Abstract
Glycoconjugate vaccines based on capsular polysaccharides (CPSs) of pathogenic bacteria such as Streptococcus pneumoniae successfully protect from disease, but suffer from incomplete coverage, are troublesome to manufacture from isolated CPSs and lack efficacy against certain serotypes. Defined, synthetic oligosaccharides are an attractive alternative to isolated CPSs but require the identification of immunogenic and protective oligosaccharide antigens. Here, we describe a medicinal chemistry strategy based on a combination of automated glycan assembly (AGA), glycan microarray-based monoclonal antibody (mAb) reverse engineering and immunological evaluation in vivo to uncover a protective glycan epitope (glycotope) for S. pneumoniae serotype 8 (ST8). All four tetrasaccharide frameshifts of ST8 CPS were prepared by AGA and used in glycan microarray experiments to identify the glycotopes recognized by antibodies against ST8. One tetrasaccharide frameshift that was preferentially recognized by a protective, CPS-directed mAb was conjugated to the carrier protein CRM197. Immunization of mice with this semisynthetic glycoconjugate followed by generation and characterization of a protective mAb identified protective and non-protective glycotopes. Immunization of rabbits with semisynthetic ST8 glycoconjugates containing protective glycotopes induced an antibacterial immune response. Co-formulation of ST8 glycoconjugates with the marketed 13-valent glycoconjugate vaccine Prevnar 13 yielded a potent 14-valent S. pneumoniae vaccine. Our strategy presents a facile approach to develop efficient semisynthetic glycoconjugate vaccines.
Introduction
Most marketed vaccines against encapsulated pathogenic bacteria, such as Streptococcus pneumoniae, Haemophilus influenzae and Neisseria meningitidis, are based on pathogen-borne capsular polysaccharides (CPSs). CPSs are composed of repeating units that contain a variety of potentially immunogenic glycotopes that are recognized as “non-self” by the immune system (1). Some clinically approved vaccines, such as the pneumococcal vaccine Pneumovax 23, comprise mixtures of isolated CPSs to protect against some of the more than 90 S. pneumoniae serotypes. More immunogenic and efficacious pneumococcal glycoconjugate vaccines such as Prevnar 13 are based on isolated CPSs that are chemically conjugated to carrier proteins. Immunization with vaccines based on isolated polysaccharides generates a polyclonal immune response that may be directed towards multiple glycotopes. Each of these glycotopes may be either protective (when antibodies that recognize the sequence protect from disease) or non-protective. Reaction to impurities present in the isolate, to neo-epitopes introduced during isolation and conjugation or to immunodominant glycotopes as part of an immune evasion strategy by the pathogen may cause a non-protective response (2–5). Furthermore, the abundance of co-isolated impurities in crude polysaccharide preparations hampers the manufacture of polysaccharide-based vaccines, and a number of quality control steps is needed to characterize isolates (1).
Chemically defined and free from cell-derived contaminants, synthetic oligosaccharides are a powerful alternative as vaccine antigens. Synthetic oligosaccharides can be designed based on protective glycotopes so that conjugation to a carrier protein yields efficaceous semisynthetic vaccines. However, the design of oligosaccharide antigens requires information on the identity of protective glycotopes within a CPS. The conventional iterative vaccine development approach relies on the synthesis of antigens and immunological evaluation of glycoconjugates (6, 7). Thereby, the plethora of putative glycotopes in a polysaccharide challenges traditional chemical synthesis because a number of different structures are needed. A more recent glycotope discovery approach uses structure-based reverse engineering of antigen binding sites of protective monoclonal antibodies (mAbs) and has greatly advanced the field of glycoconjugate vaccines (1, 8). However, structure elucidation of carbohydrate-mAb interactions by classical biophysical methods such as X-ray crystallography is slow and suffers from low throughput.
Two methods of chemical glycobiology are particularly suitable to address the shortcomings of glycotope discovery. First, AGA can produce complex glycans by employing an automated solid-phase oligosaccharide synthesis workflow (9, 10) and allows for the rapid generation of glycan collections. Second, glycan microarrays use glycan collections to characterize multiple binding events of proteins to surface-immobilized glycans in a single experiment (11). Combining AGA with glycan microarrays simplifies both glycan accessibility and glycotope mapping compared to conventional methods.
Here, we describe the combination of AGA and reverse engineering of a protective mAb by glycan microarray to design a semisynthetic S. pneumoniae serotype 8 (ST8) glycoconjugate vaccine candidate. Highly virulent ST8 causes frequent outbreaks of invasive disease. ST8 is part of the polysaccharide vaccine Pneumovax 23, but is not included in conjugate vaccines such as Prevnar 13. Many clinical ST8 isolates are broadly resistant to antibiotics such that vaccination to prevent rather than fight ST8 infections is advisable (12, 13). The combination of AGA, glycan microarrays and mAb reverse engineering produced semisynthetic glycoconjugate vaccines against ST8 that were combined with Prevnar 13 to form experimental 14-valent vaccines. The reported glycotope discovery approach will greatly accelerate the development of efficacious prophylactic or therapeutic vaccines based on synthetic glycans.
Results
Automated glycan assembly of all frameshifts of serotype 8 capsular polysaccharide
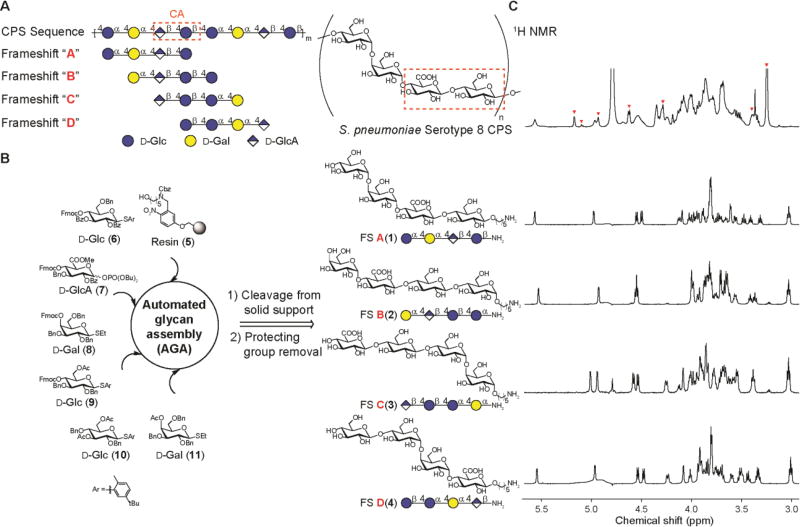
ST8 CPS consists of a linear tetrasaccharide repeating unit with the sequence [→4)-α-D-Glcp-(1→4)-α-D-Galp-(1→4)-β-D-GlcpA-(1→4)-β-D-Glcp-(1→] (Fig. 1A) (14, 15) and shares a common cellobiuronic acid disaccharide (β- d-GlcpA-(1→4)-β-d-Glcp) with S. pneumoniae serotype 3 (ST3) CPS (6, 15). The repetitive nature of ST8 CPS allows for the definition of four tetrasaccharide frameshifts A–D with different monosaccharide sequences (Fig. 1A). Octasaccharides derived from ST8 CPS hydrolysis have been found to induce protective immunity (16), but based on the findings that glycans as small as di- to tetrasaccharides induced protective immunity against other S. pneumoniae serotypes (8, 9, 17), we reasoned that a collection of all four tetrasaccharide frameshifts would cover most glycotopes in ST8 CPS that are relevant for antibody reverse engineering. We used automated glycan assembly (AGA) to synthesize conjugation-ready variants of all four frameshifts A(1), B(2), C(3) and D(4) from polystyrene resin 5 and orthogonally protected building blocks 6–11 (Fig. 1 B; fig. S1) in milligram quantities for glycan microarray screening of antibodies. Commercially available ST8 CPS is heavily contaminated with pneumococcal C-polysaccharide (18, 19), a common impurity in marketed pneumococcal glycoconjugate vaccines, whereas our chemical synthesis generated glycans devoid of cellular contaminants.
Fig. 1. Automated glycan assembly of ST8 CPS frameshift oligosaccharides.
(A) ST8 CPS structure and nomenclature of tetrasaccharide frameshifts A–D. The cellobiuronic acid (CA) moiety is highlighted. (B) Automated assembly of synthetic frameshifts A(1), B(2), C(3) and D(4) using resin 5 and building blocks 6–11. (C) Overlay of 1H NMR (600 MHz) spectra with commercially available ST8 CPS in D2O at 25 °C. Red arrowheads indicate peaks from pneumococcal C-polysaccharide as reported (18).
Glycotope mapping of ST8-binding antibodies
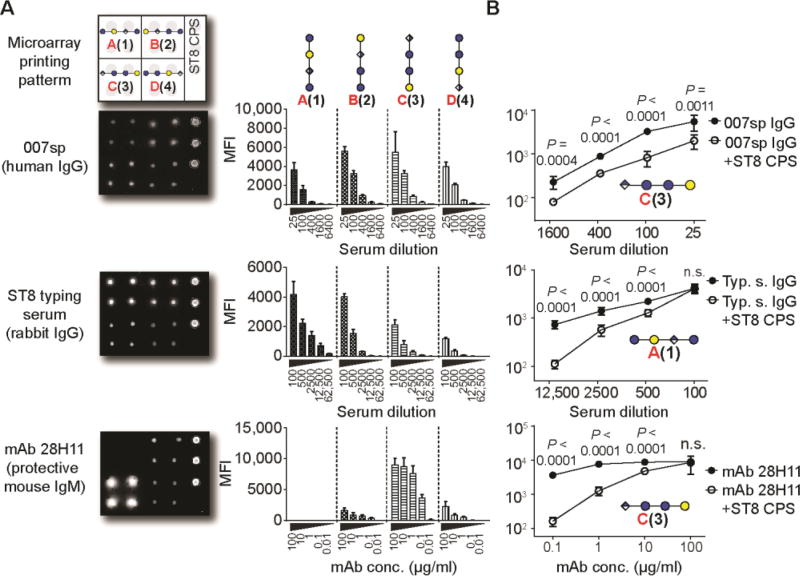
Glycan microarrays containing all ST8 CPS frameshifts served to map the glycotopes that are recognized by antibodies. Oligosaccharides 1–4 and native ST8 CPS were covalently immobilized on glass slides and probed with antibodies. We initially screened polyclonal sera raised against native ST8 CPS (Fig. 2, A and B). All four synthetic glycans as well as the native ST8 polysaccharide were recognized by both a human reference serum mixture (007sp) and a rabbit-derived ST8 typing serum (20). Thereby, the human serum bound preferentially to frameshifts B(2) and C(3), but only binding to frameshift C(3) was inhibited by antibody pre-adsorption with native ST8 CPS (Fig. 2, A and B; fig. S2). Non-ST8-dependent recognition of frameshift B may reflect the presence of naturally occurring antibodies in human sera that recognize glycotopes such as the terminal α-galactoside (21). The rabbit serum preferentially bound to frameshift A(1) and frameshift B(2) (Fig. 2A and fig. S2). Binding was abrogated by ST8 CPS pre-adsorption in both cases (Fig. 2B and fig. S2). Polyclonal antisera contain both protective as well as non-protective antibodies and therefore may not reveal the identity of protective glycotopes. We envisaged that analyzing a protective mAb would provide insights into the nature of a protective ST8 CPS glycotope. mAb 28H11, a murine IgM raised against native ST8 CPS, protects mice from infection with live ST8 pneumococci in vivo (3). Glycan microarray analysis revealed a robust interaction of mAb 28H11 with ST8 frameshift C(3) that was bound with a five times higher intensity than frameshifts B(2) and D(4) (Fig. 2A and fig. S2). mAb 28H11 did not bind frameshift A(1). Frameshift C(3) recognition of mAb 28H11 was specific, and could be blocked by adding native ST8 CPS (Fig. 2B and fig. S2). Thus, frameshift C tetrasaccharide 3 contains a protective ST8 glycotope.
Fig. 2. Differential immune recognition of synthetic ST8 CPS frameshift oligosaccharides 1–4.
(A) Glycan microarray analysis of pooled sera from Pneumovax 23-vaccinated humans (human reference serum mixture 007sp), rabbit ST8 typing serum, and a protective murine mAb 28H11 at different concentrations. Corresponding data are means + SD of 8 spots as technical replicates. (B) Inhibition of antibody binding by pre-adsorption with native ST8 CPS. Data are means + SD of 8 spots as technical replicates. P values are determined by one-tailed, unpaired t test with Welch’s correction. Data are from one representative out of at least 2 independent experiments. MFI, mean fluorescence intensity. n.s., not significant.
Evaluation of the immune response against synthetic ST8 frameshift C
To procure sufficient quantities of a frameshift C tetrasaccharide for detailed immunological evaluations, we developed a scalable solution-phase synthetic route of a frameshift C tetrasaccharide (12) (Fig. S3, A and B). Frameshift C tetrasaccharides 3 and 12 differed in the choice of reducing-end linkers that were tailored to provide sufficient distance from microarray slides (five-carbon linker in 3) or reduced hydrophobicity to facilitate protein conjugation (two-carbon linker in 12). A disaccharide fragment 13 was prepared from a synthetic intermediate to probe antibody specificity (Fig. S3C).
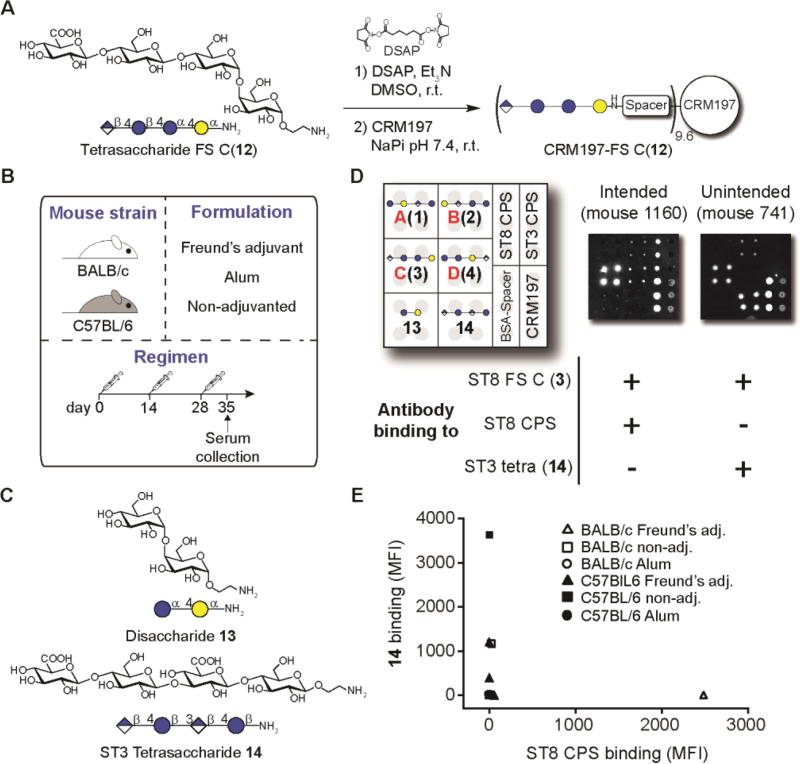
Tetrasaccharide frameshift C(12) was conjugated to the widely-used carrier protein CRM197 to establish whether immunization with this glycoconjugate would recapitulate the glycan binding pattern of mAb 28H11 (Fig. 3A). The glycoconjugate contained on average 9.6 oligosaccharide chains on each CRM197 protein (Fig. S4).
Fig. 3. Evaluation of a synthetic ST8 CRM197-frameshift C(12) glycoconjugate in mice.
(A) Conjugation of synthetic FS C tetrasaccharide 12 to CRM197. (B) Immunization strategy. Mice (n = 3 per group) were subcutaneously immunized 3 times with CRM197-frameshift C(12) using different mouse strains and formulations. Serum was collected one week after the third immunization. (C) Structures of glycans 13 and 14. (D) Glycan-directed immune responses of mice as assessed by glycan microarray, and cross-reactivity patterns towards ST8 CPS or tetrasaccharide 14. (E) Glycan binding specificities of sera reactive against ST8 frameshift C(3). DSAP, di-N-succinimidyl adipate NaPi, sodium phosphate buffer.
We performed an immunization experiment with CRM197-frameshift C(12) using different combinations of mouse strains and adjuvant formulations (Fig. 3B) and monitored the immune response by glycan microarray. We included disaccharide 13 and tetrasaccharide 14, a synthetic dimer of cellobiuronic acid that is an ST3 vaccine candidate (6, 17), in the microarray experiment to evaluate the glycan binding characteristics of immune sera (Fig. 3C). Sera immunized with the CRM197-frameshitf C (12) glycoconjugate either cross-reacted to native ST8 CPS or recognized ST3 tetrasaccharide 14 (Fig. 3D). Binding to 14 was observed more frequently, indicating that tetrasaccharides 12 and 14 share a glycotope that is immunogenic in mice. Both immune responses were mutually exclusive since no serum sample bound both ST3 tetrasaccharide 14 and ST8 CPS (Fig. 3E and fig. S5). Thus, ST8 CPS-binding antibodies can be raised by immunization with a semisynthetic frameshift C glycoconjugate, but a glycotope that is part of an ST3 CPS tetrasaccharide is more immunogenic and does not confer cross-reactivity to ST8 CPS in mice. Additionally, all sera cross-reacted with an unrelated N-acetylglucosamine-bovine serum albumin (BSA) glycoconjugate, likely due to the presence of free spacer molecules on the carrier protein as a side product of the conjugation reaction (Fig. 3D).
Development of mAbs directed against serotype 8 frameshift C
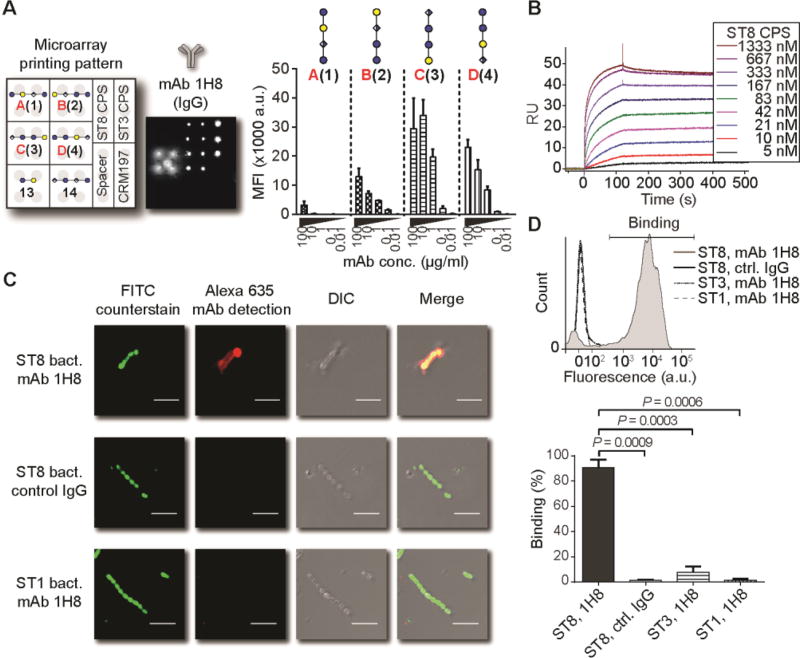
We then closely investigated the immune response invoked by the CRM197-frameshift C(12) glycoconjugate. To define protective glycotopes within the frameshift C tetrasaccharide, we generated two mAbs, (IgG1[κ]) and 1F1 (IgM[κ]), using splenocytes of a mouse that mounted an immune response against both synthetic frameshift C and ST8 CPS (no. 1160, fig. S5 and fig. S6). Both mAbs bound to immobilized ST8 CPS (Fig. S7A), and similar to mAb 28H11, mAb 1H8 showed detectable binding at antibody concentrations as low as 50 ng/ml. mAb 1H8 binding to synthetic ST8 CPS frameshifts closely resembled the binding pattern of mAb 28H11 by glycan microarray (see fig. 2), with specificity towards frameshift C(3) and less robust binding to frameshifts B(2) and D(4) (Fig. 4A). In contrast, mAb 1F1 was more promiscuous towards synthetic glycans, especially frameshifts B(2) and D(4) (Fig. S7B).
Fig. 4. Binding of ST8 CPS-derived glycans and ST8 bacteria by mAb 1H8.
(A) Glycan microarray analysis. Data are means + SD of 8 spots as technical replicates from one representative out of 3 independent experiments. (B) Surface plasmon resonance of immobilized mAb 1H8 using ST8 CPS as analyte in the indicated concentrations. (C) Immunofluorescence of inactivated, FITC-labeled ST8 or ST1 pneumococci by 1H8 or IgG1 control mAb. Scale bar: 5 µm. (D) Flow cytometry of fluorescently labeled, inactivated pneumococci. Data are a representative result with gating strategy for binding after incubation with mAb 1H8 or IgG1 control mAb and fluorescently labeled detection antibody, and cumulated results of 3 labeling experiments. Data are means + SD of positive binding from n = 3 independent experiments. P values are determined by one-tailed, paired t test. DIC, differential interference contrast; FITC, fluorescein isothiocyanate; RU, response units.
To shed light on the polysaccharide binding behavior of mAbs 1H8 and 1F1, we next used surface plasmon resonance to assess whether the mAbs bind terminal or internal glycotopes of ST8 CPS. Binding of terminal glycotopes to immobilized mAbs would lead to 1:1 interaction with detectable dissociation rates, while binding of internal glycotopes would exhibit undiscernible dissociation rates due to re-binding (22) We found that both mAbs 1H8 and 1F1 recognize internal glycotopes (Fig. 4B and fig. S7C). This finding was confirmed by the markedly accelerated dissociation of ST8 CPS fragments of intermediate (one to eight repeating units) chain lengths generated by acid-mediated depolymerization (16), as well as the 1:1 binding pattern of synthetic tetrasaccharide frameshift C(12) harboring a single repeating unit (Fig. S8). Thus, mAbs raised against ST8 frameshift C recognize internal rather than terminal glycotopes within the native polysaccharide.
Evaluation of serotype 8 frameshift C-directed mAbs in vitro and in vivo
We then investigated whether mAbs 1H8 and 1F1 bind ST8 bacteria. Immunofluorescence staining of UV-inactivated, fluorescently labeled S. pneumoniae revealed that both mAbs bound to ST8 capsules, as assessed by fluorescence microscopy (Fig. 4C) and flow cytometry (Fig. 4D; and fig. S7D). Interactions between mAbs 1H8 and 1F1 and bacteria are ST8-specific, since neither mAb bound S. pneumoniae serotype 1 (ST1) or ST3, and isotype-matched control antibodies did not bind ST8.
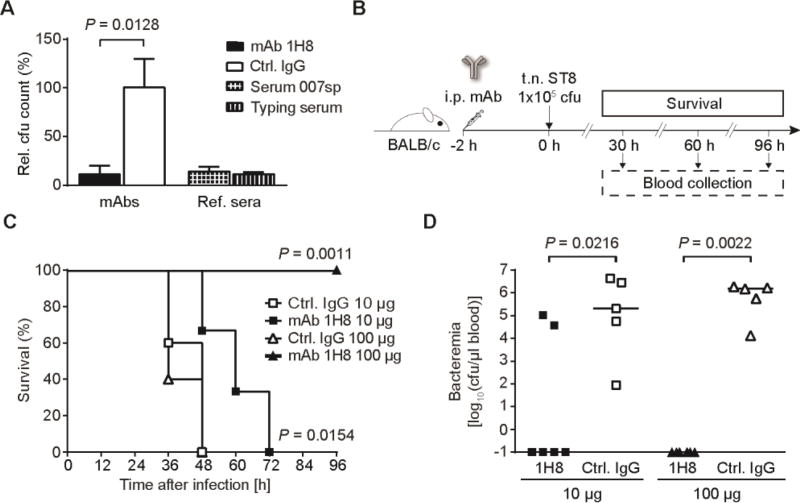
Next, we determined in vitro and in vivo whether mAbs against frameshift C could mount an anti-pneumococcal defense. mAb 1H8 rather than 1F1 was used as it binds ST8 CPS better and IgG are more relevant to the outcome of vaccinations than IgM antibodies. Antibody-dependent opsonization of bacteria is the most important defense mechanism against S. pneumoniae infections. In the presence of HL-60 cells as a phagocyte source, mAb 1H8 triggered opsonophagocytic killing at the same order of magnitude as high concentrations of reference sera (Fig. 5A) (23).
Fig. 5. Evaluation of mAb 1H8 to promote antibacterial activity in vitro and in vivo.
(A) Opsonophagocytic killing assay using differentiated HL-60 cells as a phagocyte source. Data are cfu reduction relative to control wells lacking an antibody source. Commercial ST8 antisera (1:18 dilution) are positive controls. Data are means + SD of n = 3 incubation wells of one representative out of four independent experiments. P value is determined by one-tailed, unpaired t test with Welch’s correction. (B–D) Passive immunization and lethal challenge with S. pneumoniae ST8. (B) Passive immunization strategy. Mice were treated intraperitoneally (i.p.) with 10 µg or 100 µg of mAb 1H8 or control IgG1 2 h prior to lethal transnasal (t.n.) challenge with ST8 bacteria. Blood was withdrawn at regular intervals and survival was monitored for 96 h. (C) Survival of antibody-treated mice after ST8 infection. P values are determined by Mantel-Cox log-rank test of n = 5–6 mice between groups of equal antibody doses. (D) Bacterial burden in the blood of mice 30 h after infection. Data are values from n = 5–6 individual mice and median. P values are determined by Mann-Whitney U test between groups of equal antibody doses.
To determine whether binding of mAb 1H8 to native ST8 polysaccharide is functional in a disease setting, we studied protection of mice from pneumococcal infection after passive immunization. mAb 1H8 was administered to mice prior to transnasal infection with a lethal dose of ST8 (Fig. 5B). Both survival and bacteremia were monitored and correlated with bloodstream levels of mAb 1H8 by glycan microarray analysis, making use of the distinct binding pattern of this mAb (Fig. S9, A and B). A 10 µg mAb 1H8 dose significantly prolonged survival of infected mice when compared to the control mAb (Fig. 5C), whereas a 100 µg dose of mAb 1H8 resulted in 100% survival. Survival correlated inversely with bacterial burden, and mice with measurable amounts of bacteria in the bloodstream died within twelve hours after detection of bacteremia (Fig. 5D and fig. S9, A and B). The presence of anti-capsular mAb 1H8 excluded bloodstream invasion of pneumococci (Fig. S9C).
Mapping serotype 8 frameshift C protective and non-protective glycotopes
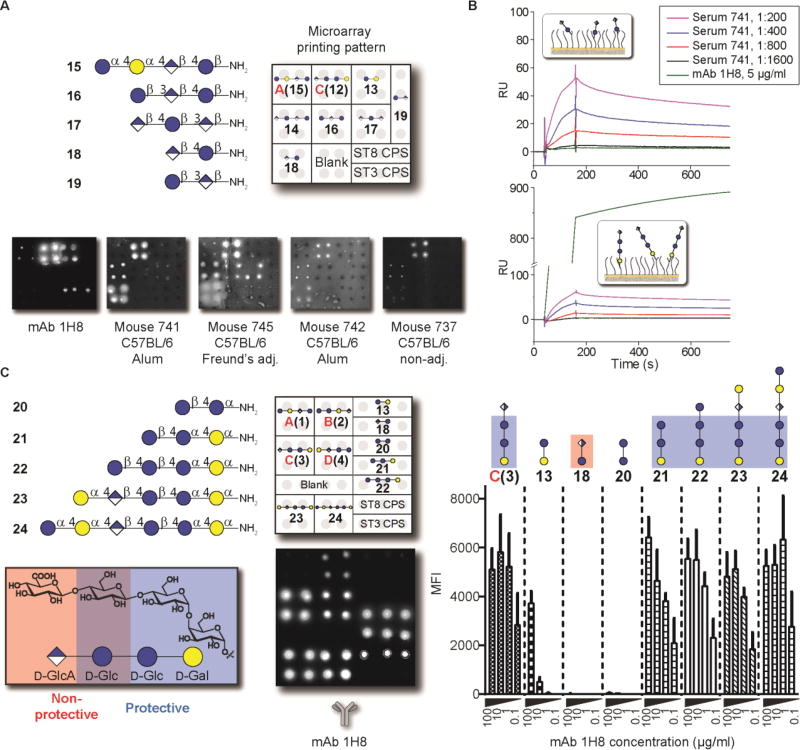
Immunization of mice with a CRM197-frameshift C(12) glycoconjugate induced immune responses either towards ST8 polysaccharide or to the cellobiuronic acid-containing tetrasaccharide 14 (see fig. 3D and E). Binding to ST8 CPS was key to protection from pneumococcal infection. We next determined the nature of protective as well as non-protective glycotopes found in ST8 frameshift C using a collection of synthetic, ST8 and ST3 CPS-related oligosaccharides (Fig. 6, A and B) (17). Antisera that were raised against frameshift C(12) and bound to ST3 tetrasaccharide 14 interacted with cellobiuronic acid 18, whereas mAb 1H8 did not recognize disaccharide 18 by glycan microarray and surface plasmon resonance (Fig. 6, A and B). Little or no binding was found to frameshifted ST3 disaccharide 21, ST3 CPS-derived trisaccharides 16 and 17, or native ST3 CPS, confirming that cellobiuronic acid is the immunogenic glycotope in ST8 frameshift C (Fig. 6A). The immune response induced by that glycotope in mice did not confer cross-reactivity towards ST8 or ST3 CPSs. Thus, the information conferring protective immunity against ST8 CPS is likely located elsewhere in the frameshift C tetrasaccharide.
Fig. 6. Mapping protective and non-protective glycotopes of ST8 frameshift C.
(A) Glycan microarray analysis comparing ST8 CPS-recognizing mAb 1H8 and antisera of mice that recognize a non-protective glycotope of frameshift C (see fig. 3) by using synthetic ST8 and ST3 CPS-derived oligosaccharides. (B) Surface plasmon resonance of mAb 1H8 and serum of mouse 741 with immobilized glycans frameshift C(12) and disaccharide 18. (C) Glycan microarray of mAb 1H8 using oligosaccharides 20–24 for glycotope mapping. Boxes mark protective (blue) and non-protective (red) glycotopes. Data are means + SD of fluorescence intensities of at least 6 spots as technical replicates from one representative out of at least 3 independent experiments.
To identify the protective glycotope in ST8 frameshift C in detail, we synthesized a panel of ST8 frameshift C-related glycans 21–24 (Fig. S10). Saccharides 21 and 22 lacked the terminal GlcA moiety that is an integral part of the non-protective cellobiuronic acid glycotope, with tetrasaccharide 22 as a “reduced” frameshift C congener containing a terminal Glc instead of GlcA. Penta- and hexasaccharides 23 and 24 served to study the effects of saccharide extensions at the non-reducing end of frameshift C. Disaccharide 20 represents the internal frameshift C disaccharide.
Glycan microarrays containing the collection of synthetic frameshift C-related oligosaccharides served to elucidate the protective glycotope of ST8 CPS recognized by mAbs 1H8 and 1F1 (Fig. 6C and fig. S12). Omission of the terminal GlcA moiety of frameshift C did not abrogate binding by either mAb, as long as the reducing-end sequence β-D-Glcp-(1→4)-α-DGlcp-(1→4)-α-D-Galp was intact; both trisaccharide 21 and tetrasaccharide 22 were recognized with comparable intensities to frameshift C(3). Further glycan extension at the non-reducing end in oligosaccharides 23 and 24 did not improve binding. In contrast, truncation of trisaccharide 21 by a single monosaccharide residue at either reducing or non-reducing ends (20 and 13, respectively) led to almost complete ablation of binding. All interactions were abrogated by mAb pre-adsorption to native ST8 CPS (Fig. S12). Thus, the trisaccharide β-D-Glcp-(1→4)-α-D-Glcp-(1→4)-α-D-Galp, represented by glycan 21, is the smallest structure containing a protective epitope of ST8 frameshift C.
To probe a possible correlation between binding preferences for anti-ST8 mAbs and affinities, we measured the dissociation constants (KD) of mAb 1H8 and synthetic ST8 CPS-derived oligosaccharides (Fig. S13). Similar KD values of 2.0 – 5.8 µM were measured for mAb 1H8 binding of ST8 frameshift C(12), trisaccharide 21 and tetrasaccharide 22, while binding of disaccharide 13 was approximately fivefold weaker. These results are in good agreement with the microarray data on the respective mAb-oligosaccharide interactions (see fig. 6C) and confirm the role of the trisaccharide β-D-Glc-(1→4)-α-D-Glc-(1→4)-α-D-Gal found at the reducing end of frameshift C as a protective glycotope of ST8 CPS.
Development of a semisynthetic S. pneumoniae serotype 8 glycoconjugate vaccine
With information on protective and non-protective glycotopes of ST8 frameshift C in hand, we developed a semisynthetic glycoconjugate vaccine against ST8. The initial immunogenicity screening experiments in fig. 3 revealed high spacer and weak glycan immunogenicity as the main challenges en route to an ST8 vaccine candidate. To address spacer immunogenicity, we changed the reagent used for glycan-protein conjugation from a bis-N-hydroxysuccinimide ester to a more stable bis-nitrophenol ester (24), thereby reducing the amount of free spacer on the final glycoconjugate (Fig. S14). CRM197 glycoconjugates of trisaccharide 21 and tetrasaccharide 22 that contained the protective glycotope but lacked the non-protective cellobiuronic acid substantially reduced the immune response against free spacer during immunization experiments in mice (Fig. S15). However, immunogenicity of tetrasaccharide 22 was low in mice, and a CRM197-21 glycoconjugate did not induce a ST8 CPS-directed immune response (Fig. S15).
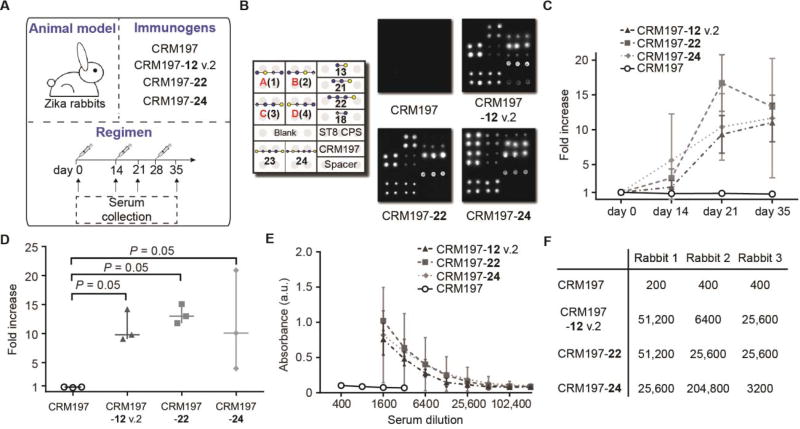
In contrast to mice, semisynthetic ST8 glycoconjugates induced reliable immune responses against native ST8 CPS in rabbits. We immunized rabbits with CRM197 glycoconjugates prepared by bis-nitrophenol ester conjugation of synthetic ST8 frameshift C(12) (termed CRM197-12 v.2), tetrasaccharide 22 as well as hexasaccharide 24 in the presence of Alum (Fig. 7A and fig. S16). Importantly, all glycoconjugates, but not CRM197 alone, induced a robust immune response against ST8-related glycans, including ST8 CPS, in all rabbits, as assessed by glycan microarray (Fig. 7B and fig. S16). Polysaccharide ELISA indicated a time course-dependent immune response as well as robust binding to ST8 CPS (Fig. 7, C–F). Importantly, tetrasacharide 22 induced an ST8-directed immune response with similar ELISA endpoint titers as frameshift C(12) and hexasaccharide 24 (Fig. 7F), underlining the nature of the reducing-end trisaccharide as a protective glycotope of frameshift C.
Fig. 7. Evaluation of semisynthetic glycoconjugates as vaccine candidates against ST8 in rabbits.
(A) Immunization strategy. Rabbits (n = 3 per group) were s.c. immunized 3 times with CRM197 glycoconjugates or CRM197. Serum was collected at regular intervals. (B) Immune responses of one representative rabbit per group at day 35, as assessed by glycan microarray. (C–F) Immune response against ST8 CPS as assessed by polysaccharide ELISA. (C) Time course of ST8 CPS binding by rabbit sera (1:1600 dilutions). Data are mens ± SD of n = 3 rabbits per group. (D) Evaluation of ST8 CPS binding by rabbit sera at day 35 (1:1600 dilution). Data are medians ± range of n = 3 rabbits per group. P values are determined by one-tailed Mann-Whitney U test. (E) ST8 CPS binding of rabbit sera at different dilutions. Data are means ± SD of n = 3 rabbits per group. (F) Endpoint titers of rabbit sera (n = 3 per group) against ST8 CPS.
Addition of semisynthetic S. pneumoniae serotype 8 glycoconjugates extends the 13-valent vaccine Prevnar 13
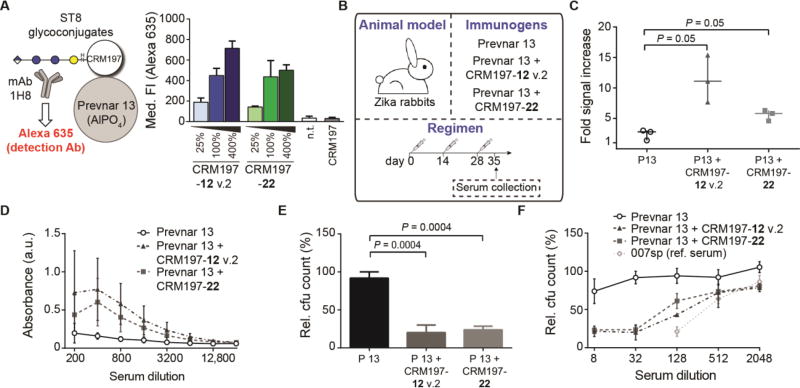
ST8 is not contained in current polysaccharide-based glycoconjugate vaccines such as Prevnar 13. Combination of these vaccines with semisynthetic glycoconjugates may be relevant to expand current formulations, or to replace serotypes that are not efficiently targeted due to difficulties during polysaccharide isolation and conjugation. To assess whether semisynthetic oligosaccharide-based ST8 glycoconjugates are compatible with marketed polysaccharide-based vaccines, we co-formulated Prevnar 13 with ST8 glycoconjugates to create 14-valent vaccines. Adsorption of CRM197 glycoconjugates containing frameshift C(12) or tetrasaccharide 22 to the AlPO4-based Prevnar 13 emulsion was confirmed by flow cytometry, using mAb 1H8 to detect these glycans (Fig. 8A and fig. S17, A and B). ST8 glycoconjugates were adsorbed to Prevnar 13 in a dose-dependent manner (Fig. 8A). Orthogonal detection of ST1 CPS with rabbit-derived ST1 typing serum indicated that ST8 glycoconjugate adsorption does not abrogate adsorption of other serotypes in Prevnar 13, such as ST1 (Fig. S17, C and D).
Fig. 8. Co-formulation of semisynthetic ST8 glycoconjugates and Prevnar 13.
(A) Adsorption of ST8 glycoconjugates to Prevnar 13, as assessed by flow cytometry. ST8 glycoconjugates were adsorbed to Prevnar 13 particles using 25%, 100% or 400% of a full dose equivalent, and detected by flow cytometry using mAb 1H8 and an Alexa Fluor 635-labeled detection antibody. Controls are non-treated and CRM197-treated Prevnar 13 particles. Data are means + SD of the median fluorescence intensities of n = 3 independent experiments. (B) Immunization strategy. Rabbits (n = 3 per group) were s.c. immunized 3 times with Prevnar 13 with or without co-adsorbed CRM197 glycoconjugates. Serum was collected at day 35. (C–D) Immune response to ST8 CPS as assessed by polysaccharide ELISA. (C) Evaluation of ST8 CPS binding by rabbit sera at day 35 (1:200 dilution). Data are medians ± range from n = 3 individual rabbits per group. P values are determined by one-tailed Mann-Whitney U test. (D) Comparison of ST8 CPS binding of rabbit sera from different groups. Data are means ± SD of n = 3 rabbits per group. (E) Comparison of opsonophagocytic killing of ST8 pneumococci by pooled rabbit sera. Data are means + SD of cfu reduction relative to control wells of n = 3 incubation wells as biological replicates at a 1:32 serum dilution. P values are determined by one-tailed, unpaired t test with Welch’s correction. (F) Comparison of opsonophagocytic killing by pooled rabbit sera and human reference serum 007sp at different serum dilutions. Data are means ± SD of cfu reduction relative to control wells of n = 3 incubation wells. Data in (E) and (F) are from one representative out of 3 independent experiments.
Rabbits were then immunized with 14-valent co-formulations of Prevnar 13 and CRM197-12 v.2 or CRM197-22 (Fig. 8B). The ST8 glycan dose (2.2 µg) was equal to that of most individual serotypes in the commercial vaccine. Both 14-valent vaccines, but not Prevnar 13 alone, induced a pronounced immune response against ST8 CPS (Fig. 8, C and D). Sera of rabbits immunized with the 14-valent vaccines mediated opsonophagocytic killing of ST8 pneumococci, in contrast to sera from Prevnar 13-immunized rabbits (Fig. 8, E and F). Serum dilutions between 1:32 and 1:128 still induced 50% killing, well above the criteria of 1:8 for protective immunity against pneumococci (25) and similar to the opsonophagocytic killing efficacy of 007sp, the reference serum established by the World Health Organization to evaluate new pneumococcal glycoconjugate vaccines (20). Co-formulation with ST8 glycoconjugates did not impair the immunogenicity of other Prevnar 13-specific glycoconjugates, as the immune response of rabbits towards six Prevnar 13-specific CPSs was not markedly decreased (Fig. S18, and table S2). Thus, semisynthetic ST8 glycoconjugates induce a robust, antibacterial immune response when co-formulated with a multivalent, polysaccharide-based glycoconjugate vaccine.
Discussion
Glycoconjugate vaccines have had tremendous success in preventing infections from encapsulated bacteria, and are part of childhood immunization schedules. Despite the advances made in covering an increasing number of serotypes, polysaccharide-based pneumococcal vaccines fail to induce an immune response with acceptable opsonophagocytic killing properties against certain serotypes (26, 27) and do not cover some of the most invasive types at all. Certain bacterial polysaccharides fail to induce a protective immune response in their native form, and alternative strategies are needed to develop efficacious vaccines (5, 28). Synthetic antigens are free of cellular impurities, structurally defined and can be reproducibly manufactured in large quantities, as illustrated by a glycoconjugate vaccine against shigellosis that is entering clinical trials (29). A tremendous advantage of synthetic over isolated antigens is the possibility to include protective while omitting non-protective glycotopes to optimize the efficacy and advance the production of glycoconjugate vaccines. However, identifying these glycotopes from first principles is cumbersome and one reason why only few semisynthetic glycoconjugate vaccines have entered clinical evaluation stages.
We describe here a strategy for the rapid discovery of protective glycotopes to aid the generation of efficacious semisynthetic glycoconjugate vaccines. ST8 served as a model since very few synthetic ST8 glycans have been prepared thus far (30). Our approach relies on the availability of synthetic glycan collections to drive glycotope discovery. AGA provided straightforward access to all four frameshifts of the native ST8 polysaccharide, and pairing AGA with glycan microarrays allows for rapid antibody reverse engineering. With the ongoing commercialization of AGA and the development of methods to facilitate synthesis (31), our glycotope discovery strategy can be implicated in the design of glycoconjugate vaccines on a broader scale.
Uncovering the frameshift of a polysaccharide that is bound by a mAb may not directly reveal the corresponding minimal protective glycotope. Accordingly, we identified the cellobiuronic acid moiety in frameshift C to be non-protective, but immunodominant in mice to the protective glycotope β-D-Glcp-(1→4)-α-D-Glcp-(1→4)-α-D-Galp in the same frameshift. Although a cellobiuronic acid disaccharide antigen was found to confer protective immunity against ST3 (6), no ST3-directed immune response was found after immunization of mice with CRM197-frameshift C(12), possibly due to the different presentation of cellobiuronic acid within the ST8 sequence. Thus, while the availability of all ST8 frameshifts was crucial to get insight into the presence of protective glycotopes, a more comprehensive glycan collection was required to exclude non-protective glycotopes. In-depth characterization of protective frameshift C-directed mAbs revealed a trisaccharide glycotope that was weakly immunogenic in mice, but induced a robust antibacterial immune response as a vaccine antigen in rabbits. Little is known about the species-specific differences in anti-carbohydrate immune responses. Despite engaging a unique mechanism of creating antibody diversity (32), rabbits are phylogenetically closer to humans than rodents and have been used extensively in the development of glycoconjugate vaccines (33, 34). The fact that rabbits develop more reproducible immune responses than mice against synthetic ST8-related glycans may hint at a more favorable general antibody architecture in rabbits (35). Alternatively, the antibody repertoire in rabbits, but not mice, may allow for the preferential generation of antibodies that recognize internal glycotopes as part of native polysaccharides.
Albeit immune responses correlated well between rabbits and primates in the evaluation of a polysaccharide-based glycoconjugate vaccine (33), optimized protective glycotopes may vary between species. The efficacy of semisynthetic glycoconjugate vaccines against ST8 in humans will be revealed during clinical evaluation. Reverse engineering human instead of murine mAbs is a promising avenue to circumvent species-specific antigen preferences if such antibodies are available.
Several parameters have to be optimized before translation of semisynthetic glycoconjugates to the clinic. These parameters include the antigen/carrier ratio and dose that may have an effect on immunogenicity (36), the adjuvant and excipient used for formulation, glycoconjugate stability and toxicity and the immunogenicity in humans. Guidelines on the development of glycoconjugate vaccines are mostly tailored towards isolated polysaccharide antigens (1, 37). Most quality control steps in the current vaccine manufacturing process aim to reduce co-isolated impurities, and antigen characterization is often performed by wet chemistry. In contrast, the generation of homogeneous synthetic glycan antigens is no different from small molecule therapeutics, including the development of process scale synthesis routes and standardized analytical procedures. The advent of semisynthetic glycoconjugate vaccines in pre-clinical and clinical stages of development requires the implementation of appropriate quality control guidelines, which will likely be much more concise than existing ones.
Synthetic vaccine antigens against a number of encapsulated, pathogenic bacteria have been reported. The combination of synthetic oligosaccharide antigens with existing, efficient polysaccharide antigens in the same vaccine has great potential to increase the number of pathogens covered, but requires information on the compatibility of both. Our finding that semisynthetic ST8 glycoconjugates can be combined with a marketed, polysaccharide-based pneumococcal vaccine while retaining the immunogenicity of both components is crucial for vaccine expansion. Development, in vivo immunological testing and compatibility assessment of glycan antigens against other serotypes will allow for the rapid generation of protective vaccines against a multitude of different pathogens.
Materials and Methods
Study Design
Predefined study components
Sample sizes were calculated using G*Power 3.1.9.2 (38). For active immunization experiments, a signal increase of 10 ± 2.5 in polysaccharide ELISA was targeted with respect to control groups or pre-immune serum (33), resulting in an effect size of d = 3.6 with α = 0.05 and a power of 1-β = 0.8, hence n = 3 (one-tailed, unpaired Mann-Whitney U test). This sample size was changed to n = 6 in immunizations of mice with CRM197-22 after prior immunizations had revealed a high variability in anti-glycan immune responses of mice. Mice were kept in cages of 3 animals. During active immunization experiments, mice were examined for their appearance, behavior, grooming, and body weight. Predefined criteria for premature euthanasia included >20% weight loss as well as the general criteria impaired reflexes, pain, bleeding and lethargy. Mice were humanely sacrificed after the end of the experiment by cervical dislocation. For passive immunization experiments, the bacterial burden (log cfu/µl blood) after infection was determined as 6 ± 1.5. A reduction of 50% of this value was targeted by mAb treatment, resulting in an effect size of d = 2.0 with α = 0.05 and a power of 1-β = 0.8, hence n = 5 (one-tailed, unpaired Mann-Whitney U test). Mice were kept in cages of 5 animals. Mice were humanely sacrificed when they reached at least one of the predefined criteria ((i) body temperature <30 °C, (ii) body weight loss >20%, (iii) cumbersome breathing, (iv) accelerated breathing in combination with staggering, pain or paleness) by exsanguination via the caudal vena cava after intraperitoneal injection of ketamine (160 mg/kg body weight) and xylazine (75 mg/kg). No data was excluded.
Negative fluorescence intensities in glycan microarray experiments indicated absence of binding and were arbitrarily set to 0.
Rationale and design of study
The aim of this work was to design a semisynthetic glycoconjugate vaccine against S. pneumoniae ST8. All four frameshifts of the tetrasaccharide repeating unit of the ST8 CPS were generated by AGA and used to reverse engineer a protective mAb against ST8 CPS. Synthetic tetrasaccharide antigen frameshift C(12) was applied as a vaccine hapten in mice. Since, in addition to an immune response against the native ST8 CPS, this antigen frequently induced a non-protective immune response, mAbs were generated to map the precise glycotopes needed for protective immunity. A mAb raised against 12 protected mice from lethal pneumococcal infection and revealed the protective glycotope of 12. On that basis, semisynthetic glycoconjugates containing the protective glycotope were used to induce an antibacterial immune response in rabbits. It was further shown that ST8 glycoconjugates can be combined with a marketed vaccine against S. pneumoniae against other serotypes.
Randomization/blinding
During passive immunization, mice receiving different doses of the same antibody were co-housed in the same cage. No blinding was carried out.
Replication
The number of replicates for each experiment is specified in the figure legends.
Relevance of the work on the replacement, reduction or refinement (3R principle) of animal experiments
Compared to traditional glycotope discovery strategies, mAb reverse engineering alleviates the need of iterative antigen generation and immunization, and therefore potentially lowers the number of experimental animals used. ARRIVE guidelines for animal research were followed.
Chemical synthesis
Saccharides were synthesized from protected precursor building blocks by either automated glycan assembly or solution-phase oligosaccharide synthesis, as described in Supplementary Methods and figs. S1, S3, S10, S11, and table S1.
Glycan microarray analysis
Synthetic oligosaccharides and natural polysaccharides were spotted on N-hydroxysuccinimide activated glass slides as described in Supplementary Methods. Slides were quenched, blocked and treated with the corresponding antibody samples. Binding was visualized by fluorescence read-out after incubation with fluorophor-labeled secondary antibodies,
Glycoconjugate immunizations
All mouse experiments were approved by local institutional (Charité - Universitätsmedizin Berlin) and governmental authorities (Landesamt für Gesundheit und Soziales Berlin, approval IDs G0128/12, G0135/14 and A0305/12). Animal housing and experiments were in strict accordance with the regulations of the Federation of European Laboratory Animal Science Associations (FELASA) and recommendations for the care and use of laboratory animals. All mice were housed under specific pathogen-free conditions. Mice (6–8 week old female BALB/c or C57BL/6N mice, Charles River) were immunized subcutaneously with the CRM197-frameshift C(12) glycoconjugate (4 µg synthetic glycan/dose) formulated either as a 1:1 (v/v) emulsion with Complete Freund’s Adjuvant (CFA, Sigma-Aldrich), a 1:1 (v/v) suspension with Alum (Alhydrogel, Brenntag), a 1:1 (v/v) suspension with Alum containing 50 µg/ml monophosphoryl lipid A (Avanti Polar Lipids) or without adjuvant at a total volume of 100 µl. Alum mixtures were incubated overnight at 4 °C before use. Booster doses were given at days 14 and 28 using the same strategy. Mice primed with CFA received booster doses with Incomplete Freund’s Adjuvant (Sigma-Aldrich). Blood (50 µl) was withdrawn once a week from the tail vein or the facial vein to retrieve serum.
Rabbit immunization experiments were performed by Biogenes GmbH. Rabbits were housed and handled according to international animal regulations (EU Directive 2010/63/EU) and sanctioned by governmental authorities (Landesamt für Landwirtschaft, Lebensmittelsicherheit und Fischerei Mecklenburg-Vorpommern). Rabbits (female Zika rabbits, 10–12 weeks, 2.5–3 kg, n = 3 per group) were immunized subcutaneously at days 0, 14 and 28 at four different sites. Sera were collected at days 0, 14, 21 and 35. For immunogenicity assessment, glycoconjugate (10 µg glycan per dose) or CRM197 (100 µg) were formulated in 400 µl per dose containing 200 µl Alum and incubated overnight at 4 °C. For Prevnar 13 + ST8 glycoconjugate co-formulations, ST8 glycoconjugate (2.2 µg glycan per dose) in 20 µl PBS was admixed to a full dose Prevnar 13 for each immunization dose and incubated overnight at 4 °C. Prevnar 13 was used for control immunizations.
Opsonophagocytic killing assay
An opsonophagocytic killing assay (OPKA) was performed as described (24). Briefly, HL-60 cells were differentiated for one week with N,N-dimethylformamide, washed twice with Hank’s balanced sterile saline supplemented with 0.1% (w/v) gelatin (OPKA buffer) and diluted to a density of 107 cells/ml in the same buffer directly before use. Bacteria were grown in growth medium at 37 °C/5% CO2 to log phase (OD600 approx. 0.2–0.3), diluted in freezing medium to a density of 106 cfu/ml and frozen in 0.5-ml aliquots at −80 °C. Bacteria were diluted with OPKA buffer and aliquoted (1000 cfu in 20 µl each) in a 96 well-plate. Bacterial suspensions were treated with mAb solutions or antiserum dilutions in triplicates and incubated for 15 min at 37 °C. Complement source (10% (v/v) of the total volume, rabbit complement, CedarLane) and differentiated HL-60 cell suspension (40 µl, phagocyte/bacteria ratio 400:1) were added and the suspensions were incubated for 45 min at 37 °C with shaking. An aliquot of each well was plated on Columbia Agar plates with 5% (v/v) sheep blood, and cfu were counted after incubation at 37 °C/5% CO2 overnight. Control wells lacked either antibody or complement. Killing was calculated relative to wells lacking antiserum. Antibody concentration in graphs relates to the initial opsonization stage (mAbs) or dilution after addition of all assay components (antisera).
Passive immunization and lethal pneumococcal challenge
Streptococcus pneumoniae ST8 was cultured in gowth medium at 37 °C/5% CO2 to log phase, harvested and suspended in sterile PBS. Female BALB/c mice (12 weeks, 20–22 g, Charles River) were treated i.p. with mAbs 1H8 or anti-Y. pestis 1E12 at doses of either 10 or 100 µg in 100 µl sterile PBS 2 h prior to infection. Mice were anaesthetized by i.p. ketamine (80 mg/kg, Ketavet, Pfizer) and xylazine (25 mg/kg, Rompun, Bayer) and t.n. inoculated with 1×105 cfu S. pneumoniae in 20 µl PBS. Disease severity was evaluated at 12 h intervals (more often if severely ill) for 96 h after bacterial infection to assess appearance, behavior, grooming, respiration, body weight and rectal temperature. Blood samples (max. 20 µl) were removed from tail veins of surviving mice 30 h and 60 h post infection. Ninety-six h after infection, surviving mice were anaesthetized with i.p. ketamine (160 mg/kg) and xylazine (75 mg/kg). After heparinization, blood was drawn from the vena cava caudalis and lungs were removed.
Serial dilutions of blood samples were plated on Columbia agar plates with 5% (v/v) sheep blood and incubated at 37 °C under 5% CO2 overnight to count cfu. Blood antibody levels were monitored by glycan microarray analysis after 0.2 µm sterile filtration of 1:20 dilutions of blood samples and comparison of the frameshift C(3) signal with a series of different concentrations of mAb 1H8.
To assess the residual bacterial burden of mice 96 h p.i., lungs were homogenized by passage through a cell strainer (100 µm, BD Bioscience). Serial dilutions of lung homogenates were assessed for cfu growth.
Statistical analysis
Statistical analysis was performed using Prism 6 (Graphpad Software Inc.). Tests used are stated in the corresponding figure legends. Non-parametric tests are used to assess the outcome of rabbit immunizations.
Supplementary Material
Fig. S1. Automated glycan assembly of tetrasaccharides 1–4.
Fig. S2. Differential immune recognition of synthetic ST8 CPS frameshifts.
Fig. S3. Solution-phase syntheses of ST8 CPS frameshift C(12) and disaccharide 13.
Fig. S4. Characterization of the CRM197-frameshift C(12) glycoconjugate.
Fig. S5. Time course of anti-glycan immune responses after immunization with CRM197-frameshift C(12).
Fig. S6. Characterization of mAbs raised against ST8 frameshift C(12).
Fig. S7. Binding of ST8-related glycans by mAbs 1H8, 28H11 and 1F1.
Fig. S8. Qualitative comparison of binding kinetics of mAb 1H8 towards ST8 CPS-derived glycans of different chain lengths.
Fig. S9. Correlation between mAb 1H8 levels and bacterial burden in blood after passive immunization and lethal pneumococcal challenge.
Fig. S10. Divergent total syntheses of ST8 frameshift C-related glycans 21–24 from versatile precursor 32.
Fig. S11. Total synthesis of ST8 tetrasaccharide 15.
Fig. S12. Binding characterization of mAbs 1H8 and 1F1 to ST8 CPS-derived substructures by glycan microarray.
Fig. S13. Determination of affinities of mAb 1H8 towards synthetic, ST8 CPS-related oligosaccharides.
Fig. S14. Conjugation of synthetic oligosaccharides to CRM197 using the bifunctional spacer bis(4-nitrophenyl)adipate (DNAP).
Fig. S15. Evaluation of CRM197 glycoconjugates of trisaccharide 21 and tetrasaccharide 22 in mice.
Fig. S16. Immune response against semisynthetic ST8 glycoconjugates in rabbits.
Fig. S17. Adsorption of ST8 glycoconjugates to Prevnar 13, as assessed by flow cytometry.
Fig. S18. Effect of co-formulation of semisynthetic ST8 glycoconjugates with Prevnar 13 on the immune response against several pneumococcal CPSs.
Table S1. Sequences of automated assembly of protected ST8 CPS-related tetrasaccharide frameshifts.
Table S2. Anti-polysaccharide IgG endpoint titers of rabbits immunized with Prevnar 13 alone or co-formulated with semisynthetic ST8 glycoconjugates.
Acknowledgments
The authors thank D. Barthel for valuable help during passive immunization experiments; E. Settels for HPLC analyses and excellent technical support; A. Geissner for help with microarray experiments; and F. Bröcker for help with glycoconjugate adsorption studies. We are grateful to S. Hammerschmidt (Universität Greifswald) for pneumococcal strains.
Funding: The Max Planck Society, the German Federal Ministry of Education and Research (BMBF) and the Körber Foundation provided generous financial support. This work was supported by a Kekulé doctoral fellowship by the Fonds der Chemischen Industrie (to B.S.), the German Ministry of Education and Research (e:Med and CAPSyS to M.W.), the National Institutes of Health (grants no. R56AI104234 and R01AG045044 to L.P.), a European Research Council Advanced Grant no. 227975 AUTOHEPARIN (to P.H.S.) and by the Deutsche Forschungsgemeinschaft (SFB-TR84, C3 and C6 to M.W. and C8 to P.H.S.).
Footnotes
Author contributions: B. S., H. S. H., S. G. P., K. R., L.-a. P., M. W., C. L. P., C. A., and P. H. S. designed research. B. S., S. G. P. and S. G. performed chemical syntheses, H. S. H. performed automated glycan assembly, B. S. and P. K. ran glycan-antibody interaction experiments, B. S. and A. W. performed immunizations and generated mAbs, K. R. and B. S. carried out passive immunization, L.-a. P. prompted research into this serotype and provided mAb 28H11, B. S., S. G. P., K. R., M. W., C. A., C. L. P. and P. H. S. analyzed data, B. S. and K. R. performed statistical analyses, B. S., K. R., M. W., C. A., C. L. P. and P. H. S. wrote the paper.
Competing interests: Synthetic oligosaccharides as vaccine antigens against ST8 are included in patents “Synthetic vaccines against Streptococcus pneumoniae serotype 8” no. PCT/EP2015/072294 filed by the inventors B. S., H. S. H., S. G. P., S. G., C. A., C. L. P. and P. H. S. and “Pneumococcal polysaccharide-protein conjugate composition” no. EP16179133 filed by the inventors B. S.,S. G. P., P. K., C. L. P. and P. H. S. P. H. S., C. L. P. and S. G. P. have a significant financial interest in “Vaxxilon” a spin-off company that is developing vaccines based on synthetic oligosaccharide antigens.
Data and materials availability: Data are available in the Supporting Information section.
References
- 1.Schumann B, Anish C, Pereira CL, Seeberger PH. In: Biotherapeutics: Recent Developments Using Chemical and Molecular Biology, RSC Drug Discovery Series No. 36. Jones L, McKnight AJ, editors. RSC Publishing; Cambridge: 2013. pp. 68–104. [Google Scholar]
- 2.Nguyen HP, Seto NO, MacKenzie CR, Brade L, Kosma P, Brade H, Evans SV. Germline Antibody Recognition of Distinct Carbohydrate Epitopes. Nat. Struct. Biol. 2003;10:1019. doi: 10.1038/nsb1014. [DOI] [PubMed] [Google Scholar]
- 3.Yano M, Pirofski LA. Characterization of Gene Use and Efficacy of Mouse Monoclonal Antibodies to Streptococcus Pneumoniae Serotype 8. Clin. Vaccine Immunol. 2011;18:59. doi: 10.1128/CVI.00368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fusco PC, Farley EK, Huang CH, Moore S, Michon F. Protective Meningococcal Capsular Polysaccharide Epitopes and the Role of O-Acetylation. Clin. Vaccine Immunol. 2007;14:577. doi: 10.1128/CVI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerca N, Jefferson KK, Maira-Litran T, Pier DB, Kelly-Quintos C, Goldmann DA, Azeredo J, Pier GB. Molecular Basis for Preferential Protective Efficacy of Antibodies Directed to the Poorly Acetylated Form of Staphylococcal Poly-N-Acetyl-Beta-(1–6)-Glucosamine. Infect. Immun. 2007;75:3406. doi: 10.1128/IAI.00078-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benaissa-Trouw B, Lefeber DJ, Kamerling JP, Vliegenthart JFG, Kraaijeveld K, Snippe H. Synthetic Polysaccharide Type 3-Related Di-, Tri-, and Tetrasaccharide-CRM197 Conjugates Induce Protection against Streptococcus Pneumoniae Type 3 in Mice. Infect. Immun. 2001;69:4698. doi: 10.1128/IAI.69.7.4698-4701.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safari D, Dekker HAT, Joosten JAF, Michalik D, de Souza AC, Adamo R, Lahmann M, Sundgren A, Oscarson S, Kamerling JP, Snippe H. Identification of the Smallest Structure Capable of Evoking Opsonophagocytic Antibodies against Streptococcus Pneumoniae Type 14. Infect. Immun. 2008;76:4615. doi: 10.1128/IAI.00472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bundle DR, Nycholat C, Costello C, Rennie R, Lipinski T. Design of a Candida Albicans Disaccharide Conjugate Vaccine by Reverse Engineering a Protective Monoclonal Antibody. ACS Chem. Biol. 2012;7:1754. doi: 10.1021/cb300345e. [DOI] [PubMed] [Google Scholar]
- 9.Seeberger PH. The Logic of Automated Glycan Assembly. Acc. Chem. Res. 2015;48:1450. doi: 10.1021/ar5004362. [DOI] [PubMed] [Google Scholar]
- 10.Eller S, Collot M, Yin J, Hahm HS, Seeberger PH. Automated Solid-Phase Synthesis of Chondroitin Sulfate Glycosaminoglycans. Angew. Chem. Int. Ed. 2013;52:5858. doi: 10.1002/anie.201210132. [DOI] [PubMed] [Google Scholar]
- 11.Geissner A, Anish C, Seeberger PH. Glycan Arrays as Tools for Infectious Disease Research. Curr. Opin. Chem. Biol. 2014;18:38. doi: 10.1016/j.cbpa.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Sanz JC, Cercenado E, Marin M, Ramos B, Ardanuy C, Rodriguez-Avial I, Bouza E. Multidrug-Resistant Pneumococci (Serotype 8) Causing Invasive Disease in Hiv+ Patients. Clin. Microbiol. Infect. 2011;17:1094. doi: 10.1111/j.1469-0691.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 13.Ardanuy C, de La Campa AG, Garcia E, Fenoll A, Calatayud L, Cercenado E, Perez-Trallero E, Bouza E, Linares J. Spread of Streptococcus Pneumoniae Serotype 8-St63 Multidrug-Resistant Recombinant Clone, Spain. Emerg. Infect. Dis. 2014;20:1848. doi: 10.3201/eid2011.131215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones JKN, Perry MB. The Structure of the Type-Viii Pneumococcus Specific Polysaccharide. J. Am. Chem. Soc. 1957;79:2787. [Google Scholar]
- 15.Bentley SD, Aanensen DM, Mavroidi A, Saunders D, Rabbinowitsch E, Collins M, Donohoe K, Harris D, Murphy L, Quail MA, Samuel G, Skovsted IC, Kaltoft MS, Barrell B, Reeves PR, Parkhill J, Spratt BG. Genetic Analysis of the Capsular Biosynthetic Locus from All 90 Pneumococcal Serotypes. PLoS Genet. 2006;2:e31. doi: 10.1371/journal.pgen.0020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malcolm AJ. Immunostimulating Activity of Streptococcus Pneumoniae Serotype 8 Oligosaccharides. US005916571A. Alberta Research Counsil; USA: 1999
- 17.Parameswarappa SG, Reppe K, Geissner A, Ménová P, Govindan S, Calow ADJ, Wahlbrink A, Weishaupt MW, Monnanda BP, Bell RL, Pirofski LA, Suttorp N, Sander LE, Witzenrath M, Pereira CL, Anish C, Seeberger PH. A Semi-synthetic Oligosaccharide Conjugate Vaccine Candidate Confers Protection Against Streptococcus pneumoniae Serotype 3 Infection. Cell Chem. Biol. 2016;11:1407. doi: 10.1016/j.chembiol.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlsson C, Jansson PE, Skov Sorensen UB. The Pneumococcal Common Antigen C-polysaccharide Occurs in Different Forms. FEBS J. 1999;265:1091. doi: 10.1046/j.1432-1327.1999.00835.x. [DOI] [PubMed] [Google Scholar]
- 19.Poolman JT, Frasch CE, Käyhty H, Lestrate P, Madhi SA, Henckaerts I. Evaluation of Pneumococcal Polysaccharide Immunoassays Using a 22F Adsorption Step with Serum Samples from Infants Vaccinated with Conjugate Vaccine. Clin. Vaccine Immunol. 2010;17:134. doi: 10.1128/CVI.00289-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldblatt D, Plikaytis BD, Akkoyunlu M, Antonello J, Ashton L, Blake M, Burton R, Care R, Durant N, Feavers I, Fernsten P, Fievet F, Giardina P, Jansen K, Katz L, Kierstead L, Lee L, Lin J, Maisonneuve J, Nahm MH, Raab J, Romero-Steiner S, Rose C, Schmidt D, Stapleton J, Carlone GM. Establishment of a New Human Pneumococcal Standard Reference Serum, 007sp. Clin. Vaccine Immunol. 2011;18:1728. doi: 10.1128/CVI.05252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galili U. The Alpha-Gal Epitope and the Anti-Gal Antibody in Xenotransplantation and in Cancer Immunotherapy. Immunol. Cell Biol. 2005;83:674. doi: 10.1111/j.1440-1711.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- 22.Roche MI, Lu ZH, Hui JH, Sharon J. Characterization of Monoclonal Antibodies to Terminal and Internal O-Antigen Epitopes of Francisella Tularensis Lipopolysaccharide. Hybridoma. 2011;30:19. doi: 10.1089/hyb.2010.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Steiner S, Libutti D, Pais LB, Dykes J, Anderson P, Whitin JC, Keyserling HL, Carlone GM. Standardization of an Opsonophagocytic Assay for the Measurement of Functional Antibody Activity against Streptococcus Pneumoniae Using Differentiated HL-60 Cells. Clin. Diagn. Lab. Immunol. 1997;4:415. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Ling CC, Bundle DR. A New Homobifunctional p-Nitrophenyl Ester Coupling Reagent for the Preparation of Neoglycoproteins. Org. Lett. 2004;6:4407. doi: 10.1021/ol048614m. [DOI] [PubMed] [Google Scholar]
- 25.Jódar L, Butler J, Carlone G, Dagan R, Goldblatt D, Käyhty H, Klugman K, Plikaytis B, Siber G, Kohberger R, Chang I, Cherian T. Serological Criteria for Evaluation and Licensure of New Pneumococcal Conjugate Vaccine Formulations for Use in Infants. Vaccine. 2003;21:3265. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 26.Assessment Report for Synflorix. European Medicines Agency; London: 2009. [Google Scholar]
- 27.Assessment Report for Prevnar 13. European Medicines Agency; London: 2009. [Google Scholar]
- 28.Gening ML, Maira-Litrán T, Kropek A, Skurnik D, Grout M, Tsetkov YE, Nifantiev NE, Pier GB. Synthetic β-(1->6)-linked N-acetylated and Nonacetylated Oligoglucosamines used to Produce Conjugate Vaccines for Bacterial Pathogens. Infect. Immun. 2010;78:764. doi: 10.1128/IAI.01093-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Put RMF, Kim TH, Guerreiro C, Thouron F, Hoogerhout P, Sansonetti PJ, Westdijk J, Stork M, Phalipon A, Mulard LA. A Synthetic Carbohydrate Conjugate Vaccine Candidate Against Shigellosis: Improved Bioconjugation and Impact of Alum on Immunogenicity. Bioconjugate Chem. 2016;27:883. doi: 10.1021/acs.bioconjchem.5b00617. [DOI] [PubMed] [Google Scholar]
- 30.Koeman FAW, Kamerling JP, Vliegenthart JFG. Synthesis of Structural Elements of the Capsular Polysaccharide of Streptococcus Pneumoniae Type 8. Tetrahedron. 1993;49:5291. [Google Scholar]
- 31.Hahm HS, Hurevich M, Seeberger PH. Automated Assembly of Oligosaccharides Containing Multiple Cis-glycosidic Linkages. Nat. Commun. 2016;7:12482. doi: 10.1038/ncomms12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mage RG, Lanning D, Knight KL. B Cell and Antibody Repertoire Development in Rabbits: The Requirement of Gut-Associated Lymphoid Tissues. Dev. Comp. Immunol. 2006;30:137. doi: 10.1016/j.dci.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Caulfield MJ, Ahl PL, Blue JT, Cannon JL. PCT/US2011/023526. Merck Sharp & Dohme Corp.; 2011. 15-valent Pneumococcal Polysaccharide-protein Conjugate Vaccine Composition. [Google Scholar]
- 34.Graur D, Duret L, Gouy M. Phylogenetic Position of the Order Lagomorpha (Rabbits, Hares and Allies) Nature. 1996;379:333. doi: 10.1038/379333a0. [DOI] [PubMed] [Google Scholar]
- 35.Rocha R, Nunes C, Rocha G, Oliveira F, Sanches F, Gobbi H. Rabbit Monoclonal Antibodies Show Higher Sensitivity Than Mouse Monoclonals for Estrogen and Progesterone Receptor Evaluation in Breast Cancer by Immunohistochemistry. Pathol. Res. Pract. 2008;204:655. doi: 10.1016/j.prp.2008.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Mawas F, Niggemann J, Jones C, Corbel MJ, Kamerling JP, Vliegenthart JFG. Immunogenicity in a Mouse Model of a Conjugate Vaccine Made with a Synthetic Single Repeating Unit of Type 14 Pneumococcal Polysaccharide Coupled to CRM197. Infect. Immun. 2002;70:5107. doi: 10.1128/IAI.70.9.5107-5114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recommendations to Assure the Quality, Safety and Efficacy of Pneumococcal Conjugate Vaccines. World Health Organization; Geneva: 2009. [Google Scholar]
- 38.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods. 2007;39:175. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 39.Hofmann J, Hahm HS, Seeberger PH, Pagel K. Identification of Carbohydrate Anomers Using Ion Mobility-mass Spectrometry. Nature. 2015;526:241. doi: 10.1038/nature15388. [DOI] [PubMed] [Google Scholar]
- 40.Demchenko AV, Rousson E, Boons GJ. Stereoselective 1,2-Cis-galactosylation Assisted by Remote Neighboring Group Participation and Solvent Effects. Tetrahedron Lett. 1999;40:6523. [Google Scholar]
- 41.Lassaletta JM, Meichle M, Weiler S, Schmidt RR. Silyl Group Migration in 1-O-Silyl Protected Sugars Convenient Synthesis of 2-O-Unprotected Sugars. J. Carbohydr. Chem. 1996;15:241. [Google Scholar]
- 42.Kaeothip S, Yasomanee JP, Demchenko AV. Glycosidation of Thioglycosides in the Presence of Bromine: Mechanism, Reactivity, and Stereoselectivity. J. Org. Chem. 2012;77:291. doi: 10.1021/jo2019174. [DOI] [PubMed] [Google Scholar]
- 43.Beshore DC, Dinsmore CJ. Preparation of Substituted Piperazinones Via Tandem Reductive Amination-(N,N '-Acyl Transfer)-Cyclization. Org. Lett. 2002;4:1201. doi: 10.1021/ol025644l. [DOI] [PubMed] [Google Scholar]
- 44.Cancogni D, Lay L. Exploring Glycosylation Reactions under Continuous-Flow Conditions. Synlett. 2014;25:2873. [Google Scholar]
- 45.Pereira CL, Geissner A, Anish C, Seeberger PH. Chemical Synthesis Elucidates the Immunological Importance of a Pyruvate Modification in the Capsular Polysaccharide of Streptococcus pneumoniae Serotype 4. Angew. Chem. Int. Ed. 2015;54:10016. doi: 10.1002/anie.201504847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Automated glycan assembly of tetrasaccharides 1–4.
Fig. S2. Differential immune recognition of synthetic ST8 CPS frameshifts.
Fig. S3. Solution-phase syntheses of ST8 CPS frameshift C(12) and disaccharide 13.
Fig. S4. Characterization of the CRM197-frameshift C(12) glycoconjugate.
Fig. S5. Time course of anti-glycan immune responses after immunization with CRM197-frameshift C(12).
Fig. S6. Characterization of mAbs raised against ST8 frameshift C(12).
Fig. S7. Binding of ST8-related glycans by mAbs 1H8, 28H11 and 1F1.
Fig. S8. Qualitative comparison of binding kinetics of mAb 1H8 towards ST8 CPS-derived glycans of different chain lengths.
Fig. S9. Correlation between mAb 1H8 levels and bacterial burden in blood after passive immunization and lethal pneumococcal challenge.
Fig. S10. Divergent total syntheses of ST8 frameshift C-related glycans 21–24 from versatile precursor 32.
Fig. S11. Total synthesis of ST8 tetrasaccharide 15.
Fig. S12. Binding characterization of mAbs 1H8 and 1F1 to ST8 CPS-derived substructures by glycan microarray.
Fig. S13. Determination of affinities of mAb 1H8 towards synthetic, ST8 CPS-related oligosaccharides.
Fig. S14. Conjugation of synthetic oligosaccharides to CRM197 using the bifunctional spacer bis(4-nitrophenyl)adipate (DNAP).
Fig. S15. Evaluation of CRM197 glycoconjugates of trisaccharide 21 and tetrasaccharide 22 in mice.
Fig. S16. Immune response against semisynthetic ST8 glycoconjugates in rabbits.
Fig. S17. Adsorption of ST8 glycoconjugates to Prevnar 13, as assessed by flow cytometry.
Fig. S18. Effect of co-formulation of semisynthetic ST8 glycoconjugates with Prevnar 13 on the immune response against several pneumococcal CPSs.
Table S1. Sequences of automated assembly of protected ST8 CPS-related tetrasaccharide frameshifts.
Table S2. Anti-polysaccharide IgG endpoint titers of rabbits immunized with Prevnar 13 alone or co-formulated with semisynthetic ST8 glycoconjugates.