Abstract
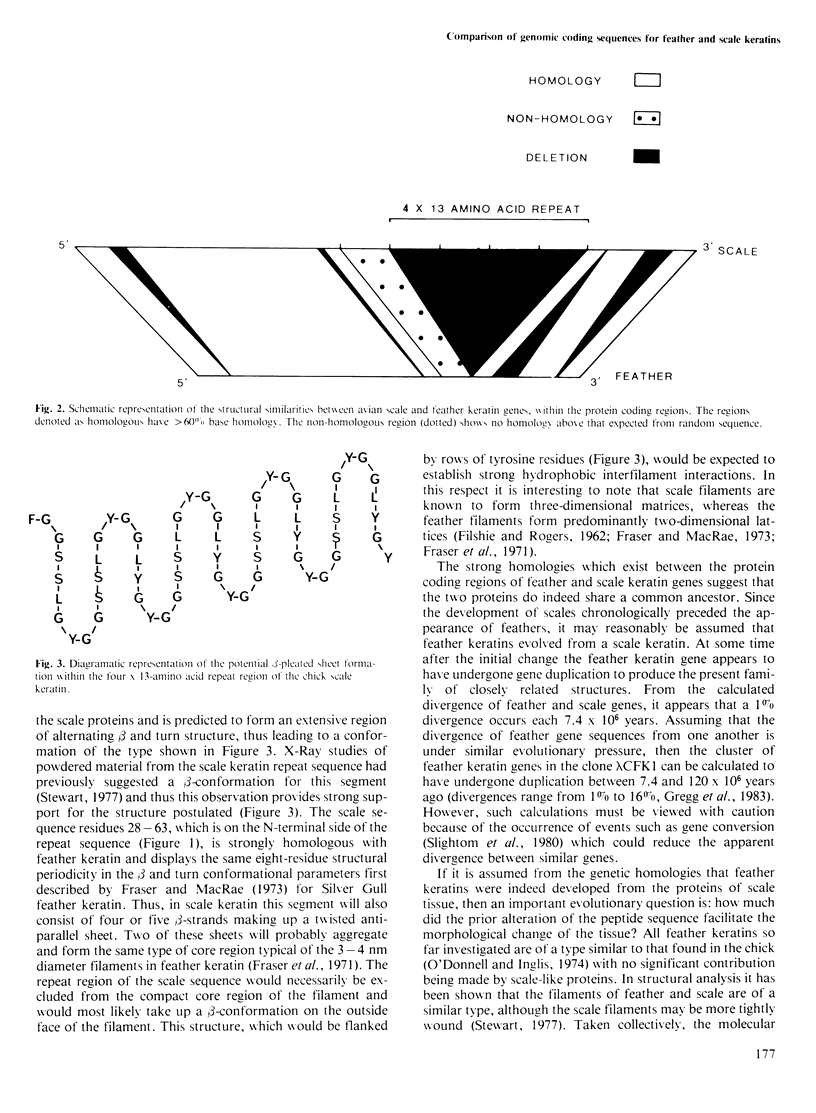
DNA sequences have been obtained for embryonic chick feather and scale keratin genes. Strong homologies exist between the protein coding regions of the two gene types and between the deduced amino acid sequences of the keratin proteins. Scale keratins are larger than feather keratins and the size difference is mainly attributable to four 13-amino acid repeats between residues 77 and 128 which compose a peptide sequence rich in glycine and tyrosine. The strong similarities between the two peptide structures for feather and scale in the homologous regions suggests a similar conformation within the protein filaments. A likely consequence is that the additional repeat region of the scale protein is located externally to the core filament. Tissue-specific features of filament aggregation may be attributable to this one striking sequence difference between the constituent proteins. It is believed that the genes share a common ancestry and that feather-like keratin genes may have evolved from a scale keratin gene by a single deletion event.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou P. Y., Fasman G. D. Prediction of beta-turns. Biophys J. 1979 Jun;26(3):367–383. doi: 10.1016/S0006-3495(79)85259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- FILSHIE B. K., ROGERS G. E. An electron microscope study of the fine structure of feather keratin. J Cell Biol. 1962 Apr;13:1–12. doi: 10.1083/jcb.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy P. L., Powell B. C., Gregg K., Barone E. D., Rogers G. E. Organisation of feather keratin genes in the chick genome. Nucleic Acids Res. 1982 Oct 11;10(19):6007–6021. doi: 10.1093/nar/10.19.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell I. J., Inglis A. S. Amino acid sequence of a feather keratin from silver gull (Larus novae-hollandiae) and comparison with one from emu (Dromaius novae-hollandiae). Aust J Biol Sci. 1974 Aug;27(4):369–382. doi: 10.1071/bi9740369. [DOI] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Stewart M. The structure of chicken scale keratin. J Ultrastruct Res. 1977 Jul;60(1):27–33. doi: 10.1016/s0022-5320(77)80038-5. [DOI] [PubMed] [Google Scholar]
- Walker I. D., Bridgen J. The keratin chains of avian scale tissue. Sequence heterogeneity and the number of scale keratin genes. Eur J Biochem. 1976 Aug 1;67(1):283–293. doi: 10.1111/j.1432-1033.1976.tb10660.x. [DOI] [PubMed] [Google Scholar]



