Abstract
Persistent infections, such as cytomegalovirus (CMV), herpes simplex virus-1 (HSV-1), Helicobacter pylori (H. pylori), and Toxoplasma gondii (T. gondii), are common in the U.S. but their prevalence varies by socioeconomic status. It is unclear if early or later life socioeconomic position (SEP) is a more salient driver of disparities in immune control of these infections. Using data from the Sacramento Area Latino Study on Aging, we examined whether early or later life SEP was the strongest predictor of immune control later in life by contrasting two life course models, the critical period model and the chain of risk model. Early life SEP was measured as a latent variable, derived from parental education and occupation, and food availability. Indicators for SEP in later life included education level and occupation. Individuals were categorized by immune response to each pathogen (seronegative, low, medium and high) with increasing immune response representing poorer immune control. Cumulative immune response was estimated using a latent profile analysis with higher total immune response representing poorer immune control. Structural equation models were used to examine direct, indirect and total effects of early life SEP on each infection and cumulative immune response, controlling for age and gender. The direct effect of early life SEP on immune response was not statistically significant for the infections or cumulative immune response. Higher early life SEP was associated with lower immune response for T. gondii, H. pylori and cumulative immune response through pathways mediated by later life SEP. For CMV, higher early life SEP was both directly associated and partially mediated by later life SEP. No association was found between SEP and HSV-1. Findings from this study support a chain of risk model, whereby early life SEP acts through later life SEP to affect immune response to persistent infections in older age.
Keywords: socioeconomic position, persistent infections, life course epidemiology, Latino health
Introduction
Lower socioeconomic position (SEP) is linked to poorer individual health outcomes, though the mechanisms underlying this association are unclear (Adler & Rehkopf, 2008). Seropositivity and immune response to persistent infections may be one important pathway by which SEP influences adult health (Roberts et al., 2010; Simanek et al., 2009). Individuals of low SEP are more likely to acquire persistent infections earlier in life and are more likely to be seropositive and to have poor immune control (i.e. higher circulating antibody levels) of these pathogens across the life course (Colugnati et al., 2007; Dowd & Aiello, 2009; Dowd et al., 2009). Common persistent infections, such as cytomegalovirus (CMV), herpes simplex-1 (HSV-1), Helicobacter pylori (H. pylori), and Toxoplasma gondii (T. gondii), have been implicated in multiple chronic diseases, including cardiovascular disease, cognitive impairment, frailty, and mortality (Aiello et al., 2006; Draborg et al., 2013; Esposito et al., 2014; Hasni, 2012; Itzhaki et al., 2004; Jeon et al., 2012; Kang et al., 2004; Schmaltz et al., 2005; Sorlie et al., 2000; Zajacova et al., 2009). Taken together, the social patterning of these infections may play an important role in perpetuating socioeconomic disparities in chronic diseases in older age. Whether early life SEP or later life SEP is most important in terms of shaping immune control of persistent infections and cumulative immune response to multiple infections later in life has not yet been elucidated.
There are several life course models by which early life SEP may be operating to influence immune response to persistent infections during adulthood. Two models, the critical period model and the chain of risk model, are strong contenders for explaining how early life SEP influences later life immune response to infection. The critical period model suggests that an event early in life, acting during a specific period of time, has a lasting independent effect on later life health (Kuh, 2004). Under this model, early life SEP may serve as a critical period exposure altering an individual's susceptibility to persistent infections as well as their likelihood of exposure due to unfavorable physical and social environments, directly impacting which infections one acquires early in life and shaping their likelihood of seropositivity and immune response to the infection as an adult (Cohen et al., 2004). Increased susceptibility and frequent exposure may not only lead to infection at a younger age among individuals of low SEP in early life, but also may influence the development of the immune system by leaving a lasting early life imprint on adult immunological function (McDade, 2005). For persistent infections, which once acquired are never cleared from the body, impaired immune control later in life may manifest as increased production of antibodies targeted against these infections and are reflective of impaired cell-mediated function (Glaser & Kiecolt-Glaser, 1994).
Alternatively, the chain of risk model posits that exposures are linked over the life course (i.e. one experience may lead to another) to influence later life health (Kuh, 2004). Under this model, early life SEP may indirectly affect immune response to persistent infections later in life by acting through later life socioeconomic exposures. Early life social disadvantage is linked to subsequent SEP in adulthood, such as educational attainment, employment grade and income level (Oakes & Kaufman, 2006). Resources and environments corresponding to SEP over the life course may, similar to early life SEP, influence exposure to persistent infections as well as contribute to immune function and the ability to maintain immunologic control over persistent infections. For this reason, low SEP in early life may influence later life immune function indirectly by setting up a chain of risk leading to low SEP in later life which then contributes to poor immune function later in life. Thus, in a chain of risk model, later life SEP may mediate the effect of early life SEP on immune response later in life.
Limiting our ability to more broadly understand how the life course influences infectious disease outcomes, most existing studies of the social patterning of persistent infections are restricted to cross-sectional analyses in which only early life or adulthood SEP was assessed (Dowd et al., 2009; Dowd et al., 2012; Staras et al., 2008; Zajacova et al., 2009). Of those studies that have looked at the early life environment, one study suggested that adversity during childhood may negatively influence the immune system, resulting in poorer control of CMV in later life (Fagundes et al., 2013). Another study showed an association between poorer family interpersonal environment during childhood and increased later life immune response to CMV (Janicki-Deverts et al., 2014). While these studies lend support for a role of early life environment in later life immunological control, they are limited to few infections, do not directly measure early life SEP, and no study has examined potential life course models by which early life SEP influences later life immunological response.
Building upon previous work, this study addresses important gaps in our understanding of the life course SEP influences on later life immune response to multiple persistent infections. Applying a life course framework, we contrast the critical period and chain of risk models to identify the most likely pathway by which early life SEP may be operating to influence immune response to individual persistent infections and cumulative immune response to multiple infections during adulthood using data on older U.S. Latinos from the Sacramento Area Latino Study on Aging (SALSA).
Methods
Sample
The Sacramento Area Latino Study on Aging (SALSA) is a longitudinal cohort study of 1,789 of Mexican Americans living in the Sacramento, California metropolitan area who were 60-101 years old at baseline in 1998-1999. Detailed information about the recruitment and study population has been described previously (Haan et al., 2003). The overall response rate was 85% and more than 89% of eligible households enumerated had a least one person who participated in the study, making SALSA highly representative of older Hispanics living in Sacramento and the surrounding suburban and rural counties (Haan et al., 2003).
Data for this study come from the baseline in-home interview. To be included in the analytic sample, SALSA participants could not have missing data for covariates (age and sex, N=1,779). Using full information maximum likelihood methods, participants who had data for at least one of the six early life SEP indicators or available infection data resulting were included, resulting in a final sample of 1,562 (87.3%) for the individual infection analyses. The final sample for the cumulative immune response analysis was limited to individuals with at least one early life SEP indictor AND infection data for all pathogens (N=1,263, 70.8%)..
Exposures
SALSA participants provided retrospective SEP information for three time points in their life course, early life, midlife and late life. First, early life SEP was constructed as a latent variable by using five different indicators used in previous SALSA studies of early life SEP recalled by the participant and meant to capture the resources and social and physical environment: father's education (reference, fixed to 1.0), mother's education, father's occupation, mother's occupation, and food availability as a child. Parental education was measured in years. Parental occupation was measured by a 3-level categorical variable (technical, professional or managerial [high]; sales, administrative support or military [middle]; and services, manual or housewives [low]) and treated as an ordered hierarchical variable. Food availability during childhood was ascertained using the question, “When growing up, how often did you not have enough to eat?” with Likert item responses and also treated as a continuous variable. Next, midlife SEP was measured by years of education completed by the SALSA participant, which is a proxy for economic resources and human capital of the participant in adulthood. Last, to measure late life SEP, the reported occupation worked by the SALSA participant for most of their life was categorized as a 4-level ordinal variable (technical, professional or managerial; sales, administrative support or military; services or manual; or housewife). Lifetime occupation is representative of later life SEP as it reflects differences in social status, earning ability and prestige.
Outcomes
Immune Response
Immune response was measured by IgG antibody level values for four pathogens, CMV, HSV-1, H. pylori, and T. gondii. Details about IgG as a serum biomarker are available in Appendix A. CMV is a beta herpesvirus and ubiquitous worldwide (Bate et al., 2010). CMV has co-evolved with humans over time and as a result has developed many mechanisms for immune evasion (Miller-Kittrell & Sparer, 2009). HSV-1 is a highly common alpha herpesvirus with adult prevalence ranging from 50 to more than 90% (Strauss & Strauss, 2008). Infections with HSV-1 are common and are characterized by recurrent oral lesions on the lips, mouth and gums (Schillinger et al., 2004). T. gondii is an obligate parasite that infects intestinal epithelial cells and establishes latency with intracellular bradyzoite cysts in muscle and brain cells (Galvan-Ramirez et al., 2010; Jones et al., 2014). T. gondii is also found worldwide with prevalence in adult populations ranging from 10% - 80% (Yolken & Torrey, 2008). H. pylori is a bacterium that colonizes the mucosal layer of the gastric epithelium (stomach) and generates a state of chronic inflammation (Lina et al., 2014). Persistent infection with H. pylori causes gastritis, peptic ulcer disease and is associated with other gastric conditions, such as cancer (Lina et al., 2014).
Serum and plasma samples from baseline were tested at the Stanley Neurovirology Laboratory at Johns Hopkins University School of Medicine using high-throughput solid-phase enzyme-linked immunosorbent assays (ELISA) to detect pathogen-specific IgG antibody levels. The ELISA methods have been described previously (Yolken et al., 2011). Briefly, diluted aliquots of serum were reacted with antigen bound to a solid-phase surface. Quantitation of IgG for each pathogen was determined by reaction of bound antibodies with enzyme labeled anti-human IgG and enzyme substrate and optical densities were read by spectrophotometric instrumentation (Dickerson et al., 2003). The sensitivity (Sn) and specificity (Sp) of the assays are as follows: CMV Sn: 97% Sp: 94%; HSV-1 Sn: 100% Sp: 96%; T. gondii Sn: 94%, Sp: 96%; H. pylori Sn: 96% Sp: 96%.
Continuous antibody level was categorized by first identifying those who were seropositive and seronegative to each infection. Seropositivity was determined by standard cutoffs for each assay such that optical density unit (ODU) values less than 1.1 were classified as seronegative and values 1.1 or greater were seropositive. Among those seropositive for a given infection, continuous antibody level values were divided into tertiles and categorized as low, middle and high antibody level with those in the highest tertile representing those with the worst immune control of the infection. Based upon both pathogen serostatus and IgG antibody response we constructed a four-level hierarchical variable representing overall immune response to each pathogen in which individuals were categorized as: seronegative, low, middle and high antibody response.
Cumulative immune response
Cumulative immune response to multiple infections may have a greater influence on disease processes than immune response towards any one infection. Recent studies suggest that traditional summary measures of cumulative immune response such as the total number of pathogens for which individuals are seropositive or exhibit elevated immune response, may not adequately capture the relative importance of particular pathogens on health outcomes of interest (Simanek et al., 2014). For this reason, we constructed a measure of cumulative immune response from a nominal latent class variable determined by the grouping of CMV, HSV-1, H. pylori, and T. gondii immune response levels. Specifically, a latent profile analysis (LPA) measurement model from continuous IgG level (log transformed) with the four infections as indicators generated latent class membership, as detailed in Appendix B. A three class solution was selected with membership as follows: the low cumulative immune response group (N=55, 4%) was seronegative to CMV and T. gondii and had low antibody levels to HSV-1 and H. pylori, the middle cumulative immune response group (N=777, 61%) was seronegative to T. gondii and had moderate CMV, HSV-1 and H. pylori antibody levels, and the high cumulative immune response group (N=431, 34%) had moderate antibody levels to all infections. Class membership was not further adjusted for potential measurement misclassification due to high entropy (Asparouhov & Muthen, 2014; Feingold et al., 2014). Predicted class membership from this LPA model was then used as a nominal dependent variable in subsequent analyses.
Covariates
Covariates of interest included age and gender. Baseline age of participants was measured in years. Participant gender was categorized as male or female, and male was treated as the referent category. Nativity was also adjusted for in sensitivity analyses with U.S. born as the referent category.
Statistical Analyses
Means, percentages and standard deviations for all variables were calculated using SAS version 9.3 (SAS Institute, Inc., Cary, NC). Figure 1 depicts the theoretical life course model connecting early life SEP to late life immune response. The early life SEP latent variable was used to test three different pathways, 1) direct, independent association of early life SEP on immune response (pathway A), 2) indirect effect of early life SEP on immune response mediated by midlife SEP alone (pathway B-C), and 3) indirect effect of early life SEP on immune response mediated by midlife and later life SEP (pathway B-D-E). We did not include a pathway from early life SEP directly to later life SEP (life-long occupation) as any effect was likely mediated by midlife SEP (education). Age and gender were then added to the model with paths extending to each SEP measurement as well as the outcome to control for potential confounding.
Figure 1. Life course model of the association between early life SEP and adult immune response to common persistent pathogens.
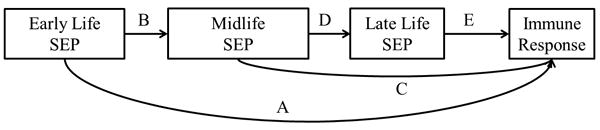
Structural equation modeling (SEM) was used to examine the life course pathways linking early life SEP to adult antibody level for CMV, HSV-1, H. pylori, T. gondii and cumulative immune response. SEM is comprised of a measurement model and a structural model. A measurement model for the latent variable early life SEP was built using five indicators, father's education and occupation, mother's education and occupation, and food availability. The early life SEP latent variable was then used in two separate structural pathway models to predict 1) ordinal immune response (i.e. seronegative, low, middle or high antibody response) for each of the four infections and 2) cumulative immune response class while adjusting for covariates.
All SEM analyses were conducted in MPlus version 7.2 (Methuén & Methuén, Los Angeles, CA). A probit link function, theta parameterization and weighted least squares estimator (WLSMV) were used to appropriately model ordered categorical outcomes and categorical mediators for the four separate infections (Muthen, 2012). Multinomial logistic regression with Monte Carlo integration was used for the cumulative immune response model with the maximum likelihood (MLR) estimator to produce parameter estimates and standard errors that were robust to non-normality (Muthen, 2012). Goodness-of-fit for the final models was obtained using the root mean squared error of approximation (RMSEA) and comparative fit index (CFI). Models with a CFI above 0.90 and an RMSEA of less than or equal to 0.05 were considered to fit well. Model goodness-of-fit measures were not available for the cumulative immune response analysis due to the use of Monte Carlo integration.
Based on previous imputation procedures we consider the data in these analyses to be missing at random (MAR) and that age, gender and education likely explained the missing data (Haan et al., 2011; Zeki Al Hazzouri et al., 2011). Age and gender may have influenced the ability or desire to give a biospecimen during data collection and consequently the individuals for whom we had infection data. Age may also be related to recall of early life SEP. Education level commonly predicts missing information (Acock, 2013). Since age, gender and education were all included in the final models, we did not include any additional auxiliary variables to meet MAR criteria (Acock, 2013). Sensitivity analyses were performed using traditional regression and mediation techniques and similar effects sizes to the SEM results were observed (Hayes, 2013).
As a sensitivity analysis, we assessed nativity as a potential confounder, assuming nativity was associated with immune response (through some unspecified mechanism independent of SEP) and was also associated with SEP. Potential mechanisms by which nativity may influence immune response include nutrition and dietary behavior differences by place of birth. We examined whether nativity was associated with early life SEP and immune function, respectively, for each infection. Potential confounding by place of birth was only indicated for T. gondii, therefore, the SEM was re-run additionally controlling for nativity.
Results
Baseline descriptive statistics of individuals included in this analysis are shown in Table 1. The sample had a mean age of 70 years and 58% were female. A total of 84% were seropositive for CMV, 91% for H. pylori, 87% for HSV-1, and 35% for T. gondii.
Table 1. Descriptive statistics for the Sacramento Area Latino Study on Aging (SALSA) at baseline.
| Variable | N | Mean or % | SD |
|---|---|---|---|
| Early Life SEP | |||
| Father's Education (years) | 732 | 3.35 | 4.18 |
| Mother's Education (years) | 827 | 3.37 | 3.12 |
| Father's Occupation | 1132 | ||
| High | 88 | 7.80 | |
| Middle | 54 | 4.80 | |
| Low | 990 | 87.50 | |
| Mother's Occupation | 1227 | ||
| High | 41 | 3.30 | |
| Middle | 15 | 1.20 | |
| Low | 1171 | 95.40 | |
| Food Availability | 1249 | 4.55 | 0.98 |
| Sibling Mortality | 1247 | 1.26 | 1.14 |
| Midlife SEP | |||
| Education (years) | 1562 | 7.53 | 5.35 |
| Late Life SEP | 1542 | ||
| Technical, Professional or Managerial | 182 | 11.80 | |
| Sales, Administrative Support or Military | 172 | 11.15 | |
| Services or Manual | 907 | 58.82 | |
| Housewives | 281 | 18.22 | |
| Covariates | |||
| Age (years) | 1562 | 70.34 | 6.93 |
| Female | 1562 | 58.00 | 0.49 |
| Seropositive | |||
| CMV | 1263 | 84.00 | 0.36 |
| T. gondii | 1263 | 35.00 | 0.48 |
| H. pylori | 1263 | 91.00 | 0.28 |
| HSV-1 | 1263 | 87.00 | 0.34 |
| Cumulative Immune Response | 1263 | ||
| Low Group | 55 | 4.00 | |
| Medium Group | 777 | 61.00 | |
| High Group | 431 | 35.00 |
Measurement Models
Unstandardized and standardized factor loadings for the early life SEP measurement model are presented in Supplemental Table 1. For early life SEP, all five indicators were statistically significant at p<0.05 and the measurement model fit was appropriate (RMSEA=0.05, CFI=0.94). The LPA measurement model for cumulative immune response from the four infection indicators produced a three class solution detailed in Appendix B, Supplemental Table 2 and Supplemental Table 3.
SEM Models: Direct, Indirect and Total Effects of Early Life SES
A general SEM path diagram is depicted in Figure 2 for immune response to each infection and in Figure 3 for cumulative immune response. Direct, indirect and total effects of early life SEP on each of the four infection outcomes, CMV, HSV-1, T. gondii, and H. pylori are summarized in Table 2. All models fit the data well. The pathway connecting early life SEP to immune response adjusting for all other pathways and covariates was not statistically significant in final models for any of the four infections. Early life SEP was found to indirectly influence immune response through pathways mediated by later life SEP for T. gondii and H. pylori. No association was found between early life SEP and immune response to HSV-1.
Figure 2. General diagram of SEM for indirect and direct effects of early life SEP on immune response.
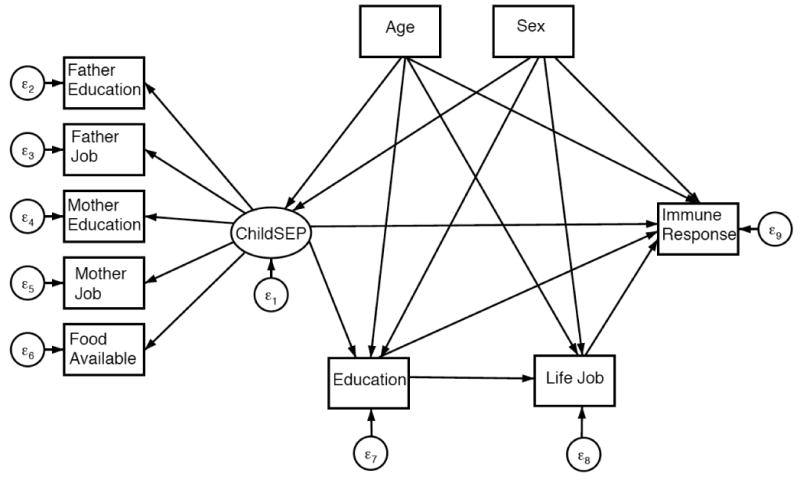
Figure 3. General diagram of SEM for indirect and direct effects of early life SEP on cumulative immune response.
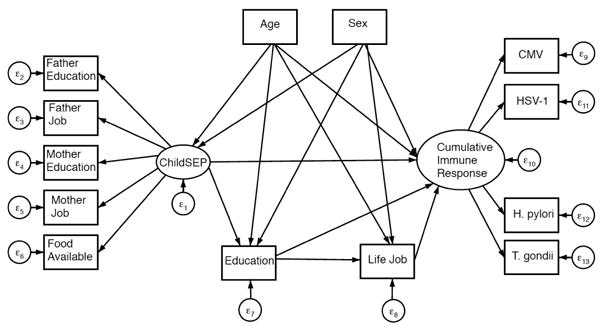
Table 2. Standardized direct and indirect effects of SEP on later life immune response, N=1562.
| Persistent Infection | ||||||||
|---|---|---|---|---|---|---|---|---|
| CMV | p-value | HSV-1 | p-value | T. gondii | p-value | H. pylori | p-value | |
| Early Life SEP | ||||||||
| Total Effect | -0.108 | 0.018 | -0.067 | 0.164 | 0.014 | 0.813 | -0.072 | 0.127 |
| Direct Effect | -0.086 | 0.185 | -0.077 | 0.254 | 0.115 | 0.165 | -0.078 | 0.231 |
| Total Indirect Effect | -0.023 | 0.405 | 0.01 | 0.725 | -0.101 | 0.003 | 0.006 | 0.831 |
| Decomposition of Indirect Effects | ||||||||
| via Education | -0.024 | 0.441 | -0.001 | 0.962 | -0.119 | 0.002 | 0.037 | 0.211 |
| via Education and Occupation | 0.001 | 0.949 | 0.011 | 0.301 | 0.067 | 0.142 | -0.031 | 0.005 |
| Midlife SEP | ||||||||
| Direct Effect of Education | -0.041 | 0.442 | -0.003 | 0.962 | -0.209 | 0.001 | 0.065 | 0.206 |
| Late Life SEP | ||||||||
| Direct Effect of Occupation | 0.003 | 0.949 | 0.042 | 0.299 | 0.067 | 0.142 | -0.114 | 0.004 |
| Model Fit | ||||||||
| RMSEA | 0.031 | 0.031 | 0.031 | 0.029 | ||||
| CFI | 0.956 | 0.962 | 0.96 | 0.965 | ||||
All models adjusted for age and sex. Bold is statistically significant at p=0.05.
Early life SEP was associated with immune response to CMV when all pathways, direct and indirect, were considered collectively (model fit indices: RMSEA=0.031, CFI=0.956). For this total effect, a one standard deviation increase in early life SEP (one standard deviation is approximately 4 years of father's education) decreased the mean of the underlying variable for CMV antibody level by 0.11 standard deviations holding all covariates constant (p=0.02).
The effect of early life SEP on T. gondii immune response was mediated by later life SEP pathways such that a one standard deviation increase in early life SEP (4 years of father's education) indirectly decreased the mean of the underlying variable for T. gondii antibody level by 0.10 standard deviations holding all covariates constant (p=0.003, model fit indices: RMSEA=0.031, CFI=0.962). When this indirect effect was decomposed into the education pathway and the education-occupation pathway, education was found to drive this association (p=0.002). Further, the pathway from education directly to T. gondii immune response was statistically significant; a one standard deviation increase in education, 5.35 years, decreased the mean of the underlying variable for T. gondii antibody level by 0.21 standard deviations, holding all else constant (p=0.001). Sensitivity analysis indicated that nativity may be a potential confounder of the SEP – T. gondii immune response association. When we additionally controlled for nativity in the SEM, effect estimates were attenuated and the indirect effect of early life SEP through the education pathway and the direct effect of education became marginally significant (p=0.07 and p=0.06 respectively) as shown in Supplemental Table 4.
Early life SEP was associated with immune response to H. pylori via the education-occupation pathway. A one standard deviation increase in early life SEP decreased the mean of the underlying variable for H. pylori antibody level by 0.03 standard deviations, holding all else constant (p=0.005, model fit indices: RMSEA=0.032, CFI=0.946). The pathway from lifetime occupation to H. pylori antibody level was statistically significant such that a one category increase in occupation decreased the mean of the underlying variable for H. pylori level by 0.11 standard deviations, holding all else constant (p=0.004).
Table 3 shows the direct, indirect and total effects of the early life SEP latent variable on cumulative immune response. In the multinomial logistic regression model, comparing the lowest cumulative immune response group to the highest cumulative immune response group and the middle cumulative immune response group to highest cumulative immune response group, no statistically significant independent direct effect of early life SEP was found on cumulative immune response class. When considering pathways mediated by later life SEP, small, statistically significant associations were observed. Through all indirect pathways, for a one unit increase in early life SEP, the odds of being in the lowest cumulative immune response group (low immune response to HSV-1 and H. pylori only) were 1.11 times the odds of being in the highest cumulative immune group (moderate immune response to all infections), holding covariates constant (p=0.04). This total indirect effect was decomposed into an indirect pathway via education only and an indirect pathway via education and lifetime occupation. The pathway via education only was shown to drive this association (OR=1.10, p=0.04). In addition, education was found to directly influence cumulative immune response class for both the lowest v. highest cumulative immune response (OR=1.09, p=0.05) and middle v. highest cumulative immune response (OR=1.06, p=0.01) comparisons.
Table 3. Life course SEP effects on later life cumulative immune response.
| Cumulative Immune Response | ||||
|---|---|---|---|---|
| Low v. High (N=1263) | Middle v. High (N=1263) | |||
|
| ||||
| OR | p-value | OR | p-value | |
| Early life SEP | ||||
| Total Effect | 1.17 | 0.06 | 0.83 | 0.11 |
| Direct Effect | 1.05 | 0.64 | 0.93 | 0.16 |
| Total Indirect Effect | 1.11 | 0.04 | 0.89 | 0.08 |
| Decomposition of Indirect Effects | ||||
| via Education | 1.10 | 0.05 | 0.92 | 0.16 |
| via Education and Occupation | 1.02 | 0.73 | 0.97 | 0.17 |
| Midlife SEP | ||||
| Direct Effect of Education | 1.09 | 0.05 | 1.06 | 0.01 |
| Late Life SEP | ||||
| Direct Effect of Occupation | 1.07 | 0.73 | 0.89 | 0.16 |
All models adjusted for Age and Sex
Standardized Estimates. Bold is statistically significant at p=0.05
Discussion
We examined the association between life course SEP and immune response to persistent infections and cumulative immune response. Two life course models, the critical period and the chain of risk, were evaluated to determine how life course SEP impacts immune response to four persistent infections associated with chronic health conditions later in life. Early life SEP was not independently associated with immune response in older age for any of the four persistent infections included in this study or the cumulative immune response to these infections. Instead, higher later life SEP, as measured by education and lifetime occupation, mediated the effect of early life SEP on immune response. These findings support a chain of risk model, whereby early life SEP influences later life SEP, which in turn, impacts immune control of these infections.
Education, in particular, may be a key link in the socioeconomic chain of risk. This may be due to the far-reaching influence of higher educational attainment on the potential for subsequent higher SEP exposures, such as higher occupational status, higher income level and increased wealth. Higher education also contributes to the development of health capital and better health behaviors, potentially reducing individual susceptibility or improving immunological function through, for example, better nutrition. In addition, higher educational attainment and higher later life SEP may act as a buffer by reducing the number and severity of stressors, which may impact the frequency of reactivation to persistent infections. Therefore, early life conditions may set the stage to enhance or prevent educational achievement, which has life-long consequences for social advantage or disadvantage, influences disparities in immune response to persistent infections, and general health overall. These findings have key public health implications because higher adult immune response to these infections has been associated with mortality and chronic health conditions (Aiello et al., 2009; Aiello et al., 2006; Dickerson et al., 2003; Esposito et al., 2014; Simanek et al., 2009). Moreover, there are significant disparities in these infections by race/ethnicity, indicating that minority populations are more likely to experience detrimental impacts of poor life course SEP on immune response which may affect overall health and increased mortality.
Of the four persistent infections examined in this study, early life SEP may be most important for later life immune response to CMV. Early life SEP was inversely associated with CMV immune response after adjusting for covariates but not mediators. Though the independent effect of early life SEP controlling for all mediating pathways was not statistically significant, the role early life may play for acquiring CMV and subsequent immune control of this infection is not diminished. CMV is generally acquired early in life through direct contact with infected body fluids (Centers for Disease Control and Prevention, 2010). Cross-sectional evidence shows racial and socioeconomic disparities exist for CMV seroprevalence and IgG antibody levels that begin at young ages (Dowd et al., 2012). In addition, immune response to CMV increases with age and lower SEP (Dowd & Aiello, 2009), and Mexican Americans on average acquire CMV 10 years earlier than non-Hispanic Whites (Colugnati et al., 2007). Low SEP in early life may result in earlier infection with CMV and fewer resources available to manage the infection, increasing the frequency of reactivation over the life course. More frequent reactivations may produce negative downstream biological effects, including inducing inflammation and the exhaustion of cell mediated immunity (immunosenescence) (Pawelec et al., 2006).
Our study findings also suggest there may be evidence for a “trigger effect” for immune response to T. gondii and H. pylori. A trigger effect occurs in a chain of risk where only the final link in the chain has a marked effect on the outcome (Ben-Shlomo & Kuh, 2002). We observed a direct effect of adulthood education on immune response to T. gondii independent of all other pathways. This may mean that educational attainment is a trigger for immune control of T. gondii later in life. The direct effect of education and the indirect effect of early life SEP through the education pathway was attenuated and made marginally significant by additionally controlling for nativity. Of note, T. gondii is transmitted through cat feces, under cooked meat, contaminated water or may be inhaled or ingested directly through soil (Torrey & Yolken, 2013). It is therefore possible that T. gondii immune response may differ by country, if these potential exposure routes are unrelated to socioeconomic differences. Better education may not only prevent exposure to T. gondii through knowledge of these transmission pathways, but it also may represent better access to other resources, such as health care, which influences overall health and immune system function later in life.
Similarly, we observed a direct effect of lifetime occupation on immune response to H. pylori independent of all other pathways and that early life SEP was only associated with this outcome through the education-occupation pathway. Occupation may, therefore, be a trigger for immune control of H. pylori later in life. This is consistent with previous studies, which have shown that manual or unskilled workers have higher odds of H. pylori infection than non-manual workers (Moayyedi et al., 2002; Rosenstock et al., 1996). Lower occupational levels may increase the virulence of H. pylori as well as interfere with the immune response to infection through increased energy expenditure or other pathways (Rosenstock et al., 1996). In addition, a recent study found that the inability for employees to make decisions affecting the experience of fairness was associated with increased long-term levels of inflammatory markers CRP and IL-6 among men (Elovainio et al., 2010). A systematic review of psychosocial job stress and immunity found that greater job satisfaction may have a positive impact on immune outcomes and that unemployment and job security are significant factors leading to reduced cellular immune response (Nakata, 2012). Thus, lower occupational status may influence immunological control of H. pylori, potentially through inflammatory pathways, and result in decreased resources to handle stress and its negative health consequences. Alternatively, jobs that do not provide health care coverage may result in fewer doctor visits associated with H. pylori infection. H. pylori is associated with peptic ulcer disease and can be treated with a combination of proton pump inhibitors and antibiotics (Grad et al., 2012). Our study did not directly diagnose H. pylori infection but IgG antibody levels to the infection indicate an initial or lasting immune response to the infection once it has cleared (Grad et al., 2012). Treatment for H. pylori or other incidental antibiotic use may result in seroreversion or decreased measurable antibody levels (Grad et al., 2012). As a result, higher SEP and health care access due to employment with health care benefits may result in lower immune response to H. pylori.
Education was also found to mediate the effect of early life SEP on cumulative immune response; those with higher education were more likely to have better cumulative immune control. Lower SEP during early life and midlife may impact individual susceptibility and patterns of exposure to these infections, as well as immunological control over the life course. Children with lower SEP may have worse housing conditions and poorer hygiene than children with higher SEP, increasing the likelihood of exposure and acquisition of multiple persistent infections. Further, co-infection may not only increase the risk of infection to other pathogens, but also increase the severity of subsequent infections (Espinola-Klein et al., 2002; Rubicz et al., 2011). Indeed, recent studies provide convincing evidence that the measles virus reduces immune response after initial infection leading to heightened susceptibility to other infections and this may explain why childhood measles vaccination led to dramatic reductions in infections with other pathogens in childhood (Griffin, 2010). Likewise, seropositvity to multiple persistent infections may contribute to morbidity over and above the influence of each infection alone through additional tolls placed on the immune system and activation of inflammatory pathways (Aiello et al., 2009). These results reinforce the importance of education for reducing exposure to persistent infections and suggest improving educational attainment as a potential target for public health intervention aimed at reducing disparities in these infections. Other potential targets may include the development of vaccinations or treatments for the pathogens that do not currently have either of these public health or medical measures.
This study has some limitations. The SALSA study may be subject to survivor bias because participants were at least 60 years of age at baseline; participants may be healthier and have better control of persistent infections than those who did not survive to enrollment, biasing our results towards the null. In addition, early life SEP measures are prone to recall bias. However, reporting of early life SEP is unlikely to vary by immune response, as individuals are unlikely to be aware of their serostatus and immunological response to infection. None of these infections are routinely diagnosed in populations unless they are associated with overt symptoms, such as peptic ulcer disease. A previous study in the SALSA cohort found that only 0.8% were taking antibiotics to treat H. pylori infection and among those seropositive to H. pylori 11% reported taking medications for acid suppression, peptic disorders, or antacids at the time of data collection for this study (Jeon et al., 2012). In this elderly cohort, poor cognition may influence the recall of early life SEP but we expect this to be non-differential with respect to level of SEP, biasing estimates toward the null. As with many studies of infections, we have no information on primary infection timing. While it is possible infections acquired earlier in life may influence later life SEP attainment, this does not prevent us from examining the role of SEP over the life course in relation to immune control later in life. Further, without infection timing, we were unable to investigate potentially protective health effects that these pathogens may have in early life (Amberbir et al., 2014; Rook, 2012). This purpose of this study was to determine the role of early life SEP in the social patterning of immune response to persistent pathogens. The clinical significance of a change in antibody level to the chronic infections examined here has no direct correlate with common measures of immune functioning (e.g. vaccine response) because there are currently no vaccinations for any of these infections. As a surrogate, we compared our results to vaccination with varicella zoster virus (VZV), which is another herpesvirus that affects children and adults. Vaccination of adults with VZV results in a geometric increase of 2.3 compared to control subjects (Gilbert et al., 2014). Interpreting our results in light of this immune response to VZV vaccine, we observed a CMV antibody level geometric mean decrease of 0.22 for those with both high early life SEP and high midlife SEP relative to all others (in the case of chronic infection, a lower antibody level is protective). This corresponds to a 10th of what one would observe when directly vaccinating an adult with VZV and is equal to a 12% lower CMV antibody level for those in the highest SEP, suggesting a potential clinically meaningful change, especially in light of the fact that we did not directly vaccinate individuals. However, this interpretation should be made with caution given that immune response to a CMV vaccine, if ever developed, may not show the same change in antibody level as VZV. VZV was not directly measured in this study because there is a vaccine available and it would be impossible to disentangle a vaccine response versus natural immune response. Natural immune response was observed for the pathogens included in this study—a strength of our work, since there are no available vaccines. It is not possible to compare surrogate vaccine responses for H. pylori or T. gondii immune response since there are no similar vaccines. Future vaccine challenge studies should consider collecting life course SEP data to assess the influence of these factors on vaccination response in adulthood with clinically significant immune response outcomes. Finally, there are additional persistent infections for which we do not have data, however, the four that we studied are important to understand because they are associated with chronic health outcomes and more prevalent among Latino populations (Aiello et al., 2006; Hasni, 2012; Simanek et al., 2009; Simanek et al., 2011; Sorlie et al., 2000; Zhu et al., 2000).
Despite these limitations, this study has many strengths. We use a life course approach to determine how early life social environment influences immune response to persistent infections later in life in a well-characterized cohort of predominantly Mexican Americans, limiting the influences of significant heterogeneity present among Latino populations. SALSA is a large cohort with rich data both on life course SEP and immune response to persistent pathogens. Samples were tested at a reliable laboratory using validated methods. Using SEM, we maximized the use of available SEP information, incorporated measurement error into the modeling process and estimated pathway specific effects and standard errors.
This study adds to the growing literature showing that childhood may not be an independent critical period for SEP exposures in relation to later life health outcomes, and instead the beginning of multiple mediating pathways that ultimately influence adult health. Future research should expand on this work to examine the effects of nativity and acculturation, important components of social and racial/ethnic disparities in health, as independent predictors of immune response, as well as modifiers of the life course social patterning of immune response to persistent infections. In addition, other life course models, such as the social mobility model, may also fit these relations of interest and provide insight into whether later life SEP partially or entirely remediates or modifies low early life SEP exposure. Lastly, given the complex relationship between the gut microbiome, infection and immune response, future research should consider additional microbial pathways that may be linked to SEP and ultimately affect immune response over the life course.
Conclusion
Numerous studies have demonstrated that disparities exist in the seroprevalence and immune control of persistent infections by SEP beginning early in life, suggesting childhood may be an important period during the life course for acquiring and establishing control of persistent infections. Our study found that higher immune response to chronic infections later in life is not just a function of disadvantage during early life, but instead reflects the end result of a chain of socioeconomic disadvantage throughout the life span. Our findings suggest that interventions targeting increased educational attainment as a modifiable risk factor may be effective for improving immune control of persistent infections later in life. Moreover, understanding the life course SEP pathways that influence immune response to persistent infections may help shed light on why chronic disease disparities associated with these infections appear to persist by SEP over time and across generations in the U.S.
Supplementary Material
Supplemental Figure 1. Diagram of SEM for standardized direct and indirect effects of early life SEP on later life immune response to CMV, adjusted for age and gender, N=1562
Supplemental Figure 2. Diagram of SEM for standardized direct and indirect effects of early life SEP on later life immune response to T. gondii, adjusted for age and gender, N=1562
Supplemental Figure 3. Diagram of SEM for standardized direct and indirect effects of early life SEP on later life immune response to H. pylori, adjusted for age and gender, N=1562
Supplemental Figure 4. Log Odds (SE) for SEM Model of Low v. High Cumulative Immune Response adjusted for age and gender, N= 1263
Supplemental Figure 5. Log Odds (SE) for SEM Model of Middle v. High Cumulative Immune Response adjusted for age and gender, N= 1263
Supplemental Table1. Measurement model for early life SEP, model fit: RMSEA=0.05, CFI=0.94
Supplemental Table 2. Measurement models for cumulative immune response from LPA with log continuous IgG level indicators for four infections (n=1263)
Supplemental Table 3. Mean IgG levels for the cumulative immune response three class measurement model solution (n=1263) Mean IgG Level (ODU)
Supplemental Table 4. Standardized direct and indirect effects of SEP on later life immune response to T. gondii controlling for age, sex and nativity, N=1562
Acknowledgments
Funding for the SALSA study was aided by a grant from the National Institute on Aging, National Institutes of Health: R01AG012975. Additional support for this work from grants: P60MD002249, R01DA022702, and R01DK087864. We thank Brisa Sanchez for her statistical consultation and the Stanley Neurovirology Laboratory at Johns Hopkins University, particularly Drs. Robert Yolken and Fuller Torrey, for performing the laboratory assays. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Appendix A
Immune Response
Lasting immune response is measured by the amount of IgG antibody to the specific pathogen circulating in the blood. IgG is a serum biological marker indicating that an individual received sufficient exposure to the pathogen to activate the adaptive immune response at some point over the life course. Recent infection is measured by IgM, however this antibody isotype is short-lived and difficult to capture in a population-based study. Moreover, recent infections with CMV, HSV-1, H. pylori and T. gondii in older age are generally rare in the U.S. as a majority of individuals are exposed at a young age (childhood-adolescence) and even earlier in Mexican Americans. Persistent infections establish residence in the body after initial infection and periodically reactivate inducing an increase in IgG antibody response. For these reasons, elevated measures of IgG levels among older age individuals are likely due to reactivation of a pathogen from exposure to stressor or age-related changes in immune competence.
Appendix B
LPA Measurement Model
Supplementary Table 1 shows the results of the LPA measurement model for cumulative immune response from the four infection indicators for a one, two, three, four and five class solution. The best class number solution was determined based on comparison of standard fit measures, including the Baysian Information Criterion (BIC), sample size adjusted BIC, the Lo, Mendell and Ruben log-likelihood ratio test, and the bootstrapped log-likelihood ratio test (BLRT), as well as judgment on the nature of the groups (mean antibody level) and their interpretability in relation to theory and previous research (Marsh et al., 2009). Fit statistics were used to first compare all possible solutions. The three and four class solutions emerged as suitable for the data as the Lo, Medell, Rubin LRT was not statistically significant for the five-class solution. Then, the mean IgG level values for each group were reviewed for the three class and four class solution. Upon considering the nature of the groups and their interpretability, as well as prior research, a three class solution was deemed most appropriate even though the Lo, Mendell, Rubin LRT and the BLRT suggested that the four class solution was a numerical improvement over the three class solution. Class membership for this solution is as follows: the low cumulative immune response group (N=55, 4%) was seronegative to CMV and T. gondii and had mean IgG antibody levels to HSV-1 and H. pylori in the lowest tertile, the middle cumulative immune response group (N=777, 61%) was seronegative to T. gondii and had mean CMV, HSV-1 and H. pylori IgG antibody levels in the middle tertile, and the high cumulative immune response group (N=431, 34%) had mean IgG antibody levels in the middle tertile to all infections. The mean IgG antibody level for each infection within each class group is reported in Supplementary Table 2. The three class solution had high entropy (0.88), indicating good class separation. Predicted class membership was extracted and then used as a nominal dependent variable in the SEM analysis with high cumulative immune response as the referent category.
Footnotes
Ethics Statement: The Sacramento Area Latino Study on Aging (SALSA) was approved by the Institutional Review Board at the University of Michigan and the University of California at San Francisco and Davis.
References
- Acock AC. Discovering Structural Equation Modeling Using Stat. College Station, TX: Stata Press; 2013. [Google Scholar]
- Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Aiello AE, Diez-Roux A, Noone AM, Ranjit N, Cushman M, Tsai MY, et al. Socioeconomic and psychosocial gradients in cardiovascular pathogen burden and immune response: the multi-ethnic study of atherosclerosis. Brain Behav Immun. 2009;23:663–671. doi: 10.1016/j.bbi.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello AE, Haan M, Blythe L, Moore K, Gonzalez JM, Jagust W. The influence of latent viral infection on rate of cognitive decline over 4 years. J Am Geriatr Soc. 2006;54:1046–1054. doi: 10.1111/j.1532-5415.2006.00796.x. [DOI] [PubMed] [Google Scholar]
- Amberbir A, Medhin G, Abegaz WE, Hanlon C, Robinson K, Fogarty A, et al. Exposure to Helicobacter pylori infection in early childhood and the risk of allergic disease and atopic sensitization: a longitudinal birth cohort study. Clin Exp Allergy. 2014;44:563–571. doi: 10.1111/cea.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asparouhov T, Muthen B. Auxiliary Variables in Mixture Modeling: Three-Step Approaches Using Mplus. Structural Equation Modeling-a Multidisciplinary Journal. 2014;21:329–341. [Google Scholar]
- Bate SL, Dollard SC, Cannon MJ. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis. 2010;50:1439–1447. doi: 10.1086/652438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31:285–293. [PubMed] [Google Scholar]
- D.o.V.D. National Center for Immunization and Resipratory Diseases, editor. Centers for Disease Control and Prevention. Cytomegalovirus and Congenitial CMV Infection. 2010. [Google Scholar]
- Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- Colugnati FA, Staras SA, Dollard SC, Cannon MJ. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect Dis. 2007;7:71. doi: 10.1186/1471-2334-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson FB, Boronow JJ, Stallings C, Origoni AE, Ruslanova I, Yolken RH. Association of serum antibodies to herpes simplex virus 1 with cognitive deficits in individuals with schizophrenia. Arch Gen Psychiatry. 2003;60:466–472. doi: 10.1001/archpsyc.60.5.466. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE. Socioeconomic differentials in immune response. Epidemiology. 2009;20:902–908. doi: 10.1097/EDE.0b013e3181bb5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE, Alley DE. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol Infect. 2009;137:58–65. doi: 10.1017/S0950268808000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Palermo TM, Aiello AE. Family poverty is associated with cytomegalovirus antibody titers in U.S. children. Health Psychol. 2012;31:5–10. doi: 10.1037/a0025337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draborg AH, Duus K, Houen G. Epstein-Barr virus in systemic autoimmune diseases. Clin Dev Immunol. 2013;2013:535738. doi: 10.1155/2013/535738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovainio M, Ferrie JE, Singh-Manoux A, Gimeno D, De Vogli R, Shipley M, et al. Organisational justice and markers of inflammation: the Whitehall II study. Occup Environ Med. 2010;67:78–83. doi: 10.1136/oem.2008.044917. [DOI] [PubMed] [Google Scholar]
- Espinola-Klein C, Rupprecht HJ, Blankenberg S, Bickel C, Kopp H, Rippin G, et al. Impact of infectious burden on extent and long-term prognosis of atherosclerosis. Circulation. 2002;105:15–21. doi: 10.1161/hc0102.101362. [DOI] [PubMed] [Google Scholar]
- Esposito S, Bosis S, Semino M, Rigante D. Infections and systemic lupus erythematosus. Eur J Clin Microbiol Infect Dis. 2014 doi: 10.1007/s10096-014-2098-7. [DOI] [PubMed] [Google Scholar]
- Fagundes CP, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Childhood adversity and herpesvirus latency in breast cancer survivors. Health Psychol. 2013;32:337–344. doi: 10.1037/a0028595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold A, Tiberio SS, Capaldi DM. New Approaches for Examining Associations With Latent Categorical Variables: Applications to Substance Abuse and Aggression. Psychology of Addictive Behaviors. 2014;28:257–267. doi: 10.1037/a0031487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan-Ramirez ML, Madriz Elisondo AL, Rico Torres CP, Luna-Pasten H, Rodriguez Perez LR, Rincon-Sanchez AR, et al. Frequency of Toxoplasma gondii in pork meat in Ocotlan, Jalisco, Mexico. J Food Prot. 2010;73:1121–1123. doi: 10.4315/0362-028x-73.6.1121. [DOI] [PubMed] [Google Scholar]
- Gilbert PB, Gabriel EE, Miao X, Li X, Su SC, Parrino J, et al. Fold rise in antibody titers by measured by glycoprotein-based enzyme-linked immunosorbent assay is an excellent correlate of protection for a herpes zoster vaccine, demonstrated via the vaccine efficacy curve. J Infect Dis. 2014;210:1573–1581. doi: 10.1093/infdis/jiu279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser J. Stress-associated immune modulation and its implications for reactivation of latent herepsviruses. In: Glaser R, Jones J, editors. Human herpesvirus infections. New York: Dekker; 1994. pp. 245–270. [Google Scholar]
- Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol. 2012;175:54–59. doi: 10.1093/aje/kwr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin DE. Measles virus-induced suppression of immune responses. Immunol Rev. 2010;236:176–189. doi: 10.1111/j.1600-065X.2010.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haan MN, Mungas DM, Gonzalez HM, Ortiz TA, Acharya A, Jagust WJ. Prevalence of dementia in older latinos: the influence of type 2 diabetes mellitus, stroke and genetic factors. J Am Geriatr Soc. 2003;51:169–177. doi: 10.1046/j.1532-5415.2003.51054.x. [DOI] [PubMed] [Google Scholar]
- Haan MN, Zeki Al-Hazzouri A, Aiello AE. Life-span socioeconomic trajectory, nativity, and cognitive aging in Mexican Americans: the Sacramento Area Latino Study on Aging. J Gerontol B Psychol Sci Soc Sci. 2011;66(Suppl 1):i102–110. doi: 10.1093/geronb/gbq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasni SA. Role of Helicobacter pylori infection in autoimmune diseases. Curr Opin Rheumatol. 2012;24:429–434. doi: 10.1097/BOR.0b013e3283542d0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis : a regression-based approach 2013 [Google Scholar]
- Itzhaki RF, Wozniak MA, Appelt DM, Balin BJ. Infiltration of the brain by pathogens causes Alzheimer's disease. Neurobiol Aging. 2004;25:619–627. doi: 10.1016/j.neurobiolaging.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Doyle WJ, Marsland AL, Bosch J. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain Behav Immun. 2014 doi: 10.1016/j.bbi.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care. 2012;35:520–525. doi: 10.2337/dc11-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Rivera HN, Price C, Wilkins PP. Toxoplasma gondii seroprevalence in the United States 2009-2010 and comparison with the past two decades. Am J Trop Med Hyg. 2014;90:1135–1139. doi: 10.4269/ajtmh.14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I, Quan T, Nolasco H, Park SH, Hong MS, Crouch J, et al. Defective control of latent Epstein-Barr virus infection in systemic lupus erythematosus. J Immunol. 2004;172:1287–1294. doi: 10.4049/jimmunol.172.2.1287. [DOI] [PubMed] [Google Scholar]
- Kuh D, Ben-Shlomo Y, editors. A Life Course Approach to Chronic Disease Epidemiology. New York, NY: Oxford University Press; 2004. [Google Scholar]
- Lina TT, Alzahrani S, Gonzalez J, Pinchuk IV, Beswick EJ, Reyes VE. Immune evasion strategies used by. World J Gastroenterol. 2014;20:12753–12766. doi: 10.3748/wjg.v20.i36.12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW. Life history, maintenance, and the early origins of immune function. Am J Hum Biol. 2005;17:81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- Miller-Kittrell M, Sparer TE. Feeling manipulated: cytomegalovirus immune manipulation. Virol J. 2009;6:4. doi: 10.1186/1743-422X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayyedi P, Axon AT, Feltbower R, Duffett S, Crocombe W, Braunholtz D, et al. Relation of adult lifestyle and socioeconomic factors to the prevalence of Helicobacter pylori infection. Int J Epidemiol. 2002;31:624–631. doi: 10.1093/ije/31.3.624. [DOI] [PubMed] [Google Scholar]
- Muthen LKMBO. Mplus Statistical Analysis with Latent Variables User's Guide. Los Angeles, CA: Muthen & Muthen; 2012. [Google Scholar]
- Nakata A. Psychosocial job stress and immunity: a systematic review. Methods Mol Biol. 2012;934:39–75. doi: 10.1007/978-1-62703-071-7_3. [DOI] [PubMed] [Google Scholar]
- Oakes JM, Kaufman JS. Methods in Social Epidemiology. San Francisco, CA: Jossey-Bass; 2006. [Google Scholar]
- Pawelec G, Koch S, Franceschi C, Wikby A. Human immunosenescence: does it have an infectious component? Ann N Y Acad Sci. 2006;1067:56–65. doi: 10.1196/annals.1354.009. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook GA. Hygiene hypothesis and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:5–15. doi: 10.1007/s12016-011-8285-8. [DOI] [PubMed] [Google Scholar]
- Rosenstock SJ, Andersen LP, Rosenstock CV, Bonnevie O, Jorgensen T. Socioeconomic factors in Helicobacter pylori infection among Danish adults. Am J Public Health. 1996;86:1539–1544. doi: 10.2105/ajph.86.11.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubicz R, Leach CT, Kraig E, Dhurandhar NV, Grubbs B, Blangero J, et al. Seroprevalence of 13 common pathogens in a rapidly growing U.S. minority population: Mexican Americans from San Antonio, TX. BMC Res Notes. 2011;4:433. doi: 10.1186/1756-0500-4-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillinger JA, Xu F, Sternberg MR, Armstrong GL, Lee FK, Nahmias AJ, et al. National seroprevalence and trends in herpes simplex virus type 1 in the United States, 1976-1994. Sex Transm Dis. 2004;31:753–760. doi: 10.1097/01.olq.0000145852.43262.c3. [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Aiello AE. Persistent pathogens linking socioeconomic position and cardiovascular disease in the US. Int J Epidemiol. 2009;38:775–787. doi: 10.1093/ije/dyn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One. 2011;6:e16103. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Zajacova A, Aiello AE. Unpacking the ‘black box’ of total pathogen burden: is number or type of pathogens most predictive of all-cause mortality in the United States? Epidemiol Infect. 2014:1–11. doi: 10.1017/S0950268814003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorlie PD, Nieto FJ, Adam E, Folsom AR, Shahar E, Massing M. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch Intern Med. 2000;160:2027–2032. doi: 10.1001/archinte.160.13.2027. [DOI] [PubMed] [Google Scholar]
- Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE, Jr, Cannon MJ. Cytomegalovirus seroprevalence and childhood sources of infection: A population-based study among pre-adolescents in the United States. J Clin Virol. 2008;43:266–271. doi: 10.1016/j.jcv.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Strauss EG, Strauss JH. Viruses and Human Disease. Boston, M.A: Elsevier; 2008. [Google Scholar]
- Torrey EF, Yolken RH. Toxoplasma oocysts as a public health problem. Trends Parasitol. 2013;29:380–384. doi: 10.1016/j.pt.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–479. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF, Lieberman JA, Yang S, Dickerson FB. Serological evidence of exposure to Herpes Simplex Virus type 1 is associated with cognitive deficits in the CATIE schizophrenia sample. Schizophr Res. 2011;128:61–65. doi: 10.1016/j.schres.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Zajacova A, Dowd JB, Aiello AE. Socioeconomic and race/ethnic patterns in persistent infection burden among U.S. adults. J Gerontol A Biol Sci Med Sci. 2009;64:272–279. doi: 10.1093/gerona/gln012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Haan MN, Kalbfleisch JD, Galea S, Lisabeth LD, Aiello AE. Life-course socioeconomic position and incidence of dementia and cognitive impairment without dementia in older Mexican Americans: results from the Sacramento area Latino study on aging. Am J Epidemiol. 2011;173:1148–1158. doi: 10.1093/aje/kwq483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, et al. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Diagram of SEM for standardized direct and indirect effects of early life SEP on later life immune response to CMV, adjusted for age and gender, N=1562
Supplemental Figure 2. Diagram of SEM for standardized direct and indirect effects of early life SEP on later life immune response to T. gondii, adjusted for age and gender, N=1562
Supplemental Figure 3. Diagram of SEM for standardized direct and indirect effects of early life SEP on later life immune response to H. pylori, adjusted for age and gender, N=1562
Supplemental Figure 4. Log Odds (SE) for SEM Model of Low v. High Cumulative Immune Response adjusted for age and gender, N= 1263
Supplemental Figure 5. Log Odds (SE) for SEM Model of Middle v. High Cumulative Immune Response adjusted for age and gender, N= 1263
Supplemental Table1. Measurement model for early life SEP, model fit: RMSEA=0.05, CFI=0.94
Supplemental Table 2. Measurement models for cumulative immune response from LPA with log continuous IgG level indicators for four infections (n=1263)
Supplemental Table 3. Mean IgG levels for the cumulative immune response three class measurement model solution (n=1263) Mean IgG Level (ODU)
Supplemental Table 4. Standardized direct and indirect effects of SEP on later life immune response to T. gondii controlling for age, sex and nativity, N=1562


