Abstract
Background
There is significant debate over whether written consent is necessary for low-risk, pragmatic randomized controlled trials (RCT).
Objective
To assess the U.S. public’s views regarding alternatives to written consent for low-risk pragmatic RCTs.
Design
National experimental survey (2-by-2 factorial design) examining support for written consent versus general notification or verbal consent in two research scenarios.
Setting
Web-based survey conducted in December 2014.
Participants
2130 U.S. adults sampled from a nationally representative probability-based online panel (response rate, 64%).
Measurements
Respondent’s recommendation to an ethics review board and personal preference as a potential participant for how to obtain consent/notification in the two research scenarios.
Results
A majority in each of the four arms (ranging from 60.3% to 71.5%) recommended written informed consent. Personal preferences generally tracked that advice. Most (78.9%) believed the pragmatic RCTs did not pose additional risks but 62.5% of these respondents would still recommend written consent. In contrast, a substantial minority in all arms (28.5% to 39.7%) recommended the alternative option (general notification or verbal consent) over written consent.
Limitations
Framing effects could impact respondents’ attitudes and non-respondents may differ in levels of trust towards research or healthcare institutions.
Conclusions
A majority of the public endorsed written informed consent over the most widely considered alternatives for low-risk pragmatic RCTs; however, a substantial minority endorsed general notification or verbal consent.
Primary Funding Source
Time-sharing Experiments in the Social Sciences and Intramural Research Program of the National Institutes of Health, Clinical Center.
Introduction
The learning healthcare system, which envisions research as a routinely integrated part of clinical practice, has the promise to improve the quality of patient care and reduce costs (1). Such research would include pragmatic randomized controlled trials (RCTs) evaluating standard-of-care interventions whose true comparative effectiveness is unknown (2) and may involve little added risk compared to normal clinical practice. Some commentators argue that written informed consent is therefore not ethically necessary and that it would make integration of research and clinical practice difficult due to the added cost, disruption of usual clinical practice, and selection bias (2–3). Instead, they argue that patients could be informed through regular notifications that the institution conducts pragmatic RCTs (4–6). However, critics respond that this approach is ethically inappropriate and may undermine public support for clinical research (7). Recent draft guidance from the U.S. Office for Human Research Protections on what counts as “reasonably foreseeable risks” that must be disclosed to research participants has further complicated this debate (8).
A recent survey by Cho et al. (9) found that most people wanted to be asked for permission to participate in pragmatic RCTs, although most would also accept verbal consent or general notification if written consent would make the research too difficult to carry out. However, the generalizability of the Cho et al. study is uncertain because it did not use a probability-based sample. Our survey evaluated the views of U.S. public regarding informed consent for pragmatic RCTs with a nationally representative probability-based sample.
Methods
Design Overview
We conducted a nationally representative online survey of the U.S. public between December 12, 2014 and December 29, 2014. Our survey assessed respondents’ attitudes towards consent procedures in two scenarios of possible pragmatic trials. This study was reviewed and deemed exempt by the National Institutes of Health Clinical Center’s Office of Human Subjects Research Protection.
Setting and Participants
We used the GfK KnowledgePanel, a probability-based online panel of adults aged 18 years or older, designed to be representative of the civilian noninstitutionalized U.S. population. The KnowledgePanel consists of about 55,000 active panel members recruited through a combination of probability-based random-digit dialing and address-based sampling to create a nationally representative panel covering 97% of households (10). When recruited, non-internet households receive a laptop and Internet service so that they can participate on online surveys. Panelists generally receive only non-survey-specific incentives through a points-based reward program amounting to approximately $4 to $6 per month. The panel has been shown to produce estimates similar to those derived from random-digit dialing telephone surveys (11–12). It is used by the National Science Foundation for its grant program involving general population experiments (13) and has been previously used to measure the public’s preferences for informed consent in clinical and research settings (14–17).
Survey Development, Options, and Administration
To develop the survey, we conducted two pretesting sessions during Empirical Research Laboratory meetings in the Department of Bioethics, National Institutes of Health and two pilot surveys (N = 101) using Amazon’s Mechanical Turk (MTurk) platform, an online, opt-in convenience sample, to ensure comprehension of the survey’s content and to obtain open-ended feedback. The use of the MTurk platform to conduct social science research has been described elsewhere (18–20). We found high levels of comprehension, with most respondents correctly answering seven true/false questions about the scenario (ranging from 88% to 100%, including the seriousness of hypertension, similarities between the treatment options, that the patient’s treatment can be changed during the study if necessary, that under general notification patients would be enrolled without being informed of the specific study, etc.). Finally, we revised our study based on critical feedback from two anonymous peer reviewers for the Time-sharing Experiments in the Social Sciences (13).
The survey (see Supplement) was designed to evaluate individuals’ views regarding consent for pragmatic RCTs with low added risks compared to standard clinical care. Key elements of the survey are described in Appendix Figure 1. Panel members were randomly assigned to receive one of two research scenarios describing a pragmatic RCT. All respondents received a vignette describing a learning healthcare system that regularly integrates research as part of providing care. We then told respondents that hypertension treatment is an area of interest for such healthcare systems given the high prevalence of hypertension in the population and serious complications associated with uncontrolled hypertension. Respondents then received one of two research scenarios describing a pragmatic RCT comparing hypertension treatment options.
RCT Scenarios
The first scenario described a Drug RCT comparing two commonly used, U.S. Food and Drug Administration approved, first-line antihypertensives with similar side-effects. The examples were based on the diuretics chlorthalidone and hydrochlorothiazide (6), although hypothetical drug names (“CTD” and “TRT”) were used to prevent response bias among those familiar with either drug.
The second scenario, intended to signal an even lower risk study and a less intrusive-seeming research intervention to a lay audience, described a Dose Timing RCT which tested the options of telling patients to take their once-daily anti-hypertensive in the morning versus at night. Respondents in this scenario were told that physicians generally do not tell patients when to take these medications. The research scenarios, as provided to survey respondents, are described in greater detail in Appendix Figure 1.
Consent options
The respondents in each RCT scenario were then randomized to a choice between written informed consent versus general notification or to a choice between written informed consent versus brief verbal consent (Appendix Figure 1). The options were described as follows:
Written consent
Contains the eight elements required by the Federal Common Rule (45 C.F.R. 46) (21). It was noted that written consent would require extra time and effort from the clinic staff and the patient, making it difficult to integrate the research as part of usual care, and, in some cases, the study would not be conducted.
General notification
All patients receive notifications (through posters, brochures, and letters) that the healthcare system regularly conducts research and eligible patients would be automatically enrolled in the study without being specifically asked if they would like to participate.
Verbal consent
The patient’s doctor would briefly explain the study, emphasize that the treatment selection would be random, and record the patient’s decision in the medical record.
Survey Administration
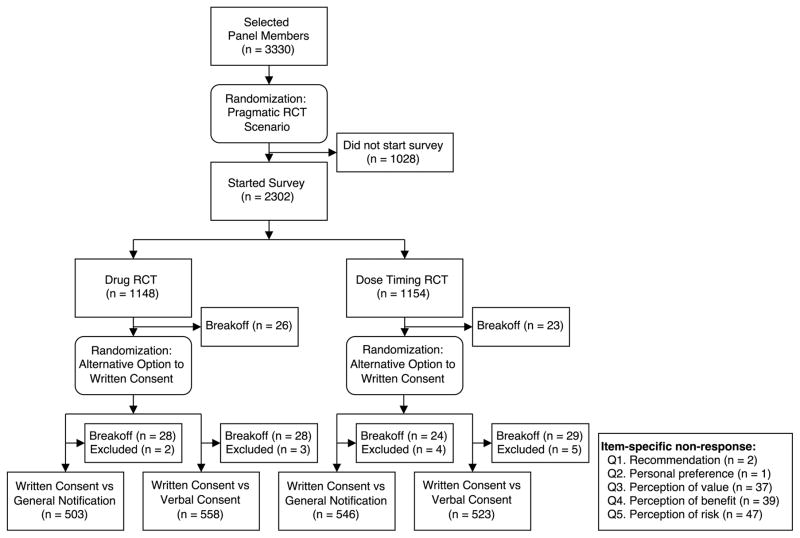
Panel members were randomly assigned to the Drug RCT or the Dose Timing RCT when they were invited to participate in the survey; respondents were further randomized to the alternative option (general notification or verbal consent) when they reached that part of the survey (Figure 1). The randomizations were computer generated and completely concealed from respondents. Simple probability-based assignment was used for randomization and neither stratification nor an imbalanced allocation scheme was used. To minimize missing data, respondents received a prompt if the main outcome measures were left blank. Because the panel’s policy is not to force respondents to answer specific questions, we decided a priori to exclude respondents who did not answer both main outcome measures.
Figure 1. Survey Design Administration.
The survey had a 2-by-2 factorial design. Panelists were randomized to receive either the Drug RCT Scenario or the Dose Timing RCT scenario when they were selected to participate in the study. Respondents were further randomized to the alternative option (general notification or verbal consent) to written consent when they arrived at that section of the study. 158 panelists started the survey but did not submit. Of those, 82 broke off in the Drug RCT scenario (26 before being randomized to the alternative option, 28 after being randomized to general notification, and 28 after being randomized to verbal consent), while 76 broke off in the Dose Timing RCT scenario (23 before being randomized to the alternative option, 24 after being randomized to general notification, and 29 after being randomized to verbal consent). 14 respondents were excluded from the analysis for nonresponse to our two primary outcome measures, ranging from 2 to 5 respondents in each arm.
Outcome and Measurements
Two questions were used to evaluate which of the pairwise consent options respondents endorsed. First, we described a debate among members of the ethics review board over how to notify or obtain consent. The primary outcome measure was respondents’ recommendation to the ethics review board: “If you were to give advice to the ethics review board, would you recommend Written Consent or [General Notification/Verbal Consent]?” The secondary outcome was respondents’ preference: “If you were a patient in this health care system, which would you personally prefer, Written Consent or [General Notification/Verbal Consent]?” Responses to both questions were measured on a four-point scale (“definitely” or “probably” for each response option).
Additionally, respondents were asked to evaluate the study’s value (“It is valuable to study whether one treatment option is more effective than the other for the treatment of high blood pressure”), risks to research participants (“Patients who participate in the randomized study face greater risks than patients who receive usual care”), and benefits to research participants (“Patients who participate in the randomized study are more likely to improve [lower] their high blood pressure than patients who receive usual care”). Response options were measured on a seven-point scale (1 = strongly disagree, 4 = neutral, 7 = strongly agree).
Statistical Analysis
We estimated that a sample size of 400 respondents in each arm of the survey would provide 80% power to detect a 10% absolute difference, for all baseline levels of support, assuming an alpha level of 0.05 (two-sided).
We dichotomized recommendations to the ethics review board and personal preferences as either in support of written consent versus support of the alternative approach. Logistic regression models were used to assess whether the research scenario and alternative consent/notification option were associated with respondents’ recommendations and personal preferences. The models included main effects for the research scenario (Drug RCT versus Dose Timing RCT) and the alternate option (general notification versus verbal consent), as well as the interaction of the two factors. The relationship between respondents’ recommendation to the ethics review board and their own personal preference was evaluated using conditional logistic regression. The effect of the research scenario on respondents’ perceptions of the study’s value, risk, and benefit, was assessed using ordered logistic regression. The association between respondents’ perceptions of the study’s risk and support for the alternative option was tested using Pearson’s χ2 test of independence corrected for the survey design.
Analysis was conducted using Stata version 11.2 (StataCorp). All analyses were conducted using Stata survey commands with post-stratification weights to account for survey nonresponse and noncoverage. Weights were provided by GfK. Statistical significance was defined as P < 0.05 and all tests were two-sided.
Role of Funding Source
This study was supported in part by the Intramural Research Program of the National Institutes of Health, Clinical Center. Funding for the data collection and survey administration was provided by Time-sharing Experiments in the Social Sciences (TESS), National Science Foundation Grant 0818839 (Jeremy Freese, PhD, and James Druckman, PhD, principal investigators). The funding sources did not participate in the design, conduct, or analysis of the study or in the decision to submit the manuscript for publication.
Results
Respondent Characteristics
The survey was sent to 3330 panel members, 2302 respondents started the survey, and 2144 respondents completed it. After excluding 14 individuals for nonresponses to both of our main outcome items, our final analysis included 2130 respondents (64.0% response rate) (Figure 1). Respondents were older (median, 51 years among responders vs 47 years for the nationally representative sample) and more commonly identified as non-Hispanic white (74.5% vs 65.5%).
25.3% (585 of 2054) reported a diagnosis of hypertension and 21.3% (501 of 2054) reported taking prescription medications for hypertension. These findings are similar to the national prevalence and treatment rates for hypertension (22). Respondent characteristics are show in Table 1.
Table 1.
Characteristics of survey respondents
| Overall (N = 2,130) | Drug RCT | Dose Timing RCT | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| General Notification (N = 503) | Verbal Consent (N = 558) | General Notification (N = 546) | Verbal Consent (N = 523) | |||
|
| ||||||
| Count | % | % | % | % | % | |
| Age, y (N = 2130) | ||||||
| 18–24 | 204 | 12.5 | 11.9 | 13.7 | 11.8 | 12.5 |
| 25–34 | 298 | 16.6 | 16.4 | 17.2 | 16.4 | 16.5 |
| 35–44 | 319 | 17.6 | 16.3 | 15.9 | 19.6 | 18.6 |
| 45–54 | 368 | 16.2 | 17.9 | 18.8 | 14.4 | 13.7 |
| 55–64 | 461 | 18.5 | 18.9 | 15.8 | 18.5 | 21.1 |
| 65–74 | 349 | 13.6 | 13.3 | 13.3 | 14.3 | 13.5 |
| 75 and over | 131 | 5.0 | 5.3 | 5.4 | 5.0 | 4.1 |
| Sex (N = 2130) | ||||||
| Male | 1,061 | 48.2 | 46.3 | 49.3 | 47.4 | 49.7 |
| Female | 1,069 | 51.8 | 53.7 | 50.7 | 52.6 | 50.3 |
| Region (N = 2130) | ||||||
| Northeast | 397 | 18.2 | 20.3 | 17.9 | 17.4 | 17.5 |
| Midwest | 502 | 21.4 | 20.8 | 22.5 | 21.1 | 20.8 |
| South | 770 | 37.1 | 38.9 | 35.5 | 35.1 | 39.1 |
| West | 461 | 23.4 | 20.0 | 24.1 | 26.4 | 22.5 |
| Race (N = 2130) | ||||||
| White, non-Hispanic | 1,587 | 65.5 | 72.4 | 61.6 | 65.1 | 63.8 |
| Black, non-Hispanic | 185 | 11.6 | 8.6 | 13.1 | 11.4 | 12.7 |
| Other, non-Hispanic | 91 | 6.5 | 5.1 | 7.9 | 7.0 | 5.6 |
| Hispanic | 203 | 15.2 | 12.5 | 16.4 | 15.4 | 16.2 |
| 2+ races, non-Hispanic | 64 | 1.3 | 1.3 | 1.1 | 1.1 | 1.7 |
| Marital Status (N = 2130) | ||||||
| Married | 1,252 | 54.1 | 56.5 | 50.4 | 55.2 | 55.0 |
| Widowed | 85 | 3.7 | 3.5 | 3.5 | 3.6 | 4.3 |
| Divorced | 192 | 8.7 | 7.2 | 8.5 | 9.4 | 9.8 |
| Separated | 27 | 1.6 | 2.3 | 1.2 | 0.9 | 2.3 |
| Never Married | 422 | 24.0 | 21.4 | 27.8 | 24.1 | 22.1 |
| Living with partner | 152 | 7.8 | 9.2 | 8.6 | 6.8 | 6.6 |
| Annual Household Income (N = 2130) | ||||||
| <$25,000 | 329 | 17.9 | 16.0 | 21.5 | 17.7 | 15.9 |
| $25,000–$50,000 | 489 | 22.5 | 21.5 | 23.2 | 22.2 | 22.8 |
| $50,000–$75,000 | 391 | 18.4 | 19.5 | 18.2 | 17.8 | 18.4 |
| $75,000+ | 921 | 41.2 | 43.0 | 37.1 | 42.4 | 43.0 |
| Employment Status (N = 2130) | ||||||
| Employed | 1203 | 56.7 | 56.3 | 55.5 | 60.4 | 54.6 |
| Retired | 480 | 18.6 | 19.0 | 16.9 | 18.8 | 19.7 |
| Disabled | 146 | 7.5 | 8.8 | 8.2 | 6.9 | 6.4 |
| Unemployed or other | 301 | 17.2 | 15.9 | 19.5 | 14.0 | 19.3 |
| Education (N = 2130) | ||||||
| <High School | 193 | 12.4 | 10.4 | 15.9 | 11.8 | 10.9 |
| Completed HS | 643 | 29.7 | 31.2 | 27.8 | 29.9 | 29.9 |
| Some College | 610 | 28.7 | 30.5 | 27.7 | 28.5 | 28.6 |
| Bachelors | 398 | 16.8 | 14.5 | 16.6 | 16.9 | 19.2 |
| Post-graduate | 286 | 12.4 | 13.3 | 12.0 | 12.9 | 11.5 |
| Religious Attendance (N = 2127) *, † | ||||||
| Regularly | 860 | 39.7 | 39.1 | 38.6 | 38.8 | 42.3 |
| Rarely | 717 | 33.9 | 32.3 | 34.0 | 37.2 | 32.0 |
| Never | 550 | 26.4 | 28.6 | 27.4 | 24.0 | 25.8 |
| Ideology (N = 2122) *, ‡ | ||||||
| Liberal | 572 | 27.8 | 25.7 | 28.0 | 26.3 | 31.1 |
| Moderate | 786 | 38.6 | 43.6 | 37.1 | 37.7 | 36.5 |
| Conservative | 764 | 33.6 | 30.7 | 34.9 | 36.0 | 32.4 |
| Diagnosed with hypertension (N = 2054) * | ||||||
| No | 1,469 | 74.6 | 74.9 | 73.6 | 72.7 | 77.7 |
| Yes | 585 | 25.3 | 25.1 | 26.4 | 27.3 | 22.3 |
| Prescription treatment for hypertension (N = 2054) * | ||||||
| No | 1553 | 78.7 | 78.4 | 77.5 | 76.8 | 82.2 |
| Yes | 501 | 21.3 | 21.6 | 22.5 | 23.3 | 17.8 |
Excludes respondents who did not answer the item.
Religious attendance was recategorized as the following: regularly = “more than once a week,” “once a week,” and “once or twice a month;” rarely = “a few times a year” and “once a year or less;” never = “never.”
Political ideology was recategorized from a seven-point scale (“extremely liberal” to “extremely conservative”).
Recommendations to Ethics Review Board
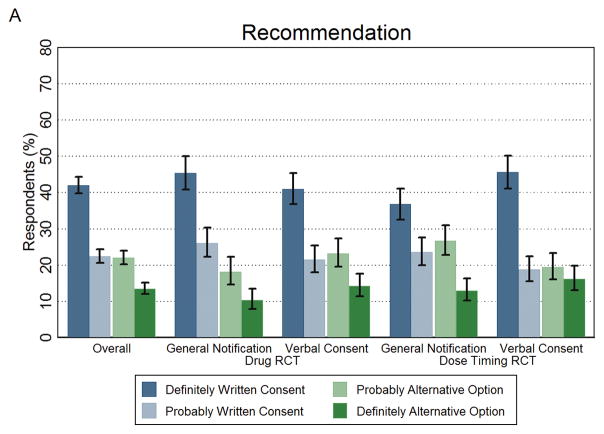
Overall, 64.4% (95% CI, 62.2%–66.6%) of respondents would (definitely or probably) recommend that the ethics committee use traditional written informed consent (Figure 2A and Appendix Table 1). In the group that received the Drug RCT scenario, 28.5% (95% CI, 24.4%–33.0%) recommended general notification over traditional written consent while 37.5% (95% CI, 33.3%–41.9%) recommended verbal consent over traditional written consent. Among respondents who received the Dose Timing RCT scenario, 39.7% (95% CI, 35.3%–44.2%) recommended general notification and 35.7% (95% CI, 31.4%–40.1%) recommended verbal consent over traditional written informed consent.
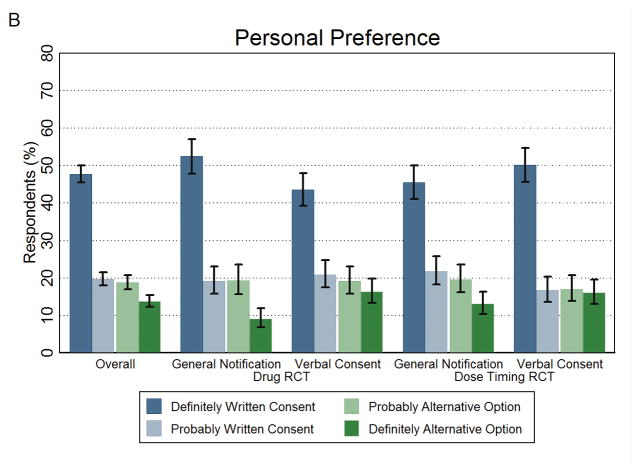
Figure 2. Recommendations to Ethics Review Board and Personal Preferences for Written Consent and the Alternative Option.
The public’s recommendation to the ethics review board and their personal preference as a patient in the healthcare system on how to obtain consent. Panel A shows the percentage of respondents who recommended written consent (definitely or probably) or the alternative option (definitely or probably). Panel B shows the percentage of respondents who personally preferred written consent (definitely or probably) or the alternative option (definitely or probably). The first set of results in both panels represents the overall study findings across the four arms. Error bars represent 95% confidence intervals.
Across all arms, 67.4% (95% CI, 65.3%–69.5%) of respondents would personally prefer written informed consent as a patient in the healthcare system (Figure 2B and Appendix Table 1). Figure 3 shows respondents’ support for the alternative option in their recommendations to the ethics review board and personal preferences for each arm of the survey. Responses to the two items were highly consistent across all arms, with most (ranging from 86.8% to 92.5%) having the same recommendation and personal preference (Appendix Table 2). Only respondents in the Dose Timing RCT scenario personally preferred general notification at a significantly lower rate than they recommended it to ethics review board (32.7% vs 39.7%, P = 0.001).
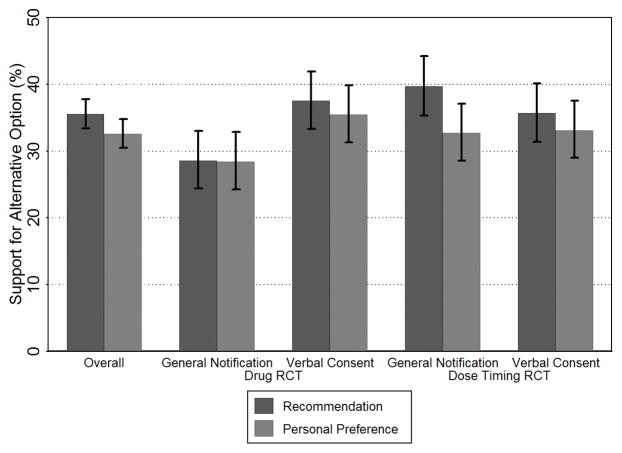
Figure 3. Support for the Alternative Options to Written Consent.
The percentage of respondents who indicated support for the alterative option (combined definitely or probably) over written consent in terms of their recommendation to the ethics review board and personal preference as patients in the healthcare system. The first set of results represents the overall study findings across the four arms. Error bars represent 95% confidence intervals.
A logistic regression model was used to test the effect of the experimental design of the survey on recommendations for using the alternative option over written consent. We found the effect of the alternative consent/notification option varied by RCT scenario (interaction, P = 0.003) (Figure 3). Respondents who received the Dose Timing RCT scenario were more likely than those who received the Drug RCT scenario to recommend general notification over written consent (39.7% vs 28.5%, P = 0.001). Similarly, respondents in the Drug RCT scenario were more likely to recommend the alternative option if they received the option of conducting the study with verbal consent compared to general notification (37.5% vs 28.5%, P = 0.004).
Similar analysis for respondents’ personal preferences found that those receiving the verbal consent option were more likely than those receiving general notification to personally prefer forgoing written consent (P = 0.023). The effects of the RCT scenario and the interaction between the RCT scenario and alternative consent/notification option on respondents’ preferences were not significant (Dose Timing RCT versus Drug RCT (reference), P = 0.164; interaction: Dose Timing RCT and verbal consent, P = 0.123).
Views of Research Scenarios
Large majorities (72.1% to 72.3%, depending on scenario) agreed that it is valuable to conduct the research, with no differences in perception between the scenarios (Table 2). A minority (19.5% to 22.7%) agreed that participants in the RCTs faced greater risks than patients receiving usual care, with those in the Drug RCT arm more likely to view the research as being riskier than usual care (P = 0.043). Few (17.2% to 18.0%) thought research participants would benefit more compared to usual care.
Table 2.
Public’s views on statements of the pragmatic RCT scenario’s social value, risk, and benefit
| Statement | Research Scenario | Disagree 1–3 | Neutral4 | Agree 5–7 | P Value* |
|---|---|---|---|---|---|
| “It is valuable to study whether one treatment option is more effective than the other for the treatment of high blood pressure” (N = 2093) | Drug RCT (n = 1032) | 5.8 | 21.9 | 72.3 | 0.80 |
| Dose Timing RCT (n = 1061) | 7.3 | 20.6 | 72.1 | ||
| “Patients who participate in the randomized study face greater risks than patients who receive usual care” (N = 2091) | Drug RCT (n = 1032) | 35.1 | 42.2 | 22.7 | 0.043 |
| Dose Timing RCT (n = 1059) | 38.9 | 41.6 | 19.5 | ||
| “Patients who participate in the randomized study are more likely to improve (lower) their high blood pressure than patient who receive usual care.” (N = 2083) | Drug RCT (n = 1030) | 29.2 | 53.6 | 17.2 | 0.062 |
| Dose Timing RCT (n = 1053) | 24.5 | 57.5 | 18.0 |
Ordered logistic regression model used to test relationship between respondents’ views (trichotimized ranking) and the research scenario.
Relationship between Risk Perception and Recommendations to the Ethics Review Board
Overall, respondents who agreed with the statement that study participants face greater risks than patients receiving usual care were less likely to recommend the alternative option to written consent (27.7%) than those who were neutral (32.5%) or disagreed (43.1%). This relationship between risk perception and recommendation to the ethics review board was individually observed in each arm of the survey (Appendix Table 3).
Discussion
Pragmatic RCTs have significant potential to improve medical care. However, there is concern that mandating written informed consent may undermine this value and preclude some studies altogether (23–24). To address these concerns, some commentators have proposed waiving written consent for studies that pose low risk compared to standard clinical care (6–7). Our study aimed to measure the public’s support for this approach (5).
The results suggest that a majority (ranging from 60.3% to 71.5% across all arms) of the public endorses written informed consent over the most widely advocated alternatives, verbal consent and general notification. This finding is important given that most respondents thought that the RCTs were valuable to conduct (72.2%), were neutral or recognized that participation did not confer additional personal benefits (82.4%), and were neutral or recognized that participation posed no additional risks (78.9%). This latter finding contradicts the claim that individuals “automatically” assume RCTs are riskier than standard clinical care (23). Importantly, a clear majority (62.5%) of those who recognized that the studies did not pose added risks nonetheless recommended written consent.
We hypothesized that the Dose Timing RCT scenario would be perceived as a more innocuous intervention for a lay audience and thus would garner more support for the alternative consent options. Although respondents were indeed more likely to recommend the use of the alternate consents in the Dose Timing RCT scenario (37.7%, average of the two alternative consents) versus the Drug RCT scenario (33.4%), the effect was small. We also hypothesized that respondents would favor verbal consent more often than general notification, since it retains crucial elements of informed consent (e.g., informing patients about the study and asking for their consent for participation). However, this was true only in the Drug RCT scenario (37.5% vs 28.5%).
Although the majority of respondents endorsed written consent, the fact that approximately a third preferred the alternative approach over written consent as a recommendation to the ethics review board (ranging 28.5% to 39.7%) and as a potential patient-participant (ranging 28.4% to 35.5%) is remarkable. For example, nearly 40% would recommend to the ethics review board general notification without specific consent for the Dose Timing RCT as the better option. This might be an underestimate since the survey portrayed RCTs in a generic learning healthcare system and not in the respondent’s own healthcare system or physician’s clinic. Additionally, it is likely that in practice verbal consent would be used in conjunction with general notification, and employing them together may have a greater effect.
Our findings are largely, if not entirely, consistent with the recent study by Cho et al. (9). In both studies the public’s support for comparative effectiveness research is high. However, most respondents wanted written consent before being enrolled in specific studies. Cho et al. found that about half of respondents preferred written consent whereas our survey found about two-thirds preferred written consent. The difference may be reflective of the Cho et al. study providing additional options for respondents to choose from or a frame of the research being conducted in the respondent’s own healthcare system. The difference may also be due to a higher selection bias in their non-probability-based sample.
Our study has several limitations. First, the response rate was 64.0%. Post-stratification weights mitigate some aspects of nonresponse bias, but cannot account for systematic differences between respondents and non-respondents, such as levels of trust towards research or healthcare institutions. Second, respondents received only brief descriptions of the research scenarios and consent options. Third, framing effects could impact respondents’ attitudes as the issues examined in this survey have not received much public deliberation. For example, we presented written informed consent as a burdensome but feasible option and required a choice of preference for one option over another. Had we presented the choice as between conducting the RCTs with an alternative consent option versus not being able to conduct the RCTs with written consent, the results most likely would have been different, as was found in the study by Cho et al. (9). Additionally, the scenarios, especially the Dose Timing RCT study, were intentionally designed to signal low risk for the purpose of this survey. Such research scenarios are likely to be more complicated in reality. Fourth, since we did not test support for general notification and verbal consent head-to-head within subjects, our findings cannot be interpreted as a direct policy preference (or indifference) among the public between verbal consent and general notification.
Our findings have two potential policy implications. First, since currently a majority of the public prefer study specific written informed consent, adoption of alternative consent options for pragmatic RCTs without extensive education would be premature and could undermine public support for this very important research. Thus, at present, rather than bypassing written informed consent, it will be important to consider ways to make such consent more efficient and less burdensome. In particular, formal written consent for pragmatic RCTs may be able to meet the federal regulatory requirements without being excessively long and time intensive (25). Second, a substantial minority of respondents saw general notification or verbal consent as better options than written consent in certain situations. It is possible that further education and public discussion about risks involved in pragmatic trials of standard therapies could lead to greater acceptance of such alternatives.
Supplementary Material
Supplement. Survey Questionnaire
Appendix Figure 1. Experimental design of survey
Shows the 2-by-2 factorial design and information presented to respondents. About half received a Drug RCT scenario comparing two first-line drugs; the others received a Dose Timing RCT scenario comparing morning versus night dosing. About half of each group compared written consent to general notification; the rest compared written consent to verbal consent.
Acknowledgments
We thank the two anonymous TESS reviewers who gave critical feedback on the design of the survey and Robert Wesley, PhD, at the National Institutes of Health for helping us with the statistical interpretation of some findings.
Funding: This research was supported in part by the Intramural Research Program of the National Institutes of Health, Clinical Center. Data collection was provided by Time-sharing Experiments in the Social Sciences (TESS), National Science Foundation Grant 0818839 (Jeremy Freese, PhD, and James Druckman, PhD, principal investigators). TESS provided data collection using the GfK KnowledgePanel; GfK provided survey weights for matching participants’ responses to the U.S. Current Population Survey.
Appendix
Appendix Table 1.
Recommendations to the ethics review board and personal preferences on how to obtain consent.
| Definitely Written Consent % (95% CI) | Probably Written Consent % (95% CI) | Probably Alternative Option % (95% CI)* | Definitely Alternative Option % (95% CI)* | |
|---|---|---|---|---|
| Recommendation to ethics review board | ||||
| Overall (N = 2128) | 42.0 (39.8 – 44.3) | 22.4 (20.6 – 24.4) | 22.0 (20.2 – 24.0) | 13.5 (12.0 – 15.2) |
| Drug RCT | ||||
| General Notification (n = 503) | 45.4 (40.8 – 50.0) | 26.1 (22.3 – 30.3) | 18.1 (14.6 – 22.3) | 10.4 (7.9 – 13.5) |
| Verbal Consent (n = 558) | 41.0 (36.7 – 45.4) | 21.5 (18.0 – 25.4) | 23.3 (19.6 – 27.3) | 14.3 (11.4 – 17.7) |
| Dose Timing RCT | ||||
| General Notification (n = 544) | 36.8 (32.6 – 41.1) | 23.6 (19.9 – 27.7) | 26.7 (22.9 – 31.0) | 13.0 (10.2 – 16.4) |
| Verbal Consent (n = 523) | 45.6 (41.1 – 50.2) | 18.8 (15.5 – 22.5) | 19.5 (16.1 – 23.4) | 16.2 (13.0 – 19.9) |
| Personal preference | ||||
| Overall (N = 2129) | 47.7 (45.5 – 50.0) | 19.7 (18.0 – 21.5) | 18.8 (17.0 – 20.7) | 13.8 (12.3 – 15.4) |
| Drug RCT | ||||
| General Notification (n = 503) | 52.4 (47.8 – 57.0) | 19.2 (15.9 – 23.1) | 19.3 (15.7 – 23.6) | 9.1 (6.9 – 11.9) |
| Verbal Consent (n = 558) | 43.6 (39.3 – 48.0) | 20.9 (17.5 – 24.8) | 19.2 (15.8 – 23.0) | 16.3 (13.3 – 19.8) |
| Dose Timing RCT | ||||
| General Notification (n = 545) | 45.5 (41.1 – 50.0) | 21.8 (18.3 – 25.8) | 19.7 (16.2 – 23.6) | 13.1 (10.3 – 16.4) |
| Verbal Consent (n = 523) | 50.2 (45.7 – 54.7) | 16.7 (13.6 – 20.3) | 17.0 (13.9 – 20.7) | 16.1 (13.1 – 19.6) |
General notification or verbal consent was given as the alternative option based on the arm of the study.
Appendix Table 2.
Cross-tabulation of respondents’ recommendation to ethics review board and personal preferences.
| Overall (N = 2127) | Drug RCT | Dose Timing RCT | |||
|---|---|---|---|---|---|
|
| |||||
| General Notification (N = 503) | Verbal Consent (N = 558) | General Notification (N = 543) | Verbal Consent (N = 523) | ||
|
| |||||
| % | % | % | % | % | |
| Recommend Written Consent; Prefer Written Consent | 60.6 | 67.8 | 57.1 | 57.3 | 61.0 |
| Recommend Written Consent; Prefer Alternative Option | 3.9 | 3.7 | 5.4 | 3.2 | 3.3 |
| Recommend Alternative Option; Prefer Written Consent | 6.9 | 3.8 | 7.4 | 10.0 | 5.9 |
| Recommend Alternative Option; Prefer Alternative Option | 28.6 | 24.7 | 30.1 | 29.5 | 29.8 |
Appendix Table 3.
Percentage of respondents recommending the alternative option based on perception of study’s risk.
| Perception of study’s risk (“Patients who participate in the randomized study face greater risks than patients who receive usual care”) | All Respondents % (95% CI) | P Value* | |||
|---|---|---|---|---|---|
|
| |||||
| Disagree 1–3 % (95% CI) |
Neutral 4 % (95% CI) |
Agree 5–7 % (95% CI) |
|||
| Overall (N =2090) | 43.1 (39.6 – 46.7) | 32.5 (29.2 – 36.0) | 27.7 (23.2 – 32.7) | 35.4 (33.2 – 37.7) | < 0.001 |
| Drug RCT | |||||
| General Notification (N = 486) | 33.9 (26.9 – 41.7) | 24.8 (18.5 – 32.4) | 22.4 (15.2 – 31.6) | 27.4 (23.2 – 31.9) | 0.094 |
| Verbal Consent (N = 546) | 43.3 (36.2 – 50.7) | 36.8 (30.4 – 43.8) | 29.0 (20.5 – 39.2) | 37.5(33.2 – 41.9) | 0.070 |
| Dose Timing RCT | |||||
| General Notification (N = 539) | 49.4 (42.5 – 56.4) | 34.7 (28.3 – 41.8) | 33.4 (23.6 – 45.0) | 40.2 (35.8 – 44.7) | 0.008 |
| Verbal Consent (N 519) | 43.9 (37.1 – 51.0) | 31.8 (25.4 – 39.0) | 26.7 (18.3 – 37.4) | 35.5 (31.3 – 40.0) | 0.010 |
Pearson’s χ2 test of independence corrected for the survey design.
Footnotes
Conflict of Interest Disclosures: All authors have no conflicts of interest to report.
Role of the Sponsors: The sponsors had no role in the design of the study; analysis and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclaimer: The opinions expressed herein are the authors’ and do not reflect the policies and positions of the National Institutes of Health, the U.S. Public Health Service, or the U.S. Department of Health and Human Services.
Reproducible Research Statement: Study protocol: Available as a Supplement. Statistical code: Available from Dr. Kim (scott.kim@nih.gov). Data set: Available from http://tessexperiments.org/ or from Dr. Kim (scott.kim@nih.gov).
References
- 1.Smith M, Saunders R, Stuckhardt L, McGinnis JM, editors. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press (US); 2013. [PubMed] [Google Scholar]
- 2.Sugarman J, Califf RM. Ethics and regulatory complexities for pragmatic clinical trials. JAMA. 2014;311(23):2381–2382. doi: 10.1001/jama.2014.4164. [DOI] [PubMed] [Google Scholar]
- 3.Truog RD, Robinson W, Randolph A, Morris A. Is informed consent always necessary for randomized, controlled trials? N Engl J Med. 1999;340(10):804–807. doi: 10.1056/NEJM199903113401013. [DOI] [PubMed] [Google Scholar]
- 4.Faden RR, Kass NE, Goodman SN, Pronovost P, Tunis S, Beauchamp TL. An ethics framework for a learning health care system: a departure from traditional research ethics and clinical ethics. Hastings Cent Rep. 2013 Jan-Feb;41(S1):S16–27. doi: 10.1002/hast.134. [DOI] [PubMed] [Google Scholar]
- 5.Faden R, Kass N, Whicher D, Stewart W, Tunis S. Ethics and informed consent for comparative effectiveness research with prospective electronic clinical data. Med Care. 2013;51(8 Suppl 3):S53–57. doi: 10.1097/MLR.0b013e31829b1e4b. [DOI] [PubMed] [Google Scholar]
- 6.Faden RR, Beauchamp TL, Kass NE. Informed consent, comparative effectiveness, and learning health care. N Engl J Med. 2014;370(8):766–768. doi: 10.1056/NEJMhle1313674. [DOI] [PubMed] [Google Scholar]
- 7.Kim SYH, Miller FG. Informed consent for pragmatic trials — The Integrated Consent Model. N Engl J Med. 2014;370(8):769–772. doi: 10.1056/NEJMhle1312508. [DOI] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. Draft guidance on disclosing reasonably foreseeable risks in research evaluating standards of care. [on 12 May 2015];Federal Register. Accessed at https://federalregister.gov/a/2014-29915.
- 9.Cho MK, Magnus D, Constantine M, Lee SS-J, Kelley M, Alessi S, et al. Attitudes toward risk and informed consent for research on medical practices: A cross-sectional survey. Ann Intern Med. 2015 doi: 10.7326/M15-0166. Epub ahead of print 14 April 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.GfK. [on 12 May 2015];KnowledgePanel Design Summary. 2012 Accessed at http://www.gfk.com/Documents/GfK-KnowledgePanel-Design-Summary.pdf.
- 11.Chang L, Krosnick JA. National surveys via rdd telephone interviewing versus the Internet: Comparing sample representativeness and response quality. Public Opinion Quarterly. 2009;73(4):641–78. [Google Scholar]
- 12.Yeager DS, Krosnick JA, Chang L, Javitz HS, Levendusky MS, Simpser A, et al. Comparing the accuracy of rdd telephone surveys and Internet surveys conducted with probability and non-probability samples. Public Opinion Quarterly. 2011;75(4):709–47. [Google Scholar]
- 13. [on 12 May 2015];Time-sharing Experiments for the Social Sciences (TESS) Accessed at tessexperiments.org.
- 14.Chiong W, Kim AS, Huang IA, Farahany NA, Josephson S. Testing the presumption of consent to emergency treatment for acute ischemic stroke. JAMA. 2014;311(16):1689–91. doi: 10.1001/jama.2014.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomlinson T, De Vries R, Ryan K, Kim H, Lehpamer N, Kim SYH. Moral concerns and the willingness to donate to a research biobank. JAMA. 2015;313(4):417–9. doi: 10.1001/jama.2014.16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grande D, Mitra N, Shah A, Wan F, Asch DA. Public preferences about secondary uses of electronic health information. JAMA Intern Med. 2013;173(19):1798–806. doi: 10.1001/jamainternmed.2013.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grande D, Mitra N, Shah A, Wan F, Asch DA. The importance of purpose: Moving beyond consent in the societal use of personal health information. Ann Intern Med. 2014;161(12):855–U37. doi: 10.7326/M14-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horton JJ, Rand DG, Zeckhauser RJ. The online laboratory: conducting experiments in a real labor market. Experimental Economics. 2011;14(3):399–425. [Google Scholar]
- 19.Buhrmester M, Kwang T, Gosling SD. Amazon’s Mechanical Turk: A new source of inexpensive, yet high-quality, data? Perspectives on Psychological Science. 2011;6(1):3–5. doi: 10.1177/1745691610393980. [DOI] [PubMed] [Google Scholar]
- 20.Berinsky AJ, Huber GA, Lenz GS. Evaluating online labor markets for experimental research: Amazon.com’s Mechanical Turk. Political Analysis. 2012;20(3):351–68. [Google Scholar]
- 21. [Accessed March 18, 2014];Protection of Human Subjects, 45 CFR part 46.116[a] 2009 Accessed at http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html.
- 22.Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States, 2011–2012. NCHS Data Brief. 2013;(133):1–8. [PubMed] [Google Scholar]
- 23.Platt R, Kass NE, McGraw D. Ethics, regulation, and comparative effectiveness research: Time for a change. JAMA. 2014;311(15):1497–1498. doi: 10.1001/jama.2014.2144. [DOI] [PubMed] [Google Scholar]
- 24.Pletcher MJ, Lo B, Grady D. Informed consent in randomized quality improvement trials: A critical barrier for learning health systems. JAMA Intern Med. 2014;174(5):668–670. doi: 10.1001/jamainternmed.2013.13297. [DOI] [PubMed] [Google Scholar]
- 25.Wendler D. “Targeted” consent for pragmatic clinical trials. J Gen Intern Med. 2015;30(5):679–682. doi: 10.1007/s11606-014-3169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement. Survey Questionnaire
Appendix Figure 1. Experimental design of survey
Shows the 2-by-2 factorial design and information presented to respondents. About half received a Drug RCT scenario comparing two first-line drugs; the others received a Dose Timing RCT scenario comparing morning versus night dosing. About half of each group compared written consent to general notification; the rest compared written consent to verbal consent.