Abstract
Infection has been hypothesized to be one of the factors associated with spontaneous preterm birth (PTB) and with the racial disparity in rates of PTB between African American and Caucasian women. However, recent findings refute the generalizability of the role of infection and inflammation. African Americans have an increased incidence of PTB in the setting of intraamniotic infection, periodontal disease, and bacterial vaginosis compared to Caucasians. Herein we report variability in infection- and inflammation-related factors based on race/ethnicity. For African American women, an imbalance in the host proinflammatory response seems to contribute to infection-associated PTB, as evidenced by a greater presence of inflammatory mediators with limited or reduced presence of immune balancing factors. This may be attributed to differences in the genetic variants associated with PTB between African Americans and Caucasians. We argue that infection may not be a cause of racial disparity but in association with other risk factors such as stress, nutritional deficiency, and differences in genetic variations in PTB, pathways and their complex interactions may produce differential inflammatory responses that may contribute to racial disparity.
Keywords: African American, health disparities, infant health, infection, prematurity
Introduction
The rate of preterm birth (<37 weeks’ gestation) is increasing in the USA, rising by as much as 30% during the last 25 years despite advances in prenatal and obstetrical care (1). The World Health Organization recently estimated the global preterm birth rate for singleton gestations as 9.6%, or approximately 13 million preterm infants born annually worldwide (2). Despite efforts to understand and prevent preterm birth, the rate increased more than 20% from 1990 to its peak in 2006, and although preterm birth rates declined slightly in 2007–2009, racial disparities have widened since 1990 (1). Because of the adverse health, neurological, and development sequelae of preterm birth, it represents a substantial problem for already overtaxed health, education, and social services (3,4).
One of the factors that complicate the understanding of preterm birth is the persistent racial/ethnic disparity in preterm birth rate among various groups. In 2009, the preterm birth rate for African Americans was 17.9%, whereas the rates were 10.8% for Asian and Pacific Islander women and 11.8% for Caucasian women (5). A significant portion of the excess in infant mortality among African Americans compared to other ethnic groups in the USA (25% increase between 1980 and 2000) is attributable to the nearly twofold greater low birthweight (LBW; <2 500 g) and preterm birth rates, and the near threefold greater rates of very low birth-weight (VLBW; <1 500 g) and very preterm births (VPTB; <28 weeks) among African Americans (6). Only a small proportion of these disparities can be explained by differences in traditional measures of socioeconomic status, psychosocial variables, and adverse health behaviors, such as smoking or drug use (7). Studies over the last 15 years have focused on elucidating individual risk factors associated with adverse birth outcomes and assessing their importance across ethnic subgroups, but this approach has neither decreased the rate of preterm birth nor eliminated the disparities (8–16). In this article series on racial disparities in preterm birth, we deal here with the role of infection, a well documented risk factor commonly associated with VPTB (17,18), as a cause of racial disparity between African Americans and Caucasians in spontaneous PTB (referred as PTB in this article).
Material and methods
We performed a literature search using spontaneous preterm birth, genetic and biomarkers, and racial disparity as key search terms on articles published between 1990 and 2010. Race was considered a risk factor of PTB and was reported by many investigators to explain the population of their studies. However, it was not considered a risk modifier in many of the early genetic studies and biomarker data analyses, and this has contributed significantly to the ambiguity and non-reproducibility of data. Many studies have reported studies based on single race but these data are not useful for understanding racial disparity. Therefore, we decided to review our own work and summarize our findings to explain the role of infection and inflammation in racial disparity associated with PTB.
We reviewed nine years of work in this field to provide an overview of racial disparity in genetic and biomarkers associated with PTB. To our best knowledge, we are the first group to initiate a series of in vitro and in vivo studies to document racial disparity associated with PTB. The data described in this review are based on (i) in vitro documentation of racial disparity in biomarker profile in response to bacterial antigens, (ii) in vivo documentation of racial disparity in amniotic fluid samples from PTB and normal term births, (iii) genetic association studies that provide explanations for some of the observed disparity with infection/inflammation signals based on genetic variations. We also provide an explanation for these facts using knowledge gained using bioinformatics tools.
Intrauterine infection associated with preterm birth
As reviewed by Romero et al., ascending infection (from the vagina and cervix) is one of the most common routes of infection (17,19). It can manifest as vaginitis, cervicitis, deciduaitis, or chorioamnionitis, eventually reaching the amniotic cavity (microbial invasion of the amniotic cavity, MIAC) and establishing intraamniotic infection (IAI). Several lines of evidence support the hypothesis that VPTB has an infectious association: (i) an inverse relation is observed between the likelihood of upper tract microbial colonization or chorioamnionitis and gestational age at delivery, (ii) the percentage of positive cultures in the chorioamnion and the amniotic fluid increases as the gestational age at delivery decreases, (iii) approximately 40% of spontaneous PTB are associated with intraamniotic infection and over 70% of VPTB are associated with infection. Establishing infection in the amniotic fluid rich in antimicrobial peptides is not an easy process as it requires compromising innate immune mechanisms (19). Host inflammatory response to IAI is overwhelming, which is hypothesized to lead to preterm labor (20,21).
IAI triggers a vicious cycle of events in the intrauterine tissues that involves cytokines, chemokines, matrix metalloproteinases, adhesion molecules, proapoptotic factors, coagulation factors, stress-related hormones (such as corticotropin-releasing hormone), and reactive oxygen radicals. These factors lead to cyclooxygenase-mediated prostanoid response (prostaglandin production) that eventually results in early labor (19–25). Although these pathways may not explain the causality and actual risk factors leading to infection, inflammation remains a major component of PTB.
Racial disparity and infection
Several findings support that many infections during pregnancy are disproportionately higher in African Americans than in Caucasians. These include asymptomatic bacteriuria, bacterial vaginosis, lower genital tract infections, MIAC and IAI, clinical and histologic chorioamnionitis, preterm premature rupture of membranes, fetal inflammatory response syndrome, and early neonatal sepsis (11,26–31).
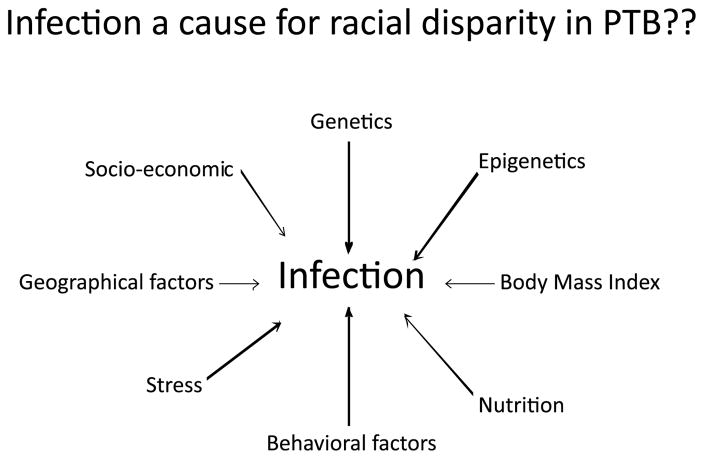
Although risk exposure may be the same for various racial/ethnic groups in a given population in a specific region, the pathophysiologic manifestations of risk factors may vary by race/ethnicity. Factors that may contribute to variable rates of infection and pregnancy outcomes for women of different races/ethnicities include, but are not limited to, genetics, epigenetics, variable exposure to infections, nutritional deficiencies, early adverse experiences, life-time exposure to chronic stress, and other behavioral, physical and psychosocial environmental factors, as well as interactions among these factors. With this framework in mind (Figure 1), the role of infection in PTB risk for African Americans and Caucasians has been examined. Research findings to date reveal some racial/ethnic differences in genetic predisposition, inflammatory response, and pregnancy outcome that may ultimately provide a better understanding of racial/ethnic differences in susceptibility factors, effectors of pregnancy outcomes (biochemical pathways), and the role of infection in the black–white disparity in PTB.
Figure 1.
Interaction of multitudes of factors with infection can contribute to pregnancy outcome. Differences in these factors (as outlined above) can contribute to racial disparity, in which case infection is likely to be a secondary phenomenon. Most of these factors are not independent and interactions do exist between them in addition to infection contributing to the complexity of preterm birth.
Racial disparity in fetal immune response to in vitro infectious stimuli
Fortunato et al. (32) initiated in vitro studies to examine and compare the immune response (expression and production of inflammatory markers of PTB) in human fetal membranes (amniochorionic membranes as fetal inflammatory response) from African American and Caucasian women in response to an infectious stimulus (32,33). We conducted further preliminary in vitro experiments using fetal membranes at term from African American and Caucasian women who were undergoing elective repeat Cesarean sections. Maternal race was self-identified, and women in the experiment were limited to those who reported having parents and grandparents of the same race.
These experiments demonstrated differential expression of various messenger RNAs (mRNA) in response to bacterial endotoxin according to maternal race/ethnicity, as summarized in Table 1 (32). Protein concentrations of several of the analytes were also determined in the culture media from the above experiments, and comparisons were made between African Americans and Caucasians in endotoxin-stimulated and -unstimulated control cultures. Whereas there were no racial/ethnic differences in these biochemical effector proteins in unstimulated membranes, stimulation resulted in varying levels of the biochemical effector proteins for African Americans and Caucasians (Table 2).
Table 1.
mRNA differences between African American (AA) and Caucasian (C) women.*
| Gene of interest | Unstimulated control membranes | Endotoxin stimulated membranes |
|---|---|---|
| IL-1β | Increased expression in C | Increased expression in AA |
| IL-6 | No difference | No difference |
| IL-8 | No difference | Increased expression in AA |
| TNF-α | No difference | Increased expression in C |
| MMP1 | Increased expression in AA | Increased expression in AA |
| MMP2 | Increased expression in AA | No difference |
| MMP 3 | No difference | Increased expression in AA |
| MMP9 | Increased expression in AA | Increased expression in AA |
| MMP 13 | Increased expression in AA | Increased expression in C |
| CRH | No difference | Increased expression in AA |
| CRH receptor 1 | Increased expression in C | Increased expression in AA |
| CRH-binding protein | Increased expression in C | Increased expression in C |
| Cox-1 | No difference | Increased expression in AA |
| Cox-2 | Increased expression in C | Increased expression in AA |
| PGDH | Increased expression in C | Increased expression in C |
From fetal membranes derived at term from women not in labor undergoing elective repeat Cesarean.
Table 2.
Differences in protein concentrations between African American (AA) and Causcasian (C) women.*
| Culture media analysis: analyte | Unstimulated control membranes | Endotoxin stimulated membranes |
|---|---|---|
| IL-1β | No difference | Higher concentration in AA |
| IL-6 | No difference | Higher concentration in C |
| IL-8 | No difference | Higher concentration in C |
| TNF-α | No difference | Higher concentration in C |
| MMP9 | No difference | Higher concentration in AA |
| CRH | No difference | Measurable only in AA |
| PGE2 | No difference | Higher concentration in AA |
From fetal membranes derived at term from women not in labor undergoing elective repeat Cesarean.
Imbalance in in vivo inflammatory response in African Americans but not in Caucasians
Tumor necrosis factor (TNF)-α, an inflammatory mediator, is elevated in infection-associated PTB. When examined in fetal membranes from the above experiments, both African American- and Caucasian-derived membranes were found to have a pronounced TNF-α response to bacterial infection. However, soluble TNF receptor responses (molecules that neutralize TNF-α from binding to TNF membrane-bound receptors) were significantly decreased in African Americans, whereas there was no change for Caucasians. Similarly, membrane-bound TNF receptors, which act to promote TNF function, were increased for African Americans but decreased for Caucasians.
These data suggest a proinflammatory pattern that favors TNF-α biological activity among African Americans. The data are indicative of differences in the expression and production of inflammatory mediators of PTB in vitro in response to infection for African American and Caucasian women. However, the underlying cause of the observed physiologic responses to infection remains undetermined, and may be related to genetic variation, epigenetic factors, variability in exposures to other risk factors, or interactions of exposures with race/ethnicity.
In vivo differences in the amniotic fluid concentrations of cytokines in preterm birth
To understand the racial disparity in PTB, a case-control study was undertaken to assess difference in inflammatory response and its underlying genetic predispositions. Cases were African American and Caucasian women who delivered preterm (between 22 and 36 weeks) after spontaneous labor with no rupture of membranes; controls included African American and Caucasian women with term labor and delivery (>37 weeks), who had intact membranes and no pregnancy-related complications. Amniotic fluid samples were subjected to multiplex-based cytokine concentration measurements for interleukin (IL)-1β, IL-6, IL-8, IL-10, TNF-α, soluble TNF receptors (sTNFR1 and sTNFR2), and corticotrophin releasing hormone (CRH). When analysis was performed without racial stratification, significant differences between cases and controls were observed for all measured cytokines and CRH, whereas no differences in soluble TNF receptor concentrations were observed. All cytokine concentrations were higher for cases than for controls. However, stratifying by race revealed that this pattern varied by race (34–38). Specifically, the case-control difference in the pooled data was driven by different races in each of the comparisons. IL-1β and TNF-α concentrations were higher in African American cases when compared with their controls, whereas IL-6 and IL-8 were elevated in Caucasian cases when compared with their controls. No racial differences were observed for IL-10 and CRH.
Studies subsequently conducted to confirm these in vitro findings demonstrated an imbalance in African American TNF-α response in response to infection. Soluble TNF receptors were higher in Caucasian cases and decreased in African American cases compared to their respective controls. This was also evident in cases with MIAC, where the molar ratio between the ligand and the soluble receptors favored higher bioavailability of TNF-α in African Americans in cases with MIAC, whereas the TNF/soluble TNF receptor response was balanced in Caucasians (34). These differences in concentration in the amniotic fluid are suggestive of different pathophysiologic processes of PTB in African Americans and Caucasians. More importantly, the biomarkers involved in driving preterm labor process may vary by race such that testing for ‘universal biomarkers’ may be futile until more is understood about the underlying causes of susceptibility to infection. Although several studies have reported biomarkers of PTB, no studies have reported stratified data analysis based on race. This factor has contributed to the lack of understanding of independent risk factors and their manifestations through specific biomarker-associated pathways.
Racial differences in genetic predispositions to PTB
Recently, the Preterm Birth International Collaborative (PREBIC) organized a systematic review of genetic association studies in preterm birth to document true associations (39). Until December 2007, 88 manuscripts documented genetic associations in spontaneous preterm birth and data were extracted from only 48 of them, as most other studies did not fit the inclusion criteria for various reasons, such as improper study designs and reporting issues. In summary, the analysis included data on 144 polymorphisms in 76 genes and 19 meta-analyses were performed. Three gene variants, two in the maternal [beta-2 adrenergic receptor (rs1042713) and interferon gamma (rs2430561)] and one in the neonatal genome [coagulation factor 2 (rs1799963)] have shown nominally significant associations, but all three have weak epidemiological credibility. None of these studies has addressed racial disparities that could have provided an explanation to our observed biomarker differences. Therefore we designed a genetic epidemiological study to address genetic differences that might explain observed disparities.
Both small-scale and large-scale candidate gene studies have been undertaken to examine the genetic contribution to the observed racial disparity in PTB between African Americans and Caucasians. Detailed reports can be found in references 40–43.
Briefly, single, multilocus, and haplotype association studies were performed in a case-control study for candidate genes (which included the above biomarkers of interest) on maternal and fetal DNA samples (as the contribution to disparity can come from mother, baby or both). Significant differences in allelic, genotypic frequencies of single nucleotide polymorphisms (SNPs) were noticed between case and controls and between races for both maternal and fetal and DNA variants. The differences were almost twofold greater (60–80%) in allelic and genotypic frequencies between African Americans and Caucasians compared to approximately 42% expected between these racial groups (http://genome.perlegen.com/). Several single locus associations in both maternal and fetal DNA samples varied between the African American and Caucasian in maternal and fetal DNA; however, none of them was significant after corrections for multiple testing. Similar differences in haplotype frequencies and associations and multilocus interactions (genetic epistasis) were noticed between African Americans and Caucasians. One possible explanation for this failure to find associations is genetic heterogeneity; namely, allelic and locus heterogeneity. Complex patterns of association caused by allelic heterogeneity and locus heterogeneity can often result from the presence of multiple rare variants and this can be addressed only by large-scale genome-wide association studies (GWAS). So far, no such studies have been reported in preterm birth, although many are underway.
Recent data demonstrate not only different biomarker associations with PTB for African Americans and Caucasians but also different associations of these cytokines with genetic variants. For example, examination of genotype–cytokine association of amniotic fluid IL-6 demonstrated a significant association with a gene promoter variant genotype in the IL-6 gene in maternal DNA in Caucasian cases, but not in Caucasian controls or in any African American pregnancies (44). Similarly, TNF and TNF receptor polymorphisms in both maternal and fetal DNA in African Americans were associated with cytokine (TNF) concentrations in amniotic fluid, whereas the interaction between the SNPs and pregnancy status was not prominent in Caucasians. This explains some of the observed disparity in cytokine concentrations between races. The presence of MIAC also demonstrated interaction with SNPs in African American fetal DNA samples, suggesting the involvement of both maternal and fetal genetic variations in producing observed differences in biomarker profiles in PTB (45). These data demonstrate two things: (i) genetic variations are associated with different pregnancy outcome and amniotic fluid cytokine concentrations in African Americans and Caucasians; (ii) the genetic association is different between clinical groups even within the same geographic population. These patterns of differences by ancestry and by clinical phenotype appear to be general, although there may be some regions of genes that harbor variants that behave similarly across groupings (46).
Pathophysiologic pathways of labor determined by genetic variants
To understand the potential biologic impact of genetic variants in determining differences in the pathophysiologic pathways according to race/ethnicity, we implemented a bioinformatics approach in our studies (32). We developed molecular interaction maps that differed between races and also between maternal and fetal genetic variants. The analysis also mapped genes to specific disease functions. Gene variants in Caucasians were implicated in disease functions associated with cervical ripening and decidual hemorrhage. In African Americans, inflammatory pathways were the most prevalent. In Caucasians, maternal gene variants showed the most prominent role in contributing to PTB, whereas in African Americans it was fetal variants that were dominantly associated with PTB. In summary, this analysis documented that differences at the genetic level can contribute to distinct disease functions and operational pathways in African Americans and Caucasians in PTB. This approach further confirmed two things: (i) in African Americans there is a dominance of inflammatory pathways contributed predominantly by the fetus, whereas disorders of coagulation cascade drive PTB in Caucasians, and (ii) the pathways and the biomarkers involved vary, bringing into question the use of biomarkers for predicting PTB without a clear understanding of the underlying etiology leading to spontaneous PTB.
Despite the fact that it is impossible based on such statistical evidence to know exactly what causes these differences in association, it is reasonable to conclude that other unmeasured factors, genetic or environmental, operate above the level of these genetic variants, impacting how the variants affect gene expression and/or protein concentrations. Such observations point to complex interactions that impact the production of biomarkers. The existence of such differences in genetic regulation across populations emphasizes the discussion in the previous section that risk factors may be differentially distributed across racial/ethnic groups in the USA.
Although animal model studies have shown that direct inoculation of bacteria can trigger labor in a controlled research environment (47), the presence of microbes in the amniotic fluid or intrauterine tissues may not be the sole causal agent for preterm labor. It is possible that MIAC and IAI are causal factors of PTB in a subset of preterm labor; however, establishment of the infection following inoculation or exposure depends on a multitude of factors. Stress is known to compromise the immune status and thereby contribute to infection (48). Similarly, hormonal changes during pregnancy (49) and nutritional deficiencies leading to depleted immune status (50) also contribute to infection. For each of these cases, the pathways that lead to preterm labor may be different; moreover, the pathway may depend upon the type, quantity, and antigenicity of the microbe (51).
Discussion
So, revisiting racial disparity in preterm birth rates: is it caused by infection or inflammatory response? Exposure to infectious agents may or may not be a causal factor of PTB, but inflammation in response to infectious (or other) exposures in an individual can likely be associated with the preterm labor process. Likewise, we suggest that infection is not a cause of the racial disparity in PTB but is a factor that variably influences pregnancy outcome depending on other factors that are associated with racial groupings. Genetic predispositions and their interaction between the infectious environment (regardless of whether infection is primary or secondary) can contribute to higher risk of PTB among women with a given genetic predisposition. We propose that the racial disparity in PTB cannot be attributed solely to infection or inflammation but rather to a number of risk factors including genetics, epigenetics, nutrition, behavioral factors, body mass index, and other risk-predisposing environmental factors (Figure 1). Interactions between these factors are likely complex and overlapping, and it is important to understand the timing of exposure and interactions during a specific stage of pregnancy (52–54). Each biological interaction may produce distinct pathways and biomarker footprints, which may be important for identifying pregnancies at high risk for delivering preterm. Given the growing body of research showing that risk factors, their interaction, and their biomarker signatures vary by race, further research must involve women of varying race/ethnicities and explore the numerous patterns of risks. Specifically, understanding the black–white racial disparity in PTB requires a thorough investigation of the multitudes of risk factors (the initiators and effectors of preterm labor process) that may be linked with infection, rather than an isolated investigation of specific infectious risks, as both exposures and susceptibility to infectious are likely to play a role in racial disparities in PTB.
Acknowledgments
This article follows a four-part seminar series on causes of racial disparities in preterm birth held in the fall of 2009 at the Rollins School of Public Health, Emory University, Atlanta, GA. The series and this work are supported in part by the National Institute of Health Reproductive, Perinatal, and Pediatric Health Training grant T32 HD052460.
Ramkumar Menon is also supported by Grants from March of Dimes, New York, NY, USA (21-FY08–557) to study genetic and biomarker differences in preterm birth racial disparity.
Footnotes
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Funding
No specific funding.
References
- 1.Births: Preliminary Data for 2005. National Center for Health Statistics; 2006. [PubMed] [Google Scholar]
- 2.Beck S, Say L, Betran AP, Merialdi M, Rubens C, Menon R, Van Roof P. WHO systematic review on maternal mortality and morbidity: The global burden of preterm birth. Bull World Health Org. 2010;88:31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.March of Dimes Peristat, 2006. March of Dimes, 2006.
- 4.International Classification of Diseases and Related Health Problems, 10th revision. Geneva: World Health Organization; 1992. [Google Scholar]
- 5.Preterm Birth: Causes, Consequences and Prevention. Washington, DC: Institute of Medicine of the Academies; 2006. [Google Scholar]
- 6.March of Dimes Peristat, 2009. March of Dimes, 2009.
- 7.Minino AM, Arias E, Kochanek KD, Murphy SL, Smith BL. National Vital Statistics Reports. 15. Vol. 50. Hyattsville, MD: National Center for Health Statistics; 2002. Deaths: Final data for 2000. [PubMed] [Google Scholar]
- 8.Demissie K, Rhoads GG, Ananth CV, Alexander GR, Kramer MS, Kogan MD, Joseph KS. Trends in preterm birth and neonatal mortality among blacks and whites in the United States from 1989 to 1997. Am J Epidemiol. 2001;154:307–15. doi: 10.1093/aje/154.4.307. [DOI] [PubMed] [Google Scholar]
- 9.Cockey CD. Premature births hit record high. AWHONN Lifelines. 2005;9:365–70. doi: 10.1111/j.1552-6356.2005.tb00863.x. [DOI] [PubMed] [Google Scholar]
- 10.Foster HW, Wu L, Bracken MB, Semanya K, Thomas J. Intergenerational effects of high socioeconomic status on low birthweight and preterm birth in African Americans. J Natl Med Assoc. 2000;92:213–21. [PMC free article] [PubMed] [Google Scholar]
- 11.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Matern Child Health J. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 12.MacDorman MF, Martin JA, Mathews TJ, Hoyert DL, Ventura SJ. Explaining the 2001–02 infant mortality increase: data from the linked birth/infant death data set. Natl Vital Stat Rep. 2005;53:1–22. [PubMed] [Google Scholar]
- 13.Martin JA, Kochanek KD, Strobino DM, Guyer B, MacDorman MF. Annual summary of vital statistics – 2003. Pediatrics. 2005;115:619–34. doi: 10.1542/peds.2004-2695. [DOI] [PubMed] [Google Scholar]
- 14.Huddy CL, Johnson A, Hope PL. Educational and behavioral problems in babies of 32–35 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;85:F23–8. doi: 10.1136/fn.85.1.F23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang ML, Dorer DJ, Fleming MP, Catlin EA. Clinical outcomes of near-term infants. Pediatrics. 2004;114:372–6. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- 16.Petrou S. The economic consequences of preterm birth during the first 10 years of life. Br J Obstet Gynaecol. 2005;112(Suppl 1):10–5. doi: 10.1111/j.1471-0528.2005.00577.x. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31:553–84. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gomez R, Romero R, Mazor M, Ghezzi F, David C, Yoon BH. The role of infection in preterm labor and delivery. In: Elder M, Romero R, Lamont R, editors. Preterm Labor. London: Churchill Livingstone; 1997. pp. 85–125. [Google Scholar]
- 20.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Espinoza J, Rogers WT, Moser A, Nien JK, Kusanovic JP, et al. Proteomic analysis of amniotic fluid to identify women with preterm labor and intra-amniotic inflammation/infection: the use of a novel computational method to analyze mass spectrometric profiling. J Matern Fetal Neonatal Med. 2008;21:367–88. doi: 10.1080/14767050802045848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JS, Park CW, Lockwood CJ, Norwitz ER. Role of cytokines in preterm labor and birth. Minerva Ginecol. 2005;57:349–66. [PubMed] [Google Scholar]
- 23.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 24.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357–65. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 25.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol. 1998;178:546–50. doi: 10.1016/s0002-9378(98)70436-3. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and premature delivery. N Engl J Med. 1998;339:313–20. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 27.Fiscella K. Race, perinatal outcome, and amniotic infection. Obstet Gynecol. 1995;51:60–6. doi: 10.1097/00006254-199601000-00022. [DOI] [PubMed] [Google Scholar]
- 28.Fiscella K. Racial disparities in preterm births. The role of urogenital infections. Public Health Rep. 1996;111:104–13. [PMC free article] [PubMed] [Google Scholar]
- 29.Smaill F. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev. 2000;2:CD000490. doi: 10.1002/14651858.CD000490. [DOI] [PubMed] [Google Scholar]
- 30.King J, Flenady V. Cochrane Library. 3. Oxford: Update Software; 2002. Antibiotics for preterm labour with intact membranes (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- 31.Carmichael SL, Iyasu S. Changes in the black–white infant mortality gap from 1983 to 1991 in the United States. Am J Prev Med. 1998;15:220–7. doi: 10.1016/s0749-3797(98)00052-x. [DOI] [PubMed] [Google Scholar]
- 32.Fortunato SJ, Lombardi SJ, Menon R. Racial disparity in membrane response to infectious stimuli: a possible explanation for observed differences in the incidence of prematurity. Am J Obstet Gynecol. 2004;190:1557–62. doi: 10.1016/j.ajog.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 33.Menon R, Merialdi M, Lombardi SJ, Fortunato SJ. Differences in the placental membrane cytokine response: a possible explanation for the racial disparity in preterm birth. Am J Reprod Immunol. 2006;56:112–8. doi: 10.1111/j.1600-0897.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 34.Menon R, Thorsen P, Vogel I, Jacobsson B, Morgan N, Jiang L, et al. Racial disparity in amniotic fluid tumor necrosis factor-α and soluble TNF receptor concentrations in spontaneous preterm birth: evidence for increased TNF mediated susceptibility in African-Americans. Am J Obstet Gynecol. 2008;198:533e1–10. doi: 10.1016/j.ajog.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Menon R, Camargo MC, Thorsen P, Fortunato SJ. Amniotic fluid interleukin-6 increase is an indicator of preterm birth in Caucasians but not in African-Americans. Am J Obstet Gynecol. 2008;198:77e1–7. doi: 10.1016/j.ajog.2007.06.071. [DOI] [PubMed] [Google Scholar]
- 36.Menon R, Arora CP, Hobel CJ, Fortunato SJ. Corticotrophin releasing hormone concentrations in lipopolysaccharide stimulated term fetal membranes and amniotic fluid from term and preterm birth in African-Americans and Caucasians. Reprod Sci. 2008;15:477–83. doi: 10.1177/1933719108315300. [DOI] [PubMed] [Google Scholar]
- 37.Menon R, Williams SM, Fortunato SJ. Amniotic fluid interleukin (IL)-1β and IL-8 concentrations: ethnic disparity in preterm birth. Repro Sci. 2007;14:253–9. doi: 10.1177/1933719107301336. [DOI] [PubMed] [Google Scholar]
- 38.Menon R, Pearce B, Velez DR, Merialdi M, Williams SM, Fortunato SJ, et al. Racial disparity in pathophysiologic pathways of preterm birth based on genetic variants. Reprod Biol Endocrinol. 2009;7:62. doi: 10.1186/1477-7827-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dolan SM, Hollegaard M, Merialdi M, Betran AP, Allen P, Abelow C, et al. Synopsis of preterm birth genetic association studies: the genomics knowledge base. Public Health Genomics. 2010;13:514–23. doi: 10.1159/000294202. [DOI] [PubMed] [Google Scholar]
- 40.Menon R, Velez DV, Simhan H, Ryckman K, Jiang L, Thorsen P, et al. Multilocus interactions at maternal TNF-α, TNF receptors, IL-6 and IL-6 receptor genes predict spontaneous preterm labor in European-American women. Am J Obstet Gynecol. 2006;194:1616–24. doi: 10.1016/j.ajog.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 41.Fortunato SJ, Menon R, Velez DR, Thorsen P, Williams SM. Racial disparity in maternal-fetal genetic epistasis in spontaneous preterm birth. Am J Obstet Gynecol. 2008;198:666e1–9. doi: 10.1016/j.ajog.2008.02.003. discussion e9–10. [DOI] [PubMed] [Google Scholar]
- 42.Velez DR, Fortunato SJ, Williams SM, Menon R. Preterm birth in African Americans is associated with infection and inflammatory response gene variants. Am J Obstet Gynecol. 2009;200:209e1–27. doi: 10.1016/j.ajog.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velez DR, Fortunato SJ, Williams SM, Menon R. Preterm birth in Caucasians is associated with coagulation pathway gene variants. PLoS ONE. 2008;3:e3283. doi: 10.1371/journal.pone.0003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Velez DR, Fortunato SJ, Williams SM, Menon R. Interleukin-6 (IL-6) and receptor (IL6-R) gene haplotypes associate with amniotic fluid protein concentrations in preterm birth. Hum Mol Genet. 2008;17:1619–30. doi: 10.1093/hmg/ddn049. [DOI] [PubMed] [Google Scholar]
- 45.Menon R, Velez DR, Morgan N, Lombardi SJ, Fortunato SJ, Williams SM. Genetic regulation of amniotic fluid TNF and soluble TNF receptor concentrations affected by race and preterm birth. Hum Genet. 2008;144:243–53. doi: 10.1007/s00439-008-0547-z. [DOI] [PubMed] [Google Scholar]
- 46.Williams SM, Velez DR, Menon R. Geographic ancestry and markers of preterm birth. Expert Rev Mol Diagn. 2010;10:27–32. doi: 10.1586/erm.09.70. [DOI] [PubMed] [Google Scholar]
- 47.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 48.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, et al. Stress, infection, and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001;15:17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- 49.Dudley DJ. Hormonal pathways of preterm birth. Am J Obstet Gynecol. 1999;180:S251–6. doi: 10.1016/s0002-9378(99)70711-8. [DOI] [PubMed] [Google Scholar]
- 50.Menon R, Peltier MR, Eckardt J, Fortunato SJ. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. Am J Obstet Gynecol. 2009;201:306.e1–6. doi: 10.1016/j.ajog.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 51.Menon R. Spontaneous preterm birth. Race and genetics in understanding the complexities of preterm birth. Expert Rev Obstet Gynecol. 2009;4:695–704. [Google Scholar]
- 52.Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600. doi: 10.1080/00016340802005126. [DOI] [PubMed] [Google Scholar]
- 53.Dudley DJ. Hormonal pathways of preterm birth. Am J Obstet Gynecol. 1999;180:S251–6. doi: 10.1016/s0002-9378(99)70711-8. [DOI] [PubMed] [Google Scholar]
- 54.Steer P. The epidemiology of preterm labor–a global perspective. J Perinat Med. 2005;33:273–6. doi: 10.1515/JPM.2005.053. [DOI] [PubMed] [Google Scholar]