicals may not provide adequate temporal control. In contrast, light-controlled diguanylate cyclases and phosphodiesterases can be quickly activated and inactivated. A red/near-infrared-light-regulated diguanylate cyclase, BphS, was engineered previously, yet a complementary light-activated c-di-GMP phosphodiesterase has been lacking. In search of such a phosphodiesterase, we investigated two homologous proteins from Allochromatium vinosum and Magnetococcus marinus, designated BldP, which contain C-terminal EAL-BLUF modules, where EAL is a c-di-GMP phosphodiesterase domain and BLUF is a blue light sensory domain. Characterization of the BldP proteins in Escherichia coli and in vitro showed that they possess light-activated c-di-GMP phosphodiesterase activities. Interestingly, light activation in both enzymes was dependent on oxygen levels. The truncated EAL-BLUF fragment from A. vinosum BldP lacked phosphodiesterase activity, whereas a similar fragment from M. marinus BldP, designated EB1, possessed such activity that was highly (>30-fold) upregulated by light. Following light withdrawal, EB1 reverted to the inactive ground state with a half-life of ∼6 min. Therefore, the blue-light-activated phosphodiesterase EB1 can be used in combination with the red/near-infrared-light-regulated diguanylate cyclase BphS for the bidirectional regulation of c-di-GMP-dependent processes in E. coli as well as other bacterial and nonbacterial cells.
IMPORTANCE Regulation of motility, attachment to surfaces, the cell cycle, and other bacterial processes controlled by the c-di-GMP signaling pathways occur at a fast (seconds-to-minutes) pace. Interrogation of these processes at high temporal and spatial resolution using chemicals is difficult or impossible, while optogenetic approaches may prove useful. We identified and characterized a robust, blue-light-activated c-di-GMP phosphodiesterase (hydrolase) that complements a previously engineered red/near-infrared-light-regulated diguanylate cyclase (c-di-GMP synthase). These two enzymes form a dichromatic module for manipulating intracellular c-di-GMP levels in bacterial and nonbacterial cells.
KEYWORDS: biofilms, c-di-GMP, diguanylate cyclase, gene expression, motility, optogenetics, phosphodiesterase, photoreceptors, photosensory reception, signal transduction
INTRODUCTION
Signaling via second messengers occurs at various time scales, from seconds to hours. To control relatively slow processes, intracellular concentrations of a second messenger can be modulated by the constitutive or inducible (over)expression of enzymes involved in its synthesis or degradation. However, such systems are inadequate for the interrogation of signaling events that occur at a faster, seconds-to-minutes time scale because of the delay involved in the activation of gene expression and the irreversibility of the process. Controlling the activities of the synthesizing and degrading enzymes via chemical inducers represents a faster option; however, the removal of inducers without major system perturbation is difficult. In addition, it is virtually impossible to achieve spatial precision, i.e., to regulate a specific subset of a cell population, by the use of diffusible chemicals.
Optogenetic (synthetic photobiology) approaches that involve light-activated enzymes for modulating the rate of synthesis or the degradation of second messengers are devoid of such deficiencies. Many naturally occurring light-activated enzymes are spontaneously inactivated after the removal of irradiation, which is attractive for interrogating processes at high temporal resolution. A light-activated enzyme comprises a chromophore-binding photoreceptor module and an enzymatic output module involved in the synthesis or degradation of a second messenger. The absorption of a photon results in conformational changes in the chromophore molecule, which in turn induce conformational changes in the surrounding chromophore-binding pocket that are subsequently transduced to the output module, ultimately increasing or decreasing its enzymatic activity. Several light-activated enzymes that control the synthesis and hydrolysis of cyclic mono- and dinucleotide second messengers (1, 2), including cyclic AMP (cAMP) (3–8), cyclic GMP (cGMP) (4, 7, 8), and cyclic di-GMP (c-di-GMP) (9–15), have been identified in microbial cells or engineered based on natural prototypes. These and similar photoactivated enzymes can complement optogenetic tools designed for regulating gene expression in bacteria that are currently more advanced (16, 17).
Recently, we described a potent red/near-infrared-light-regulated diguanylate cyclase (DGC), designated BphS (13), by using the photoreceptor module from the Rhodobacter sphaeroides bacteriophytochrome BphG1 (9) and the modified GGDEF domain from Synechocystis sp. strain Slr1143 (18). The product of DGCs, c-di-GMP, is a ubiquitous second messenger involved in regulating various aspects of bacterial physiology and behavior, from motility and biofilms to the cell cycle, differentiation, the production of secondary metabolites, and virulence (19, 20). In most bacteria where c-di-GMP-dependent signaling pathways are present, they are involved in regulating bacterial transitions from planktonic to sessile lifestyles via the inhibition of motility and the formation of adhesive surface proteins or appendages (e.g., pili). In Escherichia coli K-12, increased c-di-GMP levels inhibit swimming in semisolid (soft) agar by the “backstop break” mechanism involving the binding of the YcgR–c-di-GMP complex to the flagellum motor (21–24). High levels of c-di-GMP also induce the synthesis of curli fimbriae in E. coli B that can be readily detected by staining with Congo red dye (25, 26). Because of the ease of visual assessment, in this study, we used these phenotypes for evaluating changes in intracellular c-di-GMP levels.
Most c-di-GMP-dependent phenomena thus far have been studied at a slow time scale by using gene mutants and the constitutive or inducible (over)expression of DGCs or c-di-GMP-specific phosphodiesterases (PDEs). DGCs are associated with the GGDEF protein domains, and PDEs are associated with the EAL or HD-GYP domains (19). However, it is becoming increasingly clear that fast changes in intracellular c-di-GMP levels are no less important. For example, drastic changes in c-di-GMP levels that occur during the cell cycle have been documented for many proteobacteria, including E. coli, Pseudomonas aeruginosa, and Caulobacter crescentus (27–29). Fast (<30-s) changes in intracellular c-di-GMP levels, and swimming behavior, in response to oxygen have also been documented for Azospirillum brasilense (30). However, the means for interrogating such processes have been lacking.
The previously characterized DGC BphS can be part of the optogenetic system for the fast-pace manipulation of c-di-GMP levels; however, the complementary light-activated PDE was missing. Previously, we described a c-di-GMP PDE, BlrP1 from Klebsiella pneumoniae (10, 31), which could potentially complement BphS because it is activated by blue light. BlrP1 has the BLUF-EAL protein domain architecture, where BLUF is a sensor of blue light using flavin chromophores (32, 33). However, the photodynamic range of BlrP1, i.e., the ratio of the enzymatic activity in the light to that in the dark, is modest (3- to 4-fold in vitro) (10), which limits its utility as a tool for light-controlled c-di-GMP degradation in vivo. A low photodynamic range (∼2-fold in vitro) has also been reported for a blue-light-dependent c-di-GMP-specific PDE, SL2 from Synechococcus elongatus, which senses light via a LOV domain (11). Yet another PDE, SseB from Thermosynechococcus vulcanus, inducible by teal light, was recently described; however, its kinetic parameters and photodynamic range were not reported. The applicability of SseB is further complicated by the need to supply a cyanobacterial chromophore, phycoviolobilin, or phycoviolobilin biosynthesis genes (15).
We therefore searched for a light-activated PDE with a higher photodynamic range than that of BlrP1 and spectral parameters compatible with BphS. Here we describe one such protein, or, more precisely, a fragment of a protein, that we designated EB1. It possesses robust c-di-GMP PDE activity, is spectrally compatible with BphS, and, importantly, has the highest dynamic range among light-activated c-di-GMP PDEs described to date. BphS and EB1 form an optogenetic (synthetic photobiology) module suitable for interrogating c-di-GMP signaling processes in bacterial cells. It can also be adapted for controlling the behavior of bacterial and nonbacterial cells in biotechnological and biomedical applications.
RESULTS AND DISCUSSION
Light dependence of c-di-GMP-specific PDE activity in the multidomain EAL-BLUF domain proteins.
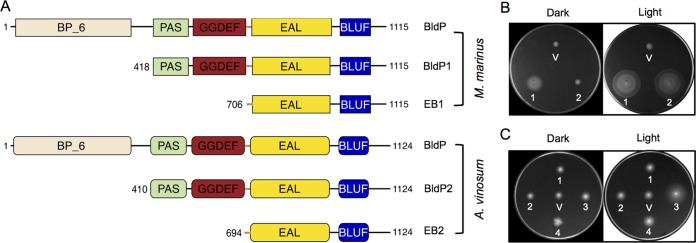
In the search for a c-di-GMP-specific PDE with light-dependent activity compatible with BphS1, we screened the Pfam database (34) for proteins containing BLUF and EAL domains. Several BLUF-EAL proteins were characterized previously. Some of them (e.g., YcgF [BluF] from E. coli) lack PDE activity, while others (e.g., BprP1 from K. pneumoniae) have only a modest photodynamic range (10, 31). Therefore, we did not explore the BLUF-EAL proteins further. Instead, we focused on proteins with an unusual EAL-BLUF domain arrangement at their C termini (Fig. 1A). The possibility that BLUF domains located downstream of the EAL domains could regulate the PDE activity of the EAL domains in a light-dependent manner was intriguing because no such proteins have been described to date. We picked two EAL-BLUF proteins, one from Magnetococcus marinus (Mmc1_2641) and another from Allochromatium vinosum (Alvin_1044), and designated them BldP (blue-light-activated diguanylate phosphodiesterase). These proteins contain periplasmic BP_6 domains separated from the PAS9-GGDEF-EAL-BLUF C termini by a transmembrane domain. To assay for PDE activities of the BldP proteins, we cloned their cytoplasmic PAS9-GGDEF-EAL-BLUF fragments as C-terminal translational fusions to maltose-binding protein (MBP), a tag known for improving protein solubility (35).
FIG 1.
Blue-light-regulated c-di-GMP PDE activity. (A) Domain architecture of the EAL-BLUF proteins and their derivatives used in this study. (B) Increase in the swim zone of the E. coli MG1655 yhjH strain expressing MBP-BldP1 and MBP-EB1 by light in semisolid agar supplemented with 0.1 mM IPTG. V, empty vector expressing MBP (pMal-c5x); 1, MBP-BldP1; 2, MBP-EB1. (C) Effect of the α-helical linker upstream of the EAL domains on light-dependent PDE activity. The EB1 and EB2 protein fragments were expressed from the pBAD vector in the MG1655 yhjH strain and spotted onto semisolid agar supplemented with 0.02% arabinose. V, empty vector (pBAD); 1, EB1 lacking the linker; 2, EB1 with the linker carrying the E712P mutation; 3, EB1 with the linker; 4, EB2 with the linker.
The truncated BldP proteins were expressed in an E. coli reporter strain, MG1655 yhjH (21), which lacks the major c-di-GMP PDE YhjH (recently renamed PdeH [36]) and is impaired in swimming in semisolid agar because of 10-fold increased intracellular c-di-GMP levels (22). The expression in this reporter strain of heterologous PDEs from diverse bacteria can partially rescue swimming in semisolid agar (37–39). The MBP fusions to both BldP1 from M. marinus (Fig. 1B, spot 1) and BldP2 from A. vinosum (Fig. 2A, air) did so. Whereas the PDE activity of MBP-BldP2 appeared to be poorly light dependent, the PDE activity of MBP-BldP1 was strongly activated by blue light, as judged by the significantly larger swim zone in the light than that in the dark (Fig. 2A, air).
FIG 2.

(A) Characterization of the light- and oxygen-dependent activities of the M. marinus BldP1 and A. vinosum BldP2 proteins. (A) The effects of oxygen and blue light on the motility of the MG1655 yhjH strain expressing the BldP1 and BldP2 proteins were assayed in semisolid agar at various oxygen levels in the absence or presence of blue light. Plates were incubated at 30°C for 12 h in air (21% O2) under micro-oxic (6 to 16% O2) or anoxic (0% O2) conditions. Dark, no light; Light, blue light irradiation (5 s of light and 60 s of dark); V, pMAL-c5x (empty vector); BldP1, pMal_BldP1; BldP2, MAL-BldP2. (B) Absorbance spectra of the purified MBP-PAS9-GGDEF protein fragment from A. vinosum BldP. The black trace shows the spectrum of the protein purified from E. coli, and the gray trace shows the spectrum after reconstitution with excess hemin in vitro. The inset shows an enlarged part of the spectrum emphasizing the Soret band (∼420 nm) indicative of the trace amounts of heme found in the “as-purified” protein.
Encouraged by these results, we made further truncations of BldP1 and BldP2. The EAL-BLUF fragments, including the predicted α-helical linkers (predicted by JPred [40]) located upstream of the EAL domains, were fused to MBP, giving rise to MBP-EB1 and MBP-EB2 (Fig. 1A). MBP-EB1 did not rescue the swimming of the MG1655 yhjH strain in the dark but did so in the light, showing a swim zone approximately as large as that of the strain containing a longer protein fragment, MBP-BldP1 (Fig. 1B, spots 1 and 2). This suggests that the EB1-BLUF fragment contains all elements for light-activated PDE activity. Furthermore, the smaller swim zone in the dark suggests that MBP-EB1 possesses lower PDE activity in the dark than does MBP-BldP1 and implies that the EB1-BLUF fragment has a higher photodynamic range than that of the longer PAS9-GGDEF-EAL-BLUF fragment. In contrast to MBP-EB1, MBP-EB2 from A. vinosum displayed no PDE activity (not shown).
To ascertain the role of the predicted 10-amino-acid (aa) α-helical linker upstream of the EAL domain for light-dependent activation, we constructed EB1 variants with and without the linker expressed as stand-alone proteins, without MBP (Table 1). We found that the construct containing the linker retained light-activated PDE activity (Fig. 1C, spot 3), while the construct lacking the linker lost activity (Fig. 1C, spot 1). To determine whether the predicted α-helical structure in the linker was important, we introduced an E712P mutation in the middle of the predicted linker, expecting proline to break the α-helix. The E712P mutation abolished, or greatly diminished, the PDE activity of EB1 (Fig. 1C, spot 2), thereby emphasizing the importance of the α-helical linker for PDE activity. These observations suggest that the EB1 fragment, either alone or as an MBP fusion, presents a good PDE candidate for the dichromatic optogenetic module.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli strains | ||
| MG1655 yhjH | MG1655 yhjH::Kmr | 24 |
| BL21(DE3) | Strain for protein overproduction and curli fimbria synthesis; chromosomally integrated T7 polymerase gene | NEB |
| DH5α | Strain for cloning and MBP fusion overexpression | NEB |
| Plasmids | ||
| pMal-c5x | Vector for overexpression of MBP fusion proteins | NEB |
| pMal_BldP1 | MBP::(PAS9-GGDEF-EAL-BLUF) from BldP1 | This study |
| pMal_EB1 | MBP::(EAL-BLUF) from BldP1 (plus α-helix; aa 706–1115) | This study |
| pBAD/Myc-HisB | pBAD series empty vector; Para | Invitrogen |
| pBAD-EB1 | pBAD::(EAL-BLUF) from BldP1 (plus α-helix; aa 706–1115) | This study |
| pBAD_EB1-L | pBAD::(EAL-BLUF) from BldP1 (minus α-helix; aa 716–1115) | This study |
| pBAD_EB1_E712P | pBAD::(EAL-BLUF) from BldP1 (plus α-helix; E712P mutation; aa 706–1115) | This study |
| pMal_BldP2 | MBP::(PAS9-GGDEF-EAL-BLUF) from BldP2 | This study |
| pMal_PAS-GGDEF | MBP::(PAS9-GGDEF) from BldP2 | This study |
| pMal_EB2 | MBP::(EAL-BLUF) from BldP2 (aa 694–1124) | This study |
| pBAD_EB2 | pBAD encoding the EAL-BLUF module (plus α-helix; aa 694–1124) of BldP2 | This study |
| pMslr1143 | MBP::Slr1143 | 17 |
| pET23a | Protein overexpression vector; PT7; Apr | Novagen |
| pbSHE | pET23a::(bphS-bphO-eb1) | This study |
| pbSHY2 | pET23a::(bphS-bphO-yhjH; RBS2) | 13 |
| pbSHY3 | pET23a::(bphS-bphO-yhjH; RBS3) | 13 |
| pbSHY4 | pET23a::(bphS-bphO-yhjH; RBS4) | 13 |
The enzymatic activity of the BldP proteins is oxygen and light dependent.
We were intrigued by the disparate behaviors of two homologous BldP proteins and decided to investigate the A. vinosum BldP2 protein further. Since the EAL-BLUF fragment of BldP2 (including the α-helical linker upstream of EAL) showed very low or no PDE activity (Fig. 1C, spot 4), we concluded that the upstream PAS9-GGDEF fragment is required for PDE activity. Interestingly, the PAS domain in the BldP proteins belongs to the PAS9 subtype (according to Pfam), whose representatives are known for their ability to noncovalently bind heme and to function as oxygen sensors (41). Coincidentally, we recently characterized the RmdA protein from Streptomyces coelicolor with a domain architecture similar to that of the BldP proteins (PAS9-GGDEF-EAL) and found that its PAS9 domain indeed binds heme (37). The presence of a potential oxygen sensory domain prompted us to investigate whether the PDE activity of BldP2 may be more strongly activated by light at lower oxygen levels than that in the air.
When the swimming of the MG1655 yhjH strain carrying BldP2 was tested under micro-oxic (6 to ∼16% oxygen) or anoxic (0% oxygen) conditions, a large light-to-dark difference between swim zones was observed, compared to the difference observed in the strain grown in air (Fig. 2A). It appears that the PDE activity of BldP2 is more strongly induced by light at lower oxygen levels primarily because it is inhibited in the dark at lower oxygen levels. Interestingly, the PDE activity of BldP1 in the dark was increased at lower oxygen levels, which is opposite from BldP2 (Fig. 2A).
To explore the potential mechanism allowing these enzymes to respond to changing oxygen levels, we turned to the PAS9 domain, which was predicted to bind heme. We overexpressed pMal_PAS-GGDEF, purified the MBP-PAS9-GGDEF protein fragment from BldP2 via amylose resin affinity chromatography, and analyzed it by UV-visible (UV-vis) absorbance spectroscopy. The protein, as purified, had a small absorbance maximum at 420 nm, reminiscent of the Soret band of heme (Fig. 2B, black trace and inset). To verify that this was not due to contamination by cellular components, we tested the ability of the MBP-PAS9-GGDEF protein to incorporate more hemin in vitro by adding high levels of hemin to the protein sample. Indeed, we found that MBP-PAS9-GGDEF was capable of binding hemin until a near-equimolar protein-to-hemin stoichiometry was reached (Fig. 2B, gray trace). This result suggests that BldP2 is a bona fide heme-binding protein, which likely explains its oxygen-sensing property. Next, we investigated whether the MBP-PAS9-GGDEF fragment possessed oxygen-dependent DGC activity. We expressed this fragment in a highly motile strain, MG1655, and analyzed its ability to inhibit swimming in semisolid agar but observed no decrease, regardless of oxygen levels (data not shown), suggesting that the PAS9-GGDEF fragment in itself is insufficient for DGC activity. It would be insightful to decipher how oxygen and light affect the enzymatic activities of the Bldp proteins; however, such studies are challenging, and even the best-understood dual (oxygen and light)-sensing proteins remain only partially characterized (42–46).
A. vinosum is a motile, anoxygenic purple sulfur bacterium that lives in sulfide-rich water and sediments (47). M. marinus is a motile, obligately microaerophilic, magnetotactic bacterium that also relies on sulfide or thiosulfate oxidation (48). For both bacteria, it is important to find the proper micro-oxic or anoxic environment. It is possible that the BldP proteins control c-di-GMP-dependent motility and/or biofilm formation in these bacteria, which facilitates their traveling to and remaining in the optimal ecological niche. We speculate that differences in oxygen sensitivities and/or metabolic capabilities in these species (e.g., A. vinosum, but not M. marinus, can grow photosynthetically under anaerobic conditions) may have contributed to the differences in BldP enzyme regulation. However, exploring the physiological roles of the BldP proteins is beyond the scope of this study.
Characterization of the EB1 fragment in vitro.
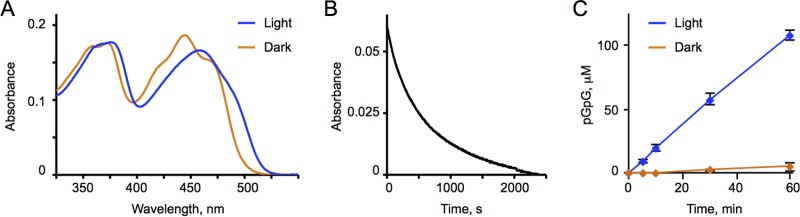
Returning to the search for the companion light-activated PDE, we can conclude that the EB1 construct with the upstream helical linker has proven to be the most promising candidate. To verify this conclusion, we proceeded with its characterization in vitro. We purified the MBP-EB1 fusion protein via amylose resin affinity chromatography and recorded its UV-vis absorption spectra. The MBP-EB1 spectrum had the typical flavin absorbance maxima observed previously in the BLUF domain proteins (31). Irradiation with blue light resulted in a spectral red shift (by ∼14 nm) (Fig. 3A), which is also characteristic of the lit states of BLUF domain photoreceptors (31). Following light withdrawal, the lit-state protein was converted to the dark state with a half-life of approximately 6 min (366 ± 6 s) at room temperature (Fig. 3B). This half-life is somewhat longer than the half-lives of the majority of the BLUF domain proteins tested thus far, which is usually on the scale of seconds to tens of seconds (31). However, previous studies revealed that the half-lives of truncated BLUF proteins are often longer than those of the full-length proteins (31). Interestingly, this seemingly long half-life observed in vitro proved inconsequential in in vivo studies (49).
FIG 3.
Spectroscopic and biochemical characterization of the MBP-EB1 protein. (A) Absorbance spectra of the dark and lit protein states. (B) Kinetics of dark recovery of EB1 from lit to dark states. Plotted are changes in the A494 over time following excitation with blue light. (C) Kinetics of c-di-GMP hydrolysis by EB1 in lit and dark states. The PDE activity assay was performed by using 5 μM MBP-EB1 and initiated by the addition of c-di-GMP.
In the dark (at room temperature and pH 8.0), the MBP-EB1 protein had very low background PDE activity. Irradiation with blue light increased the activity by ∼34-fold (Fig. 3C), the highest photodynamic range observed among light-activated c-di-GMP PDEs characterized thus far. This makes EB1 an excellent candidate to partner with BphS in a dichromatic optogenetic system for c-di-GMP regulation.
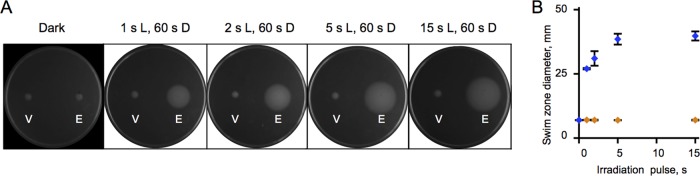
Because blue light is known to be toxic (50), decreasing cellular irradiation to a minimum is important. To determine the minimal irradiation required for activating EB1 in vivo, we tested the effects of light pulses of various durations in a swimming rescue assay (Fig. 4A). As expected, constant blue light (∼0.1 mW cm−2) strongly inhibited E. coli growth, whereas intermittent light (pulses of 15 s of light followed by 60 s of darkness) had no observable toxicity and resulted in the largest swim zones for the MG1655 yhjH strain expressing MBP-EB1 (Fig. 4B). However, light pulses as short as 1 s followed by 60 s of darkness were sufficient for inducing PDE activity detected in the swim assay (Fig. 4B). Therefore, the high photodynamic range and high absolute PDE activity of MBP-EB1 in the lit state make it suitable for applications in vivo without causing cell toxicity.
FIG 4.
Effects of various irradiation regimens on light-regulated PDE activity in E. coli. (A) Light-dependent changes in swim zones of the MG1655 yhjH strain carrying MBP-EB1. V, pMal-c5x (empty vector); E, MBP-EB1; L, light; D, dark. (B) Diameters of the swim zones (from panel A) plotted as a function of the duration of light pulses. Blue, MG1655 yhjH(pMal_EB1) strain expressing MBP-EB1; orange, MG1655 yhjH(pMAL-c5x) strain expressing MBP.
We also purified MBP-EB2 and found that its absorption spectrum and photochromic response were very similar to those of EB1; however, no PDE activity was detected in the light or dark (data not shown). These results are consistent with our conclusion derived from the E. coli motility assays that the EB2 fragment is insufficient for PDE activity.
Dichromatic optogenetic module for regulating intracellular c-di-GMP levels.
To test the possibility of using BphS and EB1 for manipulating c-di-GMP-dependent phenotypes, we expressed both proteins in an E. coli B strain. To produce billiverdin IXα, a chromophore for BphS, we also expressed the R. sphaeroides heme oxygenase gene bphO (13). In plasmid pbSHE, the engineered bphS-bphO-eb1 operon is expressed from a strong and inducible T7 promoter (Fig. 5A).
FIG 5.
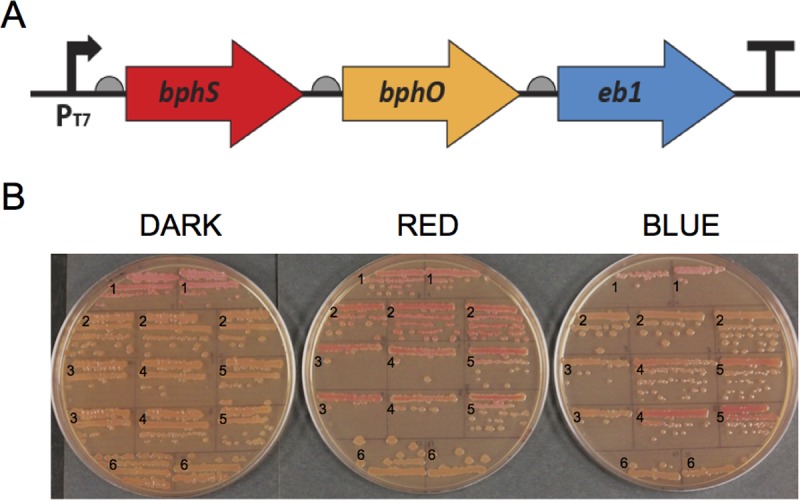
Dichromatic optogenetic module for regulating intracellular c-di-GMP levels comprising a far-red/near-infrared-light-regulated DGC, BphS, and a blue-light-activated PDE, EB1. (A) Structure of the synthetic bphS-bphO-eb1 operon. A semicircle in front of each gene indicates a RBS; the T sign at the end of the operon indicates a transcription terminator. (B) c-di-GMP-dependent curli fimbria synthesis in E. coli BL21(DE3). Fresh colonies of BL21(DE3) transformants carrying the indicated plasmids were grown on Congo red plates for 4 days at 30°C. 1, pMslr1143 (constitutive DGC; positive control); 2, pbSHE (bphS-bphO-eb1); 3, pbSHY2 (bphS-bphO-yhjH; RBS2); 4, pbSHY3 (bphS-bphO-yhjH; RBS3); 5, pbSHY4 (bphS-bphO-yhjH; RBS4); 6, pET23a (empty vector) (negative control). Dark indicates growth in the dark (plate covered in aluminum foil), red indicates colonies irradiated with pulsed (30 s of light and 120 s of dark) red (650-nm) light, and blue indicates colonies irradiated with pulsed (10 s of light and 60 s of dark) blue (465-nm) light.
The DGC and PDE activities of BphS and EB1 were assayed by using c-di-GMP-dependent curly fimbria formation (25, 26). Colonies of BL21(DE3) cells expressing the bphS-bphO-eb1 operon in the dark or with exposure to blue light were nonpigmented, which is indicative of low intracellular c-di-GMP levels (Fig. 5B, no. 2). Colonies grown in red light turned red due to the binding of Congo red to curly fimbriae, which is indicative of elevated c-di-GMP levels (Fig. 5B, no. 2). These results show that the background PDE activity of EB1 (in the dark or red light) does not interfere with the red-light-induced increase in c-di-GMP levels. Similarly, the background DGC activity of BphS (in the dark or blue light) was not observed in the presence of EB1.
We compared performance of pbSHE with those of plasmids expressing the BphS-BphO module coupled with constitutive levels of the PDE YhjH/PdeH (13). Several versions of the bphS-bphO-yhjH operon were constructed, where YhjH/PdeH levels were adjusted via different strengths of the ribosome-binding sites (RBS) upstream of yhjH. We noticed that in blue light, cells carrying pbSHY3 (RBS3) and pbSHY4 (RBS4) with lower YhjH/PdeH levels turned red (Fig. 5B, no. 4 and 5). This result is somewhat puzzling at first glance, because it suggests a blue light-induced increase in intracellular c-di-GMP levels. However, this is not that surprising. Like all bacteriophytochromes (51), BphS absorbs in the violet-blue spectral region (13), which may explain its activation by blue light. This undesired activation of BphS can be overcome by higher expression levels of YhjH (Fig. 5B, no. 3) or by expression of the blue-light-activated EB1 (Fig. 5B, no. 2). Both of these constructs resulted in nonpigmented colonies. The dichromatic module (represented here by the bphS-bphO-eb1 operon) is superior to the previously constructed system (bphS-bphO-yhjH) because it allows the bidirectional regulation of c-di-GMP levels. However, it is worth noting that when importing this module into a new organism, one needs to optimize the relative expression of the system components because expression elements (e.g., promoter efficiency, mRNA stability, and ribosome-binding-site strength) may differ among microorganisms.
In summary, EB1, identified and characterized in this study, is a robust blue-light-activated c-di-GMP PDE with a high photodynamic range. It complements the red/far-red-light-regulated DGC BphS in a module that allows the dynamic bidirectional control of intracellular c-di-GMP levels. We have tested this system in E. coli; however, it can readily be transferred to other (bacterial and nonbacterial) species. In bacteria possessing c-di-GMP signaling pathways, this system can be used to induce biofilm formation with red light or biofilm dissipation with blue light. It can also be used for studying how transient changes in c-di-GMP levels affect motility, the cell cycle, predation, and numerous other phenotypes regulated by this ubiquitous second messenger (19, 20). While the experimental setups of this study employed static conditions and “bulk” phenotypes, we anticipate that some of the most exciting applications of the engineered module will involve the fast modulation of c-di-GMP levels in cell populations, as demonstrated in the accompanying paper (49) and in single cells.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and physiological assays.
The strains used in this study are listed in Table 1. Unless indicated otherwise, strains were grown at 30°C in Luria-Bertani medium supplemented with ampicillin (100 μg ml−1). To create micro-oxic or anoxic conditions, GasPak EZ gas-generating pouch systems (BD), EZ CampyPouch and EZ Anaerobe, respectively, were used according to the manufacturer's instructions.
Swimming assays were performed by using the E. coli MG1655 yhjH strain as described previously (21). Briefly, 2 μl of a concentrated culture grown overnight (A600 of 5) was spotted onto semisolid agar (1% tryptone, 0.5% NaCl, 0.25% agar). The following inducers were added to agar media: 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for the expression of proteins from the pMal-c5x-derived plasmids, 0.02% arabinose for the expression of proteins from the pBAD-derived plasmids, or 0.01 mM IPTG for the expression of the optogenetic bphS-bphO-yhjH and bphS-bphO-eb1 modules.
For monitoring the c-di-GMP-dependent synthesis of curly fimbriae, strains were grown on YESCA agar medium supplemented with Congo red (50 μg ml−1) at 30°C for 3 to 4 days (24).
For blue light irradiation, petri dishes were placed 100 cm above All-Blue (460-nm) 225 LED Grow Light panels (total nominal power of 14 W, 30.5 by 30.5 cm; LED Wholesalers, CA), where the light irradiance was ∼0.1 mW cm−2 at the plate surface. The following irradiation regimen was used for the duration of the experiment (unless indicated otherwise): 5 s of light and 60 s of dark. For red light irradiation, petri dishes were placed 5 cm above All-Red (660-nm) LED Grow Light panel 225 (14 W, 30.5 by 30.5 cm; LED Wholesalers, CA), where the light irradiance was ∼2 mW cm−2 at the plate surface. The following irradiation regimen was used for the duration of the experiment (unless indicated otherwise): 30 s of light and 120 s of dark. Additional details on the use of light-activated enzymes for modulating c-di-GMP levels were described previously (52).
Recombinant-DNA techniques.
The plasmids used in this study are listed in Table 1. The truncated bldP1 and bldP2 genes encoding the PAS9-GGDEF-EAL-BLUF modules were amplified by PCR from genomic DNAs of M. marinus MC-1 and A. vinosum DSM 180, respectively. The amplified fragments were cloned into the pMal-c5x vector to create translational MBP fusions. The subsequent truncations were generated based on these constructs in the pMal-c5x, pBAD/Myc-HisB, or pET23a vectors by using Gibson assembly (53) according to the manufacturer's instructions (NEB).
Protein overexpression and purification.
The MBP fusion proteins were purified by using amylose resin according to the specifications of the manufacturer (NEB). The purification process was executed under dim yellow light to avoid photobleaching of the flavin chromophore. Briefly, cultures expressing MBP fusions were grown overnight in LB medium at 30°C to an A600 of 0.6. The cultures were transferred to 18°C, and protein production was induced with 0.5 mM IPTG. Following incubation for an additional 20 h with shaking at 250 rpm at 18°C, cells were collected by centrifugation at 4,000 × g for 15 min, washed, and resuspended in amylose column binding buffer (50 mM Tris-HCl [pH 8.0], 350 mM NaCl, 10 mM MgCl2, 0.5 mM EDTA, 10% glycerol). Cells were disrupted by using a French pressure cell, and cell debris were removed by centrifugation at 35,000 × g for 45 min at 4°C. Two milliliters (bed volume) of amylose resin (NEB) preequilibrated with binding buffer was added to the soluble cell extract derived from a 1.5-liter culture, and the mixture was gently agitated for 1 h at 4°C. The mix was loaded onto a column, and the resin was washed with 200 ml of column binding buffer. Fractions were eluted with 12 ml of binding buffer containing 10 mM maltose. The protein was either used immediately or stored at −80°C in 20% (vol/vol) glycerol (final concentration). Protein concentrations were measured by using a Bradford protein assay kit (Bio-Rad) with bovine serum albumin as the protein standard. Proteins were analyzed by using SDS-PAGE.
Enzymatic assays.
PDE assays were performed by measuring the rate of c-di-GMP degradation, as described previously (54). The protein incubated in PDE assay buffer (50 mM Tris-HCl [pH 8.5], 50 mM NaCl, 0.5 mM EDTA, 10 mM MgCl2) was irradiated with saturating blue light (1 mW cm−2) for activation or kept in dim yellow light. The reaction was initiated by the addition of 200 μM c-di-GMP to the mixture. Aliquots were withdrawn at different time points. To stop the reaction, CaCl2 was added to 10 mM, and samples were boiled for 5 min. The precipitated protein was removed by centrifugation at 15,000 × g for 5 min. The supernatant was filtered through a 0.22-μm-pore-size filter (MicroSolv) and analyzed by reversed-phase high-performance liquid chromatography (HPLC) as described previously (17).
Protein reconstitution with hemin and UV-vis absorption spectroscopy.
Reconstitution in vitro of the MBP-PAS9-GGDEF protein fragment from BldP2 with hemin was performed as described previously (45). Briefly, the hemin solution (50 mM) was slowly added to the protein solution at a ratio of 3:1 (hemin to protein) at 4°C. Unbound hemin was separated by using a Zeba Spin desalting column with a 7,000-molecular-weight (7K) cutoff (Thermo Scientific). UV-visible absorption spectra were recorded by using a Shimatzu UV-1601PC spectrophotometer.
ACKNOWLEDGMENTS
We are grateful to Dennis Bazylinski (University of Nevada—Las Vegas) for the gift of M. marinus MC-1 biomass and to Christiane Dahl (Rheinische Friedrich Wilhelms Universität Bonn) for the gift of A. vinosum genomic DNA. We also appreciate Dan Wall's assistance in manuscript proofreading.
This work was supported in part by National Science Foundation grant MCB1052575 (to M.G.) and the Wyoming INBRE through an institutional development award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant 2P20GM103432.
Footnotes
For a companion article on this topic, see https://doi.org/10.1128/JB.00020-17.
REFERENCES
- 1.Gomelsky M. 2011. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol 79:562–565. doi: 10.1111/j.1365-2958.2010.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kalia D, Merey G, Nakayama S, Zheng Y, Zhou J, Luo Y, Guo M, Roembke BT, Sintim HO. 2013. Nucleotide, c-di-GMP, c-di-AMP, cGMP, cAMP, (p)ppGpp signaling in bacteria and implications in pathogenesis. Chem Soc Rev 42:305–341. doi: 10.1039/C2CS35206K. [DOI] [PubMed] [Google Scholar]
- 3.Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M. 2002. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature 415:1047–1051. doi: 10.1038/4151047a. [DOI] [PubMed] [Google Scholar]
- 4.Ryu MH, Moskvin OV, Siltberg-Liberles J, Gomelsky M. 2010. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J Biol Chem 285:41501–41508. doi: 10.1074/jbc.M110.177600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stierl M, Stumpf P, Udwari D, Gueta R, Hagedorn R, Losi A, Gärtner W, Petereit L, Efetova M, Schwarzel M, Oertner TG, Nagel G, Hegemann P. 2011. Light modulation of cellular cAMP by a small bacterial photoactivated adenylyl cyclase, bPAC, of the soil bacterium Beggiatoa. J Biol Chem 286:1181–1188. doi: 10.1074/jbc.M110.185496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raffelberg S, Wang L, Gao S, Losi A, Gärtner W, Nagel G. 2013. A LOV-domain-mediated blue-light-activated adenylate (adenylyl) cyclase from the cyanobacterium Microcoleus chthonoplastes PCC 7420. Biochem J 455:359–365. doi: 10.1042/BJ20130637. [DOI] [PubMed] [Google Scholar]
- 7.Gasser C, Taiber S, Yeh CM, Wittig CH, Hegemann P, Ryu S, Wunder F, Moglich A. 2014. Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proc Natl Acad Sci U S A 111:8803–8808. doi: 10.1073/pnas.1321600111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao S, Nagpal J, Schneider MW, Kozjak-Pavlovic V, Nagel G, Gottschalk A. 2015. Optogenetic manipulation of cGMP in cells and animals by the tightly light-regulated guanylyl-cyclase opsin CyclOp. Nat Commun 6:8046. doi: 10.1038/ncomms9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tarutina M, Ryjenkov DA, Gomelsky M. 2006. An unorthodox bacteriophytochrome from Rhodobacter sphaeroides involved in turnover of the second messenger c-di-GMP. J Biol Chem 281:34751–34758. doi: 10.1074/jbc.M604819200. [DOI] [PubMed] [Google Scholar]
- 10.Barends TRM, Hartmann E, Griese J, Beitlich T, Kirienko NV, Ryjenkov DA, Reinstein J, Shoeman RL, Gomelsky M, Schlichting I. 2009. Structure and mechanism of a bacterial light-regulated cyclic nucleotide phosphodiesterase. Nature 459:1015–1018. doi: 10.1038/nature07966. [DOI] [PubMed] [Google Scholar]
- 11.Cao Z, Livoti E, Losi A, Gärtner W. 2010. A blue light-inducible phosphodiesterase activity in the cyanobacterium Synechococcus elongatus. Photochem Photobiol 86:606–611. doi: 10.1111/j.1751-1097.2010.00724.x. [DOI] [PubMed] [Google Scholar]
- 12.Savakis P, De Causmaecker S, Angerer V, Ruppert U, Anders K, Essen LO, Wilde A. 2012. Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol Microbiol 85:239–251. doi: 10.1111/j.1365-2958.2012.08106.x. [DOI] [PubMed] [Google Scholar]
- 13.Ryu MH, Gomelsky M. 2014. Synthetic second messenger module controlled by near-infrared window light. ACS Synth Biol 3:802–810. doi: 10.1021/sb400182x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enomoto G, Nomura R, Shimada T, Ni-Ni-Win, Narikawa R, Ikeuchi M. 2014. Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus. J Biol Chem 289:24801–24809. doi: 10.1074/jbc.M114.583674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enomoto G, Ni-Ni-Win, Narikawa R, Ikeuchi M. 2015. Three cyanobacteriochromes work together to form a light color-sensitive input system for c-di-GMP signaling of cell aggregation. Proc Natl Acad Sci U S A 112:8082–8087. doi: 10.1073/pnas.1504228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levskaya A, Chevalier AA, Tabor JJ, Simpson ZB, Lavery LA, Levy M, Davidson EA, Scouras A, Ellington AD, Marcotte EM, Voigt CA. 2005. Synthetic biology: engineering Escherichia coli to see light. Nature 438:441–442. doi: 10.1038/nature04405. [DOI] [PubMed] [Google Scholar]
- 17.Castillo-Hair SM, Igoshin OA, Tabor JJ. 2015. How to train your microbe: methods for dynamically characterizing gene networks. Curr Opin Microbiol 24:113–123. doi: 10.1016/j.mib.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. 2005. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into the biochemistry of the GGDEF protein domain. J Bacteriol 187:1792–1798. doi: 10.1128/JB.187.5.1792-1798.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Römling U, Galperin MY, Gomelsky M. 2013. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev 77:1–52. doi: 10.1128/MMBR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gründling A, Lee VT. 2016. Old concepts, new molecules and current approaches applied to the bacterial nucleotide signalling field. Philos Trans R Soc Lond B Biol Sci 371:20150503. doi: 10.1098/rstb.2015.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryjenkov DA, Simm R, Römling U, Gomelsky M. 2006. The PilZ domain is a receptor for the second messenger c-di-GMP: the PilZ domain protein YcgR controls motility in enterobacteria. J Biol Chem 281:30310–30314. doi: 10.1074/jbc.C600179200. [DOI] [PubMed] [Google Scholar]
- 22.Boehm A, Kaiser M, Li H, Spangler C, Kasper CA, Ackermann M, Kaever V, Sourjik V, Roth V, Jenal U. 2010. Second messenger-mediated adjustment of bacterial swimming velocity. Cell 141:107–116. doi: 10.1016/j.cell.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 23.Paul K, Nieto V, Carlquist WC, Blair DF, Harshey RM. 2010. The c-di-GMP binding protein YcgR controls flagellar motor direction and speed to affect chemotaxis by a “backstop brake” mechanism. Mol Cell 38:128–139. doi: 10.1016/j.molcel.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang X, Gomelsky M. 2010. A post-translational, c-di-GMP-dependent mechanism regulating bacterial flagellar motility. Mol Microbiol 76:1295–1305. doi: 10.1111/j.1365-2958.2010.07179.x. [DOI] [PubMed] [Google Scholar]
- 25.Hammar M, Arnqvist A, Bian Z, Olsén A, Normark S. 1995. Expression of two csg operons is required for production of fibronectin- and Congo red-binding curli polymers in Escherichia coli K-12. Mol Microbiol 18:661–670. doi: 10.1111/j.1365-2958.1995.mmi_18040661.x. [DOI] [PubMed] [Google Scholar]
- 26.Römling U. 2005. Characterization of the rdar morphotype, a multicellular behaviour in Enterobacteriaceae. Cell Mol Life Sci 62:1234–1246. doi: 10.1007/s00018-005-4557-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Christen M, Kulasekara HD, Christen B, Kulasekara BR, Hoffman LR, Miller SI. 2010. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science 328:1295–1297. doi: 10.1126/science.1188658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kulasekara BR, Kamischke C, Kulasekara HD, Christen M, Wiggins PA, Miller SI. 2013. c-di-GMP heterogeneity is generated by the chemotaxis machinery to regulate flagellar motility. eLife 2:e01402. doi: 10.7554/eLife.01402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lori C, Ozaki S, Steiner S, Böhm R, Abel S, Dubey BN, Schirmer T, Hiller S, Jenal U. 2015. Cyclic di-GMP acts as a cell cycle oscillator to drive chromosome replication. Nature 523:236–239. doi: 10.1038/nature14473. [DOI] [PubMed] [Google Scholar]
- 30.Russell MH, Bible AN, Fang X, Gooding J, Campagna S, Gomelsky M, Alexandre G. 2013. Integration of the second messenger c-di-GMP into the chemotactic signaling pathway promotes sensory adaptation. mBio 4:e00001-13. doi: 10.1128/mBio.00001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tyagi A, Penzkofer A, Griese J, Schlichting I, Kirienko NV, Gomelsky M. 2008. Photodynamics of blue-light-regulated phosphodiesterase BlrP1 protein from Klebsiella pneumoniae and its photoreceptor BLUF domain. Chem Phys 354:130–141. doi: 10.1016/j.chemphys.2008.10.003. [DOI] [Google Scholar]
- 32.Gomelsky M, Klug G. 2002. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem Sci 27:497–500. doi: 10.1016/S0968-0004(02)02181-3. [DOI] [PubMed] [Google Scholar]
- 33.Losi A, Mandalari C, Gärtner W. 2014. From plant infectivity to growth patterns: the role of blue-light sensing in the prokaryotic world. Plants (Basel) 3:70–94. doi: 10.3390/plants3010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finn D, Coggill P, Eberhardt RY, Eddy SR, Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M, Sangrador-Vegas A, Salazar GA, Tate J, Bateman A. 2016. The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terpe K. 2003. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol 60:523–533. doi: 10.1007/s00253-002-1158-6. [DOI] [PubMed] [Google Scholar]
- 36.Hengge R, Galperin MY, Ghigo JM, Gomelsky M, Green J, Hughes KT, Jenal U, Landini P. 2015. Systematic nomenclature for GGDEF and EAL domain-containing c-di-GMP turnover proteins of Escherichia coli. J Bacteriol 198:7–11. doi: 10.1128/JB.00424-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobley L, Fung RK, Lambert C, Harris MA, Dabhi JM, King SS, Lambert C, Uchida K, Basford S, Ahmad R, Aizawa SI, Gomelsky M, Sockett RE. 2012. Discrete cyclic di-GMP-dependent control of bacterial predation versus axenic growth in Bdellovibrio bacteriovorus. PLoS Pathog 8:e1002493. doi: 10.1371/journal.ppat.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hull TD, Ryu MH, Sullivan MJ, Klena NT, Johnson RC, Geiger RM, Gomelsky M, Bennett JA. 2012. Cyclic di-GMP phosphodiesterases RmdA and RmdB are involved in regulating colony morphology and development in Streptomyces coelicolor. J Bacteriol 194:4642–4651. doi: 10.1128/JB.00157-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen LH, Köseoğlu VK, Güvener ZT, Myers-Morales T, Reed JM, D'Orazio SEF, Miller KW, Gomelsky M. 2014. Cyclic di-GMP-dependent signaling pathways in the pathogenic firmicute Listeria monocytogenes. PLoS Pathog 10:e1004301. doi: 10.1371/journal.ppat.1004301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drozdetskiy A, Cole C, Procter J, Barton GJ. 2015. JPred4: a protein secondary structure prediction server. Nucleic Acids Res 43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gilles-Gonzalez MA, Gonzalez G. 2004. Signal transduction by heme-containing PAS-domain proteins. J Appl Physiol 96:774–783. doi: 10.1152/japplphysiol.00941.2003. [DOI] [PubMed] [Google Scholar]
- 42.Gomelsky M, Kaplan S. 1998. AppA, a redox regulator of photosystem formation in Rhodobacter sphaeroides 2.4.1 is a flavoprotein: identification of a novel FAD binding domain. J Biol Chem 273:35319–35325. doi: 10.1074/jbc.273.52.35319. [DOI] [PubMed] [Google Scholar]
- 43.Braatsch S, Gomelsky M, Kuphal S, Klug G. 2002. A single flavoprotein, AppA, from Rhodobacter sphaeroides integrates both redox and light signals. Mol Microbiol 45:827–836. doi: 10.1046/j.1365-2958.2002.03058.x. [DOI] [PubMed] [Google Scholar]
- 44.Masuda S, Bauer CE. 2002. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell 110:613–623. doi: 10.1016/S0092-8674(02)00876-0. [DOI] [PubMed] [Google Scholar]
- 45.Moskvin OV, Kaplan S, Gilles-Gonzalez MA, Gomelsky M. 2007. Novel heme-based oxygen sensor with a revealing evolutionary history. J Biol Chem 282:28740–28748. doi: 10.1074/jbc.M703261200. [DOI] [PubMed] [Google Scholar]
- 46.Winkler A, Heintz U, Lindner R, Reinstein J, Shoeman RL, Schlichting I. 2013. A ternary AppA-PpsR-DNA complex mediates light regulation of photosynthesis-related gene expression. Nat Struct Mol Biol 20:859–867. doi: 10.1038/nsmb.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weissgerber T, Zigann R, Bruce D, Chang YJ, Detter JC, Han C, Hauser L, Jeffries CD, Land M, Munk AC, Tapia R, Dahl C. 2011. Complete genome sequence of Allochromatium vinosum DSM 180(T). Stand Genomic Sci 5:311–330. doi: 10.4056/sigs.2335270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefèvre CT, Bazylinski DA. 2013. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol Mol Biol Rev 77:497–526. doi: 10.1128/MMBR.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O'Neal LD, Ryu M-H, Gomelsky M, Alexandre G. 2017. Optogenetic manipulation of cyclic di-GMP (c-di-GMP) levels reveals the role of c-di-GMP in regulating aerotaxis receptor activity in Azospirillum brasilense. J Bacteriol 199:e00020-17. doi: 10.1128/JB.00020-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dai T. 4 January 2017. The antimicrobial effect of blue light: what are behind? Virulence doi: 10.1080/21505594.2016.1276691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rockwell NC, Su YS, Lagarias JC. 2006. Phytochrome structure and signaling mechanisms. Annu Rev Plant Biol 57:837–858. doi: 10.1146/annurev.arplant.56.032604.144208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryu MH, Fomicheva F, O'Neal L, Alexandre G, Gomelsky M. Using light-activated enzymes for modulating intracellular c-di-GMP levels in bacteria. In Sauer K. (ed), c-di-GMP signaling, in press. Humana Press, New York, NY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA III, Smith HO. 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat Methods 6:343–345. doi: 10.1038/nmeth.1318. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt AJ, Ryjenkov DA, Gomelsky M. 2005. The ubiquitous protein domain EAL is a c-di-GMP-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol 187:4774–4781. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]