Abstract
Autoimmune disorders are long-term diseases that adversely affect the quality of life for patients, and they are one of the top ten leading causes of death. While each autoimmune disorder is unique, they all are caused by a breakdown of tolerance against endogenous proteins. This leads to auto-inflammatory events that promote the destruction of organs in a humoral and cellular immune mediated manner. Treatment options for autoimmunity can involve the use of chemical and biologic agents that suppress inflammation. While these treatment options for patients have shown to be beneficial in autoimmunity, they can result in patients being vulnerable to opportunistic infections. Newer therapies aim to identify methods to specifically block auto-inflammatory immune cells while allowing for an intact immune response to other antigens. T regulatory (Treg) cells are a subtype of the adoptive immune cell that is capable of suppressing inflammatory events in an antigen-specific manner, but they are often poorly functioning within autoimmune patients. Treg cells have been well characterized for their immune modulating capabilities and preclinical and early clinical studies support their therapeutic potential for antigen-specific immune suppression. This review will examine the current understanding of Treg cell function and the therapeutic potential of enhancing Treg cells in patients with inflammatory disorders.
Autoimmune Diseases
Autoimmune diseases are estimated to affect between 3–5% of individuals in western societies (Cooper et al., 2009). These diseases can be life-long diagnosis that adversely affect the health-related quality of life of patients and are one of the leading causes for death. It is unclear what triggers the initial event that breaks down tolerance to self-antigens and allows for the activation of auto-reactive immune cells (Goodnow et al., 2005). There are positive associations between specific human leukocyte antigen (HLA) haplotypes and the presentation of specific autoantigens via the major histocompatibility complexes (MHCs) associated with these HLA haplotypes (Gough and Simmonds, 2007). The activation of the adaptive immune response against endogenous antigens allows for the targeting of disease specific organs (e.g., brain, kidney, liver) via antibody dependent cellular cytotoxicity (ADCC) and complement dependent cytotoxicity (Hampe, 2012). Additionally, adoptive transfer of autoantigen specific T cells is capable of initiating disease in animal models, indicating that T lymphocytes also play key roles (Ohashi, 2002). While it is debatable which cell type plays a more important role in different autoimmune disorders, once these autoreactive T and B cells are activated, both can play key roles in the progression of the disease-specific pathology.
Therapies for autoimmune disorders aim to inhibit the proinflammatory immune response by depleting specific adaptive immune cell populations or inhibiting the activation of immune cells in target organs (Steinman et al., 2012). While these strategies are helpful in limiting the proinflammatory immune response against endogenous proteins and tissues, they also inhibit protective immune responses and can leave patients immunocompromised and susceptible to various infections. Newer therapies are being designed to utilize the suppressive capabilities of regulatory T (Treg) cells to suppress autoimmune cells in an antigen-specific manner (von Boehmer and Daniel, 2013). Treg cells are diverse (Josefowicz et al., 2012), being generated in the thymus [natural Tregs (nTregs)] or in the periphery [inducible Tregs (iTregs), also known as adaptive Tregs] via exposure to anti-inflammatory cytokines, such as TGF-β. Whether an nTreg or an iTreg, typically these regulatory T cells express forkhead box P3 (Foxp3), a transcription factor found to inhibit the expression of proinflammatory genes and upregulate the expression of antiinflammatory genes. Similar to proinflammatory T cells, Treg cells are activated via their T cell receptor (TCR) and the costimulatory molecule CD28. Because the specificity of the TCR against endogenous peptides is an important driver of the inflammatory response in autoimmune disorders, utilizing Treg cells that are specific for autoimmune peptides is a potentially valuable therapy because of the T cells potential to traffic to the site of inflammation and suppress the ongoing autoimmune response (Vandenbark and Offner, 2008). In this review, we will summarize the approaches and prospects for improving therapy for autoimmune diseases through manipulations of Treg cells.
Treg Mechanisms of Suppression in the Context of Autoimmunity
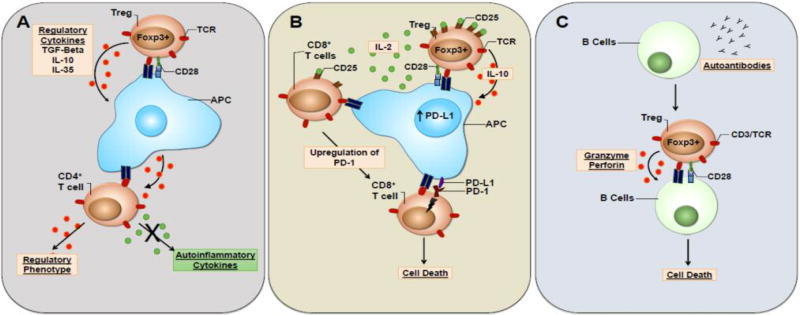
Treg cells have multiple suppressor mechanisms to mediate autoinflammatory events in patients. When activated, Treg cells secrete anti-inflammatory cytokines, such as TGF-β, IL-10, and IL-35 (Bettini and Vignali, 2009). These regulatory cytokines can affect multiple cell types at the site of inflammation. One mechanism by which Tregs are able to target autoreactive CD4+ T effector memory cells is through the generation of tolerogenic APCs (Figure 1a). When APCs are exposed to TGF-β and IL-10, they express a tolerogenic phenotype that promotes anergy of memory T cells that bind to their MHC molecules (Torres-Aguilar et al., 2010a; 2010b). Tolerogenic APCs are also able to induce IL-10 secreting T cells, but it is unclear whether these T cells were naive or memory cells prior to interacting with the tolerogenic APC. This mechanism allows for the targeting of antigen-specific memory T cells when cells become reactivated by tolerogenic APCs at the site of inflammation.
Figure 1.
Treg cell mechanism of suppression in autoimmunity. (A) When activated, Treg cells are capable of driving the formation of tolerogenic APCs via the secretion of regulatory cytokines (e.g., TGF-β and IL-10). Tolerogenic APCs activate antigen-specific CD4+ T cells and skew them towards a regulatory phenotype. (B) IL-2 cytokine depletion drives the upregulation of PD-1 on activated CD8+ T cells. Upregulation of PD-L1 by tolerogenic APCs, leave CD8 T cells vulnerable to cell death in a PD-1/PDL-1 dependent manner. (C) B cells are capable of presenting antigens to Treg cells. Once activated by B cells, Tregs suppress the secretion of autoantibodies via direct killing of autoinflammatory B cells in a perforin and granzyme dependent manner.
In addition to causing anergy in memory CD4 T cells and activation of IL-10 secreting Tregs, tolerogenic APCs upregulate programmed death ligand 1 (PD-L1) (Wolfle et al., 2011). The programmed death (PD-1)-PD-L1 signaling pathway is one mechanism essential for the suppression of T cells post activation in autoimmunity (Riella et al., 2012). Post antigen activation, exhausted CD8+ T cells upregulate the expression of certain cell surface markers, such as PD-1 (Chikuma et al., 2009). This upregulation of PD-1 leaves CD8 T cells susceptible to PD-L1 dependent anergy (Figure 1b). The generation of exhausted PD-1+CD8+ T cells involves the depletion or blockade of IL-2 in the environment. Treg express higher amounts of the high affinity IL-2 receptor (CD25) and are capable of depleting local cytokines (Pandiyan et al., 2007). In the case of autoimmunity, Treg cells are expected to soak up IL-2 at the site of inflammation leading to exhaustion of CD8+ T cell and leaving them susceptible to PD-1-PD-L1 mediated cell death.
B cells play a critical role in autoimmune disease pathology via the secretion of autoantibodies. These antibodies bind to endogenous proteins and allow for direct targeting of specific cell types by Fc receptor and complement mediated mechanisms (Yanaba et al., 2008). The effector mechanism of autoantibodies has been verified via adoptive transfer of autoantibodies into animal models in which they exacerbate tissue pathology in an analogous manner as in the human disease (Yan et al., 2014; Saadoun et al., 2010). While regulatory cytokines such as TGF-β induce apoptosis in B cells (Spender et al., 2009), Treg cells are also capable of directly suppressing autoantibody secreting B cells via cell-to-cell contact (Figure 1c) (Lim et al., 2005). Animal model studies have shown that depleting Treg cells leads to increase autoantibody production (Liu et al., 2014). Patients deficient in functional FoxP3, a disease called immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome, lack nTreg cells and suffer from a number of serious autoimmune and inflammation disorders that are lethal if not treated by bone marrow transplantation (van der Vliet and Nieuwenhuis, 2007). When Treg cells are activated by B cells that secrete autoantibodies, Tregs can inhibit proliferation of B cells and drive cell death in a perforin and granzyme-dependent manner (Wang and Zheng, 2013).
Imbalance of Regulatory T cells in Human Autoimmune Diseases
While Treg cells are present in patients with autoimmune disorders (Putheti et al., 2004; Viglietta et al., 2004), this population of cells appear to lack auto-antigen specificity as well as suppressive capabilities (Venken et al., 2008; Viglietta et al., 2004; Ehrenstein et al., 2004). While the number of Tregs may vary from patient to patient and differ in activity between various autoimmune diseases, it is clear that the Treg suppressive phenotype is limited within patients with autoimmune disorders (Long and Buckner, 2011). Because of this observation, multiple therapies have been designed and tested to expand Treg cells in animal disease models and in patients suffering with autoimmune disorders. The following section will focus upon notable therapies that increase the number of Treg cells when administered in vivo.
IL-2 therapy rescues Treg population
When originally discovered, interleukin-2 (IL-2) was seen as a proinflammatory cytokine, which supported the proliferation and activation of naive T cells into T helper cell subsets (Robb, 1984). Although Foxp3+ T cells are present and functionally suppressive in IL-2−/− and IL-2rα−/− mice, IL-2 is an important cytokine in regulating the transcription of growth and metabolic genes in the context of Treg cells (Fontenot et al., 2005). Clinical trials have begun with autoimmune and inflammatory disorders to examine whether low dose IL-2 treatment promotes the formation of Treg cells in patients (Koreth et al., 2011; Saadoun et al., 2011). Because of their expression of a high affinity IL-2R, it is thought that Tregs may preferentially expand in conditions of low amounts of IL-2. Low dose IL-2 treatment results in an increase in the percentage of peripheral blood CD4+CD25HighFoxp3+ T cells with increased suppressive function. In graft-versus-host-disease (GVHD) patients that received a 12-month treatment with IL-2, researchers observed an increase in the number of CD4+ Treg cells (Koreth et al., 2011). In patients with hepatitis C-induced vasculitis, Treg cell numbers were restored after low dose IL-2 therapy and 8 of 10 patients saw clinical improvement of their autoimmune disorder (Saadoun et al., 2011). It is of note that no effect was observed on T effector cell populations in patients from either of these clinical studies.
Therapies utilizing autoantigens to induce Treg suppression
Screening autoantibodies isolated from the plasma of patients, a large array of autoantigen epitopes have been identified for various autoimmune disorders (Lernmark, 2001). Utilizing this knowledge, methods have been tested to identify procedures that expand antigen-specific Treg cells that are capable of inducing tolerance against autoantigens and inhibiting autoimmune inflammatory events from a polyclonal T cell population (Xu et al., 2013). Autoantigens have been utilized in two different methods to produce tolerogenic T cells ex vivo: (i) antigen bound directly to biodegradable particles (Maldonado et al., 2015), or (ii) antigen fixed onto APCs to trigger the formation and expansion of Foxp3+ T cells and the secretion of the regulatory cytokines IL-10 and TGF-β (Prasad et al., 2012b).
Nanoparticle technology has been developed in the past decade to expand T cell populations (Steenblock and Fahmy, 2008; Park et al., 2011). Biodegradable nanoparticles with autoantigens and rapamycin bound to their surface are capable of generating Foxp3+ Treg and B regulatory cells in multiple animal models (Maldonado et al., 2015). Under these conditions, the nanoparticle taken up by APCs led to the presentation of the autoantigens on MHC molecules. The use of rapamycin directed the APCs to express a tolerogenic phenotype that allowed for the activation of inducible Treg cells. Nanoparticle treatment given to mice via subcutaneous or intravenous injection showed promising results in ameliorating disease in the relapsing-remitting multiple sclerosis animal model (Maldonado et al., 2015). This therapy relies on rapamycin bound to the nanoparticle interacting with the APCs. Loss of the rapamycin on the nanoparticle results in the inability to generate the Treg cell population.
Another approach to generate autoantigen-specific Treg cells is by cross-linking autoantigens to the cell surface of APCs with the chemical ethylene carbodimide (ECDI) (Miller et al., 2007). Adoptive transfer of APCs ECDI-crosslinked with diabetes associated autoantigens induces tolerance to antigens and inhibits the development of spontaneous type-1 diabetes (T1D) due to the generation of antigen-specific regulatory T cells in the NOD diabetes model (Prasad et al., 2012a). Additionally, this treatment in concert with islet cell transplantation leads to the restoration of normoglycemia in mice (Kheradmand et al., 2011). Treated mice show an increase in IL-10 secretion when cells were restimulated ex vivo. Additional studies show that there is an increase in CD4+Foxp3+ T cells in the spleen and draining lymph nodes following treatment (Kheradmand et al., 2012). A first-in-man clinical trial examined the safety of antigen-ECDI-cell treatment in patients with multiple sclerosis (MS) (Lutterotti et al., 2013). MS patients whose T cell responses were specific for myelin peptides were given identical peptides chemically coupled to autologous PBMCs. While the higher doses of the treatment showed a reduction in autoantigen-specific T effector cells, treatment did not appear to have any effect on the peripheral Treg cell population.
Adoptive Treg cell therapy to mediate inflammation
In GHVD and autoimmune animal models, early Treg cell treatment inhibits the development of the disease (Roncarolo and Battaglia, 2007). In Hori et al. (2002), researchers utilized a RAG-1 deficient TCR transgenic mouse model with a TCR specific for myelin basic protein (MBP) that spontaneously developed CNS autoimmunity (Hori et al., 2002). Adoptive transfer of MBP-specific Treg cells into this model prior to the development of disease symptoms led to suppression of clinical phenotypes. Additionally, this study also showed that adoptive transfer of non-MBP specific Treg cells was capable of suppressing the disease phenotype. Studies performed in an animal model for type 1 diabetes has shown similar results when autoantigen-specific T cells were adoptively transferred into NOD Rag−/− mice prior to the development of clinical symptoms (Masteller et al., 2005; Tang et al., 2004). These studies suggested that reconstituting the immune system with autoantigen-specific Tregs prior to the onset of clinical disease would prove beneficial at inhibiting the early auto-inflammatory events that initiate the disease. Studies performed in GVHD models suggest that adoptive transfer of Treg cells post reconstitution of the immune system decreases the efficacy of Treg adoptive cell therapy (ACT) (Nguyen et al., 2007). A 2007 study showed that mice who received an infusion of Treg cells on day 0 versus on day 21 post bone marrow transplant had significantly less occurrence of GVHD and a greater chance of survival over the long term (Nguyen et al., 2007). While many of these preclinical studies showed that early adoptive transfer of Treg cells proved most effective in preventing disease, Tang et al. (2004) indicated that ACT of Treg cells into the diabetic NOD mouse model during the early stages of diabetes showed efficacy in suppressing the progression of the disease (Masteller et al., 2005). All of these preclinical studies provide some proof-of-concept that ACT of Treg cells could be an effective therapeutic option for autoimmunity and GVHD. The efficacy of the treatment relies primarily on the antigen specificity of the Tregs and whether the cells are given during early or late stages of disease progression, with earlier treatment being more effective.
One of the greatest hurdles to overcome in generating an effective ACT Treg cell therapy in humans is the need to transfer a large number of Treg cells into patients. Treg cells are of a very low percentage of leukocytes in the blood, so isolating enough Treg cells to generate a suppressive response has proven challenging. Several laboratories have generated in vitro protocols that expand small populations of Treg cells and maintain their suppressor phenotype (Hoffmann et al., 2004; Putnam et al., 2009). The expanded Treg cells retained their suppressive capabilities (secretion of IL-10 and Foxp3 expression) and had low proinflammatory characteristics (as measured by IFN-γ or IL-4 secretion post activation) (Bluestone et al., 2015). Starting in 2009, multiple early stage clinical trials examined the safety and efficacy of utilizing ACT Treg cell therapy in GVHD patients and autoimmune disease patients (Bluestone et al., 2015; Brunstein et al., 2011; Desreumaux et al., 2012; Marek-Trzonkowska et al., 2012; Trzonkowski et al., 2009). These first-in-man studies verified that ACT Treg cell therapy is tolerable in humans, with patients showing minimal negative side-effects associated with treatment.
In cases of autoimmunity, treatment with Tregs did not show the same efficacy as what was previously demonstrated in studies using animal models. Bluestone et al. (2015) reported a clinical study examining a dose escalation of ex vivo expanded polyclonal Tregs and their effect on conditions in early onset type 1 diabetes in adults (Bluestone et al., 2015). Treatment with the highest dose of Tregs (2.6 × 109 cells/patient) showed no significant difference in the progression of disease. This study showed that despite Treg cells having a suppressive capacity post ex vivo expansion, adoptively transferred Treg cells did not survive long term in patients. When isolated from the patients, the Treg cells had lost their activation phenotype. The use of antigen specific Treg cells may be critical for efficacy in autoimmune patients. Desremaux et al. (2012) reported the use of ovalbumin-specific Treg cells for the treatment of patients suffering from refractory Crohn’s disease (Desreumaux et al., 2012). Due to the site of inflammation being predominantly within intestinal tissues, the researchers generated clonal Tregs specific for ovalbumin. They theorized that since ovalbumin is commonly consumed, the antigen would be presented to Treg cells in intestinal tissue. Twenty patients received a single dose of ovalbumin-specific Tregs ranging from 106 to 109 cells. Of the 20 patients tested, 8 patients showed a reduction of Crohn’s disease activity index. Taken together, these two studies suggest that the antigen specificity of the Treg may play an important role in designing future Treg cell based therapies.
ACT of Tregs has been further characterized in studies performed in hematopoietic stem cell transplantation (HSCT), with the goal of preventing GVHD. In patients that receive bone marrow infusions, there is a 30–60% chance that the transferred immune cells will generate an immune response against the host’s proteins (Barton-Burke et al., 2008). The immune response of grafted cells against endogenous proteins leads to continuous inflammation and damage to tissues and organs. When treating acute leukemia patients with HSCT, the infusion of allogeneic bone marrow cells is meant to reconstitute the immune system post high dose irradiation treatment (Rezvani and Barrett, 2008). One side effect of HSCT therapy, however, is that donor T cells meant to protect the patients from pathogens will also target host tissues and mediate GVHD despite HLA matching due to the many non-HLA differences between host and donor. Clinical studies have examined whether donor-derived Foxp3+ T cells can prevent GVHD without affecting donor T cell derived protection from infections (Di Ianni et al., 2011; Martelli et al., 2014). Di Ianni et al. (2011) showed that 26 out of 28 patients achieved donor-engraftment with only 2 of the 26 patients developing ≥ grade 2 GVHD. Martelli et al. (2014) showed that of the 43 patients that received donor Treg cells four days prior to receiving HSCT, 95% donor engraftment was achieved with only 15% of patients developing ≥ grade 2 GVHD. With the positive outcomes being observed-with these studies, other groups have examined additional methods to protect against GVHD following HSCT. In 2014, Bacchetta et al. (2014) describes a clinical trial in which patients received HSCT in combination with alloantigen-specific Treg cells. The alloantigen-specific Tregs were generated by culturing donor derived peripheral blood mononuclear cells (PBMCs) with host APCs in the presence of IL-10. Researchers theorized that ACT of Treg cells would lead to anergy against host-derived alloantigens but still allow for protection against bacterial or viral antigens (Bacchetta et al., 2010). Of the 12 patients given the treatment, 4 patients showed a decrease in alloantigen-specific cytotoxic T cell populations following the adoptive transfer of IL-10 anergized Treg cells. These patients also showed stable donor chimerism and long-term immune reconstitution.
Conclusions
It is possible that one of the major driving forces of autoimmunity is the lack of regulatory T cells against disease-specific autoantigens. Clinical trials in GVHD patients show a positive correlation with the reconstitution of autoantigen-specific regulatory T cells and a decrease in disease progression. The available data do not support the notion that reconstitution of the immune response with polyclonal Tregs can inhibit disease progression. It may turn out that for ACT of Tregs to be of clinical benefit, Tregs must become activated in vivo against an antigen present within the auto-inflammatory environment. With the limited number of Tregs found in patients, future studies may examine the use of non-autologous Tregs from haplotype similar individuals or genetically modifying autoantigen-specific T effector cells to become Treg cells themselves. Treg cells that are present and activated during auto-inflammatory disease may be capable of inhibiting the progression of disease.
Disease progression in autoimmune disorders is highly variable between patients diagnosed with the same disorder. For immune suppression to occur in patients suffering from an autoimmune disorder, identifying the opportune stage of disease to intervene may be critical for the efficacy of treatment. For ACT of Tregs to prevent GVHD, Treg cells are transferred along with the bone marrow graft, allowing for the early suppression of T effector cells that can trigger GVHD. Early suppression of inflammatory cells is key for beneficial effects to be observed. For autoimmune diseases, early treatment methods or more effective Treg cells must be generated for ACT of Tregs to become an effective approach to inhibit the progression of clinical disease.
Acknowledgments
Support for this work was provided in part by the Dartmouth Immunology Program and an NIH T32 Training Grant (AI 003763) and funds from the Center for Synthetic Immunity.
Footnotes
Disclosure
B.A. and C.L.S. have intellectual property related to immune cell engineering and regulatory cells. These are managed in compliance with the policies of Dartmouth College. D.J.G. has nothing to disclose.
References
- Bacchetta R, Gregori S, Serafini G, Sartirana C, Schulz U, Zino E, Tomiuk S, Jansen U, Ponzoni M, Paties CT, Fleischhauer K, Roncarolo MG. Molecular and functional characterization of allogantigen-specific anergic T cells suitable for cell therapy. Haematologica. 2010;95(12):2134–2143. doi: 10.3324/haematol.2010.025825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchetta R, Lucarelli B, Sartirana C, Gregori S, Lupo Stanghellini MT, Miqueu P, Tomiuk S, Hernandez-Fuentes M, Gianolini ME, Greco R, Bernardi M, Zappone E, Rossini S, Janssen U, Ambrosi A, Salomoni M, Peccatori J, Ciceri F, Roncarolo MG. Immunological outcome in haploidentical-HSC transplanted patients treated with IL-10-anergized donor T cells. Front Immunol. 2014;5:16. doi: 10.3389/fimmu.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton-Burke M, Dwinell DM, Kafkas L, Lavalley C, Sands H, Proctor C, Johnson E. Graft-versus-host disease: a complex long-term side effect of hematopoietic stem cell transplant. Oncology. 2008;22(11 Suppl):31–45. Nurse Ed. [PubMed] [Google Scholar]
- Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21(6):612–618. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7(315):315ra189. doi: 10.1126/scitranslmed.aad4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunstein CG, Miller JS, Cao Q, Mckenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P, Mcglave PB, Blazar BR, Wagner JE. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117(3):1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikuma S, Terawaki S, Hayashi T, Nabeshima R, Yoshida T, Shibayama S, Okazaki T, Honjo T. PD-1-mediated suppression of IL-2 production induces CD8+ T cell anergy in vivo. J Immunol. 2009;182(11):6682–6689. doi: 10.4049/jimmunol.0900080. [DOI] [PubMed] [Google Scholar]
- Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33(3–4):197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desreumaux P, Foussat A, Allez M, Beaugerie L, Hebuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, Ticchioni M, Duchange A, Morel-Mandrino P, Neveu V, Clerget-Chossat N, Forte M, Colombel JF. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143(5):1207–1217. e1201–1202. doi: 10.1053/j.gastro.2012.07.116. [DOI] [PubMed] [Google Scholar]
- Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D, Aloisi T, Perruccio K, Ruggeri L, Balucani C, Pierini A, Sportoletti P, Aristei C, Falini B, Reisner Y, Velardi A, et al. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117(14):3921–3928. doi: 10.1182/blood-2010-10-311894. [DOI] [PubMed] [Google Scholar]
- Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200(3):277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6(11):1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Goodnow CC, Sprent J, Fazekas De St Groth B, Vinuesa CG. Cellular and genetic mechanisms of self tolerance and autoimmunity. Nature. 2005;435(7042):590–597. doi: 10.1038/nature03724. [DOI] [PubMed] [Google Scholar]
- Gough SC, Simmonds MJ. The HLA Region and Autoimmune Disease: Associations and Mechanisms of Action. Curr Genomics. 2007;8(7):453–465. doi: 10.2174/138920207783591690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampe CS. B Cell in Autoimmune Diseases. Scientifica (Cairo) 2012;2012 doi: 10.6064/2012/215308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25high regulatory T cells. Blood. 2004;104(3):895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]
- Hori S, Haury M, Coutinho A, Demengeot J. Specificity requirements for selection and effector functions of CD25+4+ regulatory T cells in anti-myelin basic protein T cell receptor transgenic mice. Proc Natl Acad Sci U S A. 2002;99(12):8213–8218. doi: 10.1073/pnas.122224799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand T, Wang S, Bryant J, Tasch JJ, Lerret N, Pothoven KL, Houlihan JL, Miller SD, Zhang ZJ, Luo X. Ethylenecarbodiimide-fixed donor splenocyte infusions differentially target direct and indirect pathways of allorecognition for induction of transplant tolerance. J Immunol. 2012;189(2):804–812. doi: 10.4049/jimmunol.1103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheradmand T, Wang S, Gibly RF, Zhang X, Holland S, Tasch J, Graham JG, Kaufman DB, Miller SD, Shea LD, Luo X. Permanent protection of PLG scaffold transplanted allogeneic islet grafts in diabetic mice treated with ECDI-fixed donor splenocyte infusions. Biomaterials. 2011;32(20):4517–4524. doi: 10.1016/j.biomaterials.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth J, Matsuoka K, Kim HT, Mcdonough SM, Bindra B, Alyea EP, 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. doi: 10.1056/NEJMoa1108188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lernmark A. Autoimmune diseases: are markers ready for prediction? J Clin Invest. 2001;108(8):1091–1096. doi: 10.1172/JCI14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175(7):4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu A, Iikuni N, Xu H, Shi FD, La Cava A. Regulatory CD4+ T cells promote B cell anergy in murine lupus. J Immunol. 2014;192(9):4069–4073. doi: 10.4049/jimmunol.1302897. [DOI] [PubMed] [Google Scholar]
- Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187(5):2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterotti A, Yousef S, Sputtek A, Sturner KH, Stellmann JP, Breiden P, Reinhardt S, Schulze C, Bester M, Heesen C, Schippling S, Miller SD, Sospedra M, Martin R. Antigen-specific tolerance by autologous myelin peptide-coupled cells: a phase 1 trial in multiple sclerosis. Sci Transl Med. 2013;5(188):188ra175. doi: 10.1126/scitranslmed.3006168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, Lamothe RA, Ferrari JD, Zhang AH, Rossi RJ, Kolte PN, Griset AP, O’neil C, Altreuter DH, Browning E, Johnston L, Farokhzad OC, Langer R, Scott DW, Von Andrian UH, Kishimoto TK. Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance. Proc Natl Acad Sci U S A. 2015;112(2):E156–165. doi: 10.1073/pnas.1408686111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A, Mysliwska J, Trzonkowski P. Administration of CD4+CD25highCD127− regulatory T cells preserves beta-cell function in type 1 diabetes in children. Diabetes care. 2012;35(9):1817–1820. doi: 10.2337/dc12-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, Pierini A, Massei MS, Amico L, Urbani E, Del Papa B, Zei T, Iacucci Ostini R, Cecchini D, Tognellini R, Reisner Y, Aversa F, Falini B, Velardi A. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014;124(4):638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- Masteller EL, Warner MR, Tang Q, Tarbell KV, Mcdevitt H, Bluestone JA. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J Immunol. 2005;175(5):3053–3059. doi: 10.4049/jimmunol.175.5.3053. [DOI] [PubMed] [Google Scholar]
- Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7(9):665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack A, Contag CH, Negrin RS. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109(6):2649–2656. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- Ohashi PS. T-cell signalling and autoimmunity: molecular mechanisms of disease. Nat Rev Immunol. 2002;2(6):427–438. doi: 10.1038/nri822. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8(12):1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Park J, Gao W, Whiston R, Strom TB, Metcalfe S, Fahmy TM. Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery. Mol Pharm. 2011;8(1):143–152. doi: 10.1021/mp100203a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Kohm AP, Mcmahon JS, Luo X, Miller SD. Pathogenesis of NOD diabetes is initiated by reactivity to the insulin B chain 9–23 epitope and involves functional epitope spreading. J Autoimmun. 2012a;39(4):347–353. doi: 10.1016/j.jaut.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad S, Xu D, Miller SD. Tolerance strategies employing antigen-coupled apoptotic cells and carboxylated PLG nanoparticles for the treatment of type 1 diabetes. Rev Diabet Stud. 2012b;9(4):319–327. doi: 10.1900/RDS.2012.9.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putheti P, Pettersson A, Soderstrom M, Link H, Huang YM. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J Clin Immunol. 2004;24(2):155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58(3):652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani K, Barrett AJ. Characterizing and optimizing immune responses to leukaemia antigens after allogeneic stem cell transplantation. Best Pract Res Clin Haematol. 2008;21(3):437–453. doi: 10.1016/j.beha.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riella LV, Paterson AM, Sharpe AH, Chandraker A. Role of the PD-1 pathway in the immune response. Am J Transplant. 2012;12(10):2575–2587. doi: 10.1111/j.1600-6143.2012.04224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb RJ. Interleukin 2: the molecule and its function. Immunol Today. 1984;5(7):203–209. doi: 10.1016/0167-5699(84)90224-X. [DOI] [PubMed] [Google Scholar]
- Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7(8):585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- Saadoun D, Rosenzwajg M, Joly F, Six A, Carrat F, Thibault V, Sene D, Cacoub P, Klatzmann D. Regulatory T-cell responses to low-dose interleukin-2 in HCV-induced vasculitis. N Engl J Med. 2011;365(22):2067–2077. doi: 10.1056/NEJMoa1105143. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of neuromyelitis optica immunoglobulin G and human complement produces neuromyelitis optica lesions in mice. Brain. 2010;133(Pt 2):349–361. doi: 10.1093/brain/awp309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spender LC, O’brien DI, Simpson D, Dutt D, Gregory CD, Allday MJ, Clark LJ, Inman GJ. TGF-beta induces apoptosis in human B cells by transcriptional regulation of BIK and BCL-XL. Cell Death Differ. 2009;16(4):593–602. doi: 10.1038/cdd.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenblock ER, Fahmy TM. A comprehensive platform for ex vivo T-cell expansion based on biodegradable polymeric artificial antigen-presenting cells. Mol Ther. 2008;16(4):765–772. doi: 10.1038/mt.2008.11. [DOI] [PubMed] [Google Scholar]
- Steinman L, Merrill JT, Mcinnes IB, Peakman M. Optimization of current and future therapy for autoimmune diseases. Nat Med. 2012;18(1):59–65. doi: 10.1038/nm.2625. [DOI] [PubMed] [Google Scholar]
- Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, Mcdevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199(11):1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Aguilar H, Aguilar-Ruiz SR, Gonzalez-Perez G, Munguia R, Bajana S, Meraz-Rios MA, Sanchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. J Immunol. 2010a;184(4):1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- Torres-Aguilar H, Sanchez-Torres C, Jara LJ, Blank M, Shoenfeld Y. IL-10/TGF-beta-treated dendritic cells, pulsed with insulin, specifically reduce the response to insulin of CD4+ effector/memory T cells from type 1 diabetic individuals. J Clin Immunol. 2010b;30(5):659–668. doi: 10.1007/s10875-010-9430-5. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Bieniaszewska M, Juscinska J, Dobyszuk A, Krzystyniak A, Marek N, Mysliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127− T regulatory cells. Clin Immunol. 2009;133(1):22–26. doi: 10.1016/j.clim.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Van Der Vliet HJ, Nieuwenhuis EE. IPEX as a result of mutations in FOXP3. Clin Dev Immunol. 2007;2007:89017. doi: 10.1155/2007/89017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbark AA, Offner H. Critical evaluation of regulatory T cells in autoimmunity: are the most potent regulatory specificities being ignored? Immunology. 2008;125(1):1–13. doi: 10.1111/j.1365-2567.2008.02900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180(9):6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199(7):971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Boehmer H, Daniel C. Therapeutic opportunities for manipulating T(Reg) cells in autoimmunity and cancer. Nat Rev Drug Discov. 2013;12(1):51–63. doi: 10.1038/nrd3683. [DOI] [PubMed] [Google Scholar]
- Wang P, Zheng SG. Regulatory T cells and B cells: implication on autoimmune diseases. Int J Clin Exp Pathol. 2013;6(12):2668–2674. [PMC free article] [PubMed] [Google Scholar]
- Wolfle SJ, Strebovsky J, Bartz H, Sahr A, Arnold C, Kaiser C, Dalpke AH, Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41(2):413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]
- Xu D, Prasad S, Miller SD. Inducing immune tolerance: a focus on Type 1 diabetes mellitus. Diabetes Manag (Lond) 2013;3(5):415–426. doi: 10.2217/dmt.13.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan W, Nguyen T, Yuki N, Ji Q, Yiannikas C, Pollard JD, Mathey EK. Antibodies to neurofascin exacerbate adoptive transfer experimental autoimmune neuritis. J Neuroimmunol. 2014;277(1–2):13–17. doi: 10.1016/j.jneuroim.2014.09.012. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Matsushita T, Magro CM, St Clair EW, Tedder TF. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]