Abstract
Background
Several widely-used risk scores for cardiovascular disease (CVD) incorporate sex effects, yet there has been no systematic summary of the role of sex in clinical prediction models (CPMs). To better understand the potential of these models to support sex-specific care, we conducted a field synopsis of sex effects in CPMs for CVD.
Methods and Results
We identified CPMs in the Tufts Predictive Analytics and Comparative Effectiveness (PACE) CPM Registry, a comprehensive database of CVD CPMs published from 1/1990–5/2012. We report the proportion of models including sex effects on CVD incidence or prognosis, summarize the directionality of the predictive effects of sex, and explore factors influencing the inclusion of sex. Of 592 CVD-related CPMs, 193 (33%) included sex as a predictor or presented sex-stratified models. Sex effects were included in 78% (53/68) of models predicting incidence of CVD in a general population, versus only 35% (59/171), 21% (12/58) and 17% (12/72) of models predicting outcomes in patients with coronary artery disease (CAD), stroke, and heart failure, respectively. Among sex-including CPMs, women with heart failure were at lower mortality risk in 8/8 models; women undergoing revascularization for CAD were at higher mortality risk in 10/12 models. Factors associated with the inclusion of sex effects included the number of outcome events and using cohorts at-risk for CVD (rather than with established CVD).
Conclusions
While CPMs hold promise for supporting sex-specific decision making in CVD clinical care, sex effects are included in only one third of published CPMs.
Keywords: cardiovascular diseases, women, risk model, prevention, prognosis, sex, gender differences
The study of sex differences is necessary to optimize preventive, diagnostic and therapeutic strategies for cardiovascular disease (CVD) in men and women, and should be viewed as part of the larger initiative to provide patient-centered care.1–3 Sex-specific analyses are especially important in the context of CVD with recognized sexual dimorphism in risk, prognosis, and potential for treatment benefit and harm.4–8 While the majority of CVD prevention guidelines are similar for men and women, there is growing appreciation that there may be sex differences in the magnitude of relative and absolute benefits and harms of preventive interventions.9 For instance, while aspirin is associated with a reduction in the risk of CVD events in both men and women, the specific types of benefit appear to differ by sex, with some evidence that aspirin therapy lowers myocardial infarction risk in men (but not women), and ischemic stroke risk in women (but not men).10
Clinical prediction models (CPMs), algorithms that estimate the probability of clinical outcomes using patient-specific characteristics, can enable clinicians to personalize medical decisions for individual patients,11 and thus have the potential to support sex-specific care. Several CVD-related CPMs that are widely used in clinical practice for primary and secondary prevention incorporate sex-specific risk algorithms.12–14 However, while there is an abundance of CPMs for CVD outcomes in the literature,15 their potential to support sex-specific prevention and treatment strategies in men and women remains unexplored, and the frequency and directionality of predictive sex effects in these models has not been systematically described.
To explore the potential of these models to inform sex-specific prevention and treatment decisions in cardiovascular care, we conducted a field synopsis of the role of sex effects in CVD-related prediction models using the Tufts Predictive Analytics and Comparative Effectiveness (PACE) CPM Registry. We aimed to describe the frequency with which sex effects are included in these models, determinants of the inclusion of sex effects, and the directionality of predictive sex effects.
Methods
The Tufts CPM Registry
The Tufts PACE CPM Registry is based on a systematic review of PubMed for English-language articles containing CPMs for CVD published from 1/1990–5/201215. CVD included coronary heart disease, heart failure, arrhythmias, stroke, venous thromboembolism and peripheral vascular disease. Detailed descriptions of article inclusion and exclusion criteria are described elsewhere.15 Briefly, articles were included if: (1) the primary aim was to develop a CPM; (2) they contained a model predicting binary clinical endpoints (either CVD incidence or prognosis); (3) the model contained at least two predictor variables; and (4) the model allowed calculation or categorization of outcome risk for an individual patient.
Selection of models
The Tufts CPM Database includes 796 total CPMs extracted from 506 articles related to CVD. From each article, if multiple CPMs were presented for a unique index condition-outcome pair, a single model was selected as a primary model. Primary models were (1) those designated as primary by the authors of the published article; (2) where no model was so specified, the most clinically-oriented model (e.g., versus model extensions with laboratory or radiographic information); or (3) by consensus among extractors if none of the above applied.
In order to account for correlation between how sex effects are included in models derived from previously published models, the present analysis was limited to de novo models, those defined as newly derived CPMs that report a method to calculate an individual’s absolute risk for a binary outcome. Recalibration CPMs (previously described models with a revised intercept and/or slope to better fit a new population) and adaptation CPMs (previously described CPMs revised to predict a different outcome) were excluded.
Study and model-level descriptive characteristics
From each included article, information was extracted to describe key study design, cohort and model characteristics. Data were extracted in duplicate in electronic forms to ensure consistency; discrepancies were resolved by consensus involving a third investigator. Study and cohort characteristics included author names, year of publication, study design, cohort sample size, cohort or trial enrollment period, the number and proportion of women in the cohort or trial, and the age distribution (mean (SD) or median (IQR) age, and range).
For each model, the index condition was first categorized using a broad primary definition, and further categorized at a secondary level for some conditions. Primary index condition categories included population sample (populations at risk for incident CVD), congestive heart failure (CHF), stroke, coronary artery disease (CAD), aortic disease, venous thromboembolism (VTE), cardiac surgery, and arrhythmias. For the CAD primary index condition, secondary level index condition categories were stable CAD, acute coronary syndromes (ACS), cardiac surgery, and revascularization procedures (i.e., coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI)). Model outcomes were categorized as mortality, morbidity, or a composite of morbidity and mortality. Models were further categorized by index condition-outcome pairings. We also extracted model sample size, number of outcome events, covariates, parameter estimates (e.g., log-odds ratios or log-hazard ratios) with corresponding standard errors, intercept or baseline hazard, and the model’s discriminative ability (e.g., concordance statistic or area under the receiver operating characteristic curve (AUC)).
Classification of sex effects in CVD-related CPMs
Each CPM was classified according to how sex effects were incorporated: (1) as a covariate; (2) as a stratification variable where male- and female-specific models were presented separately (with intercepts, covariates, and parameter estimates allowed to vary by sex); (3) whether the model was built from a sex-restricted cohort of only men or only women; or (4) none of the above.
For models not including sex effects, the articles were reviewed with respect to whether sex was reported as a candidate for inclusion based on statistical or clinical criteria. Statistical criteria were (1) exploration of the univariable relation between sex and the outcome, and/or (2) evaluation of sex as a candidate in the final multivariable model. A description of the distribution (e.g., proportion) of males or females in the cohort was not counted as evidence of statistical consideration. Clinical rationale consisted of a description of a lack of clinical or biological plausibility of a relationship between sex and outcome risk, typically supported by author opinion or citation of external literature.
Statistical analysis
Descriptive statistics (counts and proportions) were used to summarize how sex effects were included in the total sample of models, and among models stratified by the top four most frequent primary index conditions. A pair of sex-stratified models (1 male and 1 female) within a study was counted as one model. For all subsequent analyses, models developed from sex-restricted cohorts were excluded as sex effects would be impossible to evaluate or include. Chi-square tests were used to compare the frequency of inclusion of sex (as a covariate or stratification variable vs. not included) by index condition. For the top three most frequently occurring models by secondary index condition-outcome pair, the magnitude and directionality of the effect of sex on outcome risk was summarized when sex was included as a covariate. To summarize the predictive effects of sex, forest plots were used to display the effect estimates (odds ratio or hazard ratio with associated 95% confidence intervals (CI)). CPMs that included point scores or nomograms, but did not report coefficients for the effect of sex in statistical models, were excluded from this summary.
In order to identify study- and model-related factors associated with the inclusion of sex effects in prediction models, univariable odds ratios, 95% CI, and associated p values were calculated using logistic regression. Stepwise multivariable logistic regression analysis was performed using all variables from the univariable analyses with an entry and exit significance level of 0.05. Regression analyses used the SAS statistical package, version 9.3 (SAS Institute, Cary, NC). Institutional Review Board approval and patient informed consent was not needed as this study was a systematic review of aggregated data from published studies.
Results
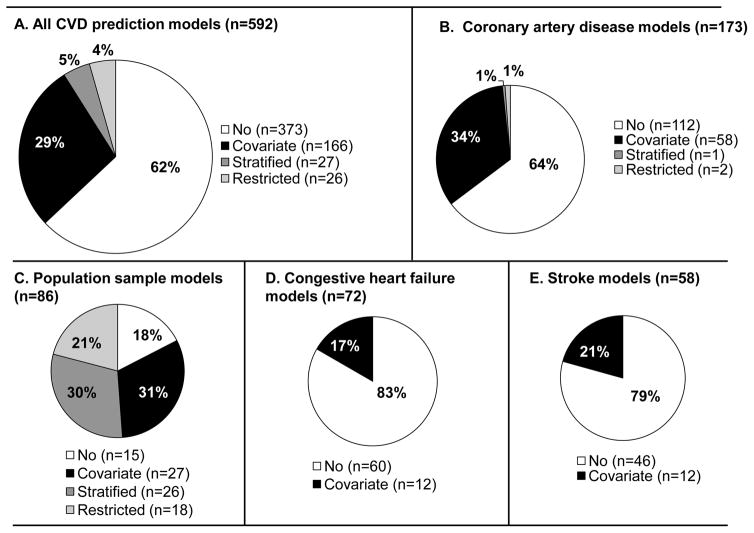
Among the 796 Tufts PACE CPM Registry models extracted from 506 articles, 592 were identified as primary, de novo models for CVD. One third (34%) of the CVD models included sex as either a covariate or presented separate models stratified by sex (Figure 1A). A minority (4%) of the models were developed from a sex-restricted cohort. Seventeen models (3%) included an interaction term between sex and another covariate. Of the 17 interaction terms, 7 were between age and sex, and 4 were between sex and diabetes status. Among the 373 CVD-related models that did not include sex as a covariate or stratification variable, 71% reported that sex was a candidate for inclusion based on clinical or statistical criteria. Four pregnancy-related models were identified – 3 models predicting a composite of adverse pregnancy outcomes (including stroke) among women with existing or congenital heart disease and 1 model predicting VTE risk and placental complications. Agreement between raters (JKP, LYHL, GR, JSL) classifying information on sex effects was high (Cohen’s kappa=92.5%).
Figure 1.
The frequency with which sex is included as either a covariate, model stratification variable, or as a cohort inclusion criteria (“restriction”) is presented for all CVD-related prediction models (Panel A), in models predicting outcomes among patients with CAD (Panel B), in models predicting incidence of CVD in an at-risk general population (Panel C), in models predicting outcomes among patients with CHF (Panel D), and in models predicting outcomes among patients who have experienced stroke (Panel E).
The likelihood of including sex as a covariate or stratification variable was significantly different depending on the index condition of the cohort (p<0.0001, Figure 1B–E). Sex effects were included in 78% (53/68) of models predicting CVD outcomes in a general population, versus only 35% (59/171), 21% (12/58) and 17% (12/72) of models developed from cohorts of patients with CAD, stroke, and CHF, respectively.
Sex effects in CVD models by index condition–outcome pair
Sex effects were infrequently included in the most commonly occurring CPMs classified by index condition-outcome pair: 36% of 83 models predicting mortality among patients with CAD, 14% of 58 models predicting mortality among CHF patients, and 36% of 47 models predicting morbidity in those with CAD (Table 1). For models developed in patients with stroke, sex effects were also included in a minority of models predicting mortality (17% of 23 models), morbidity and mortality (30% of 23 models), or morbidity alone (8% of 12 models). In contrast, for models developed in a population sample, sex effects were included in 78% of the 67 models predicting incident CVD outcomes of mortality, morbidity, or a composite of morbidity and mortality.
Table 1.
The inclusion of sex in the top 15 most frequently occurring CVD prediction models, by index condition-outcome pair (n=566)
| Index condition – outcome pair | Total number of models* | Number of models, n (%) | |||
|---|---|---|---|---|---|
| With sex incorporated | Without sex | ||||
| Covariate | Stratified | Sex considered** | Consideration not reported | ||
| CAD – Mortality | 83 | 29 (35) | 1 (1) | 37 (45) | 16 (19) |
| CHF – Mortality | 58 | 8 (14) | 0 (0) | 34 (59) | 16 (28) |
| CAD – Morbidity | 47 | 17 (36) | 0 (0) | 22 (47) | 8 (17) |
| CAD – Morbidity & Mortality | 40 | 12 (30) | 0 (0) | 21 (53) | 7 (18) |
| Population Sample – Morbidity | 32 | 10 (31) | 11 (34) | 8 (25) | 4 (13) |
| Population Sample – Morbidity & Mortality | 25 | 13 (52) | 9 (36) | 2 (8) | 1 (4) |
| Cardiac surgery – Mortality | 23 | 11 (48) | 0 (0) | 10 (43) | 2 (9) |
| Stroke – Morbidity & Mortality | 23 | 7 (30) | 0 (0) | 8 (35) | 8 (35) |
| Stroke – Mortality | 23 | 4 (17) | 0 (0) | 13 (57) | 6 (26) |
| VTE– Morbidity | 18 | 6 (33) | 0 (0) | 7 (39) | 5 (28) |
| Aortic Diseases – Mortality | 17 | 4 (24) | 0 (0) | 11 (65) | 2 (12) |
| Population sample – Mortality | 10 | 4 (40) | 6 (60) | 0 (0) | 0 (0) |
| Arrhythmia – Morbidity | 13 | 6 (46) | 0 (0) | 7 (54) | 0 (0) |
| Cardiac surgery – Morbidity | 12 | 3 (25) | 0 (0) | 8 (67) | 1 (8) |
| Stroke – Morbidity | 12 | 1 (8) | 0 (0) | 7 (58) | 4 (33) |
| Other | 130 | 32 (25) | 0 (0) | 70 (54) | 28 (22) |
| Total | 566 | 166 (29) | 27 (5) | 265 (47) | 108 (19) |
Sex restricted models excluded
Models were noted to have considered sex if assessment of sex as a candidate variable based on statistical or clinical criteria was reported in the manuscript.
CAD: coronary artery disease; CHF: congestive heart failure, VTE: venous thromboembolism
Directionality of the predictive effect of female sex on CVD outcome risk
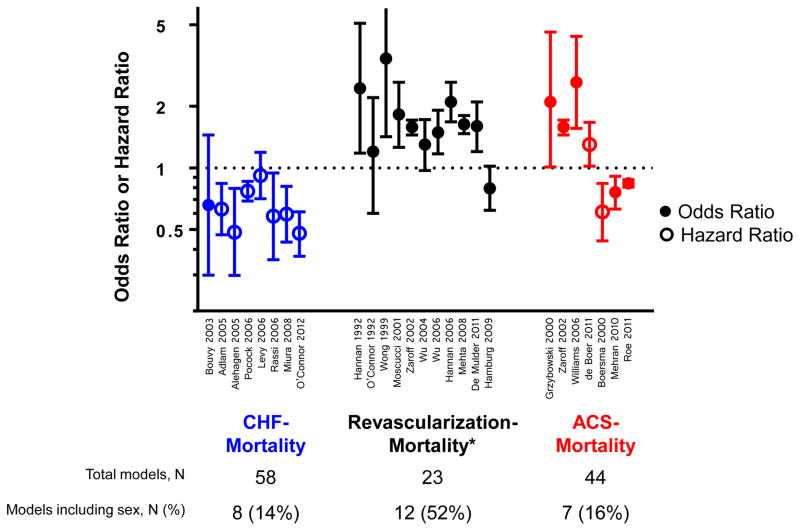
The directionality of the predictive effect of female sex on outcome risk was summarized among the top three most common models by secondary index condition-outcome pair: CHF-Mortality (n=58 models), ACS-Mortality (n=44 models), and Revascularization procedure-Mortality (n=23 models) (Figure 2). Among all 8 sex-including CPMs predicting mortality among patients with CHF, women were at lower risk of death than men. In 10 out of 12 sex-including CPMs predicting mortality among patients undergoing revascularization procedures, female sex was associated with an increased risk of death. Five of 9 CPMs predicting mortality among patients with ACS indicated women were at a lower risk of death than men, while the remainder indicated women were at higher risk.
Figure 2.
Among the top three most common models by secondary index condition-outcome pair, the predictive effect of female sex (odds ratio or hazard ratio, with corresponding 95% CI, with first author last name and year of publication) on outcome risk are presented for models that contained sex as a covariate. For example, among the 58 models predicting mortality among patients with CHF, 8 (14%) included a covariate for the effect of sex, and all 8/8 (100%) indicated women were at reduced risk of death as compared to men. References for the models including sex effects are presented in Supplementary Table 1. *: 1 of 12 revascularization models that included sex only presented a point score without a corresponding OR/HR.
Determinants of sex-specific CVD-related CPMs
Models developed from larger cohort sample sizes (n≥2000: OR=4.28, 95% CI 2.94–6.23) and with more outcome events (n≥150: OR=3.16, 95% CI 2.15–4.66) were significantly more likely to include sex as either a covariate or stratification variable (Table 2). Increasing proportions of women included in the study cohort was also significantly associated with greater odds of including sex effects in CPMs for CVD (p for trend=0.002), though the median proportion of women in study cohorts was only 35% (interquartile range: 26–49%). Advancing age of cohort participants was inversely associated with likelihood of including sex effects (p for trend= 0.001). As compared to models among populations defined by CAD, models predicting CVD among a general population sample were over 7 times more likely to include sex effects (OR=7.15, 95% CI: 3.66–13.90), while models predicting outcomes among patients with CHF (OR=0.41, 95% CI 0.21–0.80) or stroke (OR=0.48, 95% CI 0.24–0.98) were less likely to include sex effects.
Table 2.
Univariable study- and model-level characteristics and odds of including sex as a covariate or stratification variablea
| Odds Ratio (95% CI) | P valueb | |
|---|---|---|
| Sample size | ||
| Cohort ≥ 2000 participants (median), n=562 | ||
| ≥2000 participants=283 models | 4.28 (2.94–6.23) | <0.0001 |
| Number of events ≥ 150 (median), n=496 | ||
| ≥150 events = 245 models | 3.16 (2.15–4.66) | <0.0001 |
|
| ||
| Proportion women in the cohort, n=505 | ||
| 1–25%, n=123 | Ref | |
| 25–50%, n=269 | 1.83 (1.14–2.97) | |
| 50–75%, n=109 | 2.44 (1.40–4.27) | |
| 75–99%, n=4 | 9.30 (0.93–92.79) | 0.002c |
|
| ||
| Age of cohort participants | ||
| Mean or median age (quartiles), n=442 | ||
| Q1: n=108, age <60 y | Ref | |
| Q2: n=105, age 60–64 y | 0.93 (0.54–1.61) | |
| Q3: n=113, age 64–68 y | 0.77 (0.44–1.33) | |
| Q4: n=116, age ≥68 y | 0.37 (0.21–0.68) | 0.001c |
|
| ||
| Cohort index condition, n=566 | ||
| CAD, n=171 | Ref | Ref |
| Population sample, n=68 | 7.14 (3.66–13.90) | <0.0001 |
| CHF, n=72 | 0.41 (0.21–0.80) | 0.009 |
| Stroke, n=58 | 0.48 (0.24–0.98) | 0.04 |
| Other, n=175 | 0.83 (0.54–1.28) | 0.40 |
|
| ||
| AUC (Higher (>0.8) vs. Lower (0.6–0.8)), n=357 | ||
| Higher, n=125 | 0.69 (0.44–1.09) | 0.11 |
|
| ||
| Time | ||
| Cohort enrollment date <1996, n=211 out of 457 | 1.45 (0.99–2.13) | 0.06 |
| Publication year <2006, n=295 out of 566 | 0.81 (0.57–1.15) | 0.23 |
Models from restricted sex cohorts excluded
P values are from Wald tests except where indicated
P value from trend test
In multivariable analysis, only the number of outcome events (OR=2.6, 95% CI 1.60–4.35) and a cohort defined as a population sample (OR=6.15, 95% CI 2.68–14.13) were significantly associated with inclusion of sex effects.
Discussion
In a registry of 592 CPMs related to CVD risk or prognosis, sex was incorporated as a covariate or stratification variable in approximately one third of models. Sex effects were included in the majority of models predicting outcomes among a population sample at risk for incident CVD, but relatively rarely in models predicting outcomes among patients with existing CVD. The majority of models that did not include sex effects reported sex as a candidate for inclusion based on either statistical or biological criteria. Although sex was included rarely in models predicting morbidity and mortality among patients with existing CVD, the protective effect of female sex on mortality among patients with CHF, and the harmful effect among patients undergoing revascularization procedures, were (where reported) remarkably consistent.
The importance of considering sex effects when assessing risk in CVD primary prevention is evident in several commonly used CPMs, such as the Pooled Cohort Equations for 10-year atherosclerotic cardiovascular disease (ASCVD) risk12 and the Framingham model for 10 year CVD risk,13 which present sex-stratified algorithms. In our review of the CPM literature, the vast majority (~80%) of the models predicting CVD morbidity and mortality among a general population sample also included sex effects. This observation supports the notion that sex is of central importance when individualizing primary prevention efforts. Recent AHA guidelines for primary prevention in women have updated risk classification schemes for women by emphasizing the importance of CVD more broadly (rather than coronary heart disease alone) and criteria beyond 10-year risk models. These changes are meant to reflect the differential distribution of CVD by sex across the lifespan, with men having a higher prevalence of CAD under age 75, but women comprising a majority of the elderly population and having a cumulative lifetime CVD risk of 50%.9,16,17 Differences in the inclusion of sex in different age cohorts may reflect the nullification of the predictive effects of sex in the older (survivor) population. While these and several other effects are interesting (such as inclusion of sex being more frequent in cohorts with greater proportions of females), these unadjusted analyses should be cautiously interpreted.
While this field synopsis is not intended to review all investigations of the role of sex in CVD incidence and progression (such as attempts to identify causal or etiologic effects of sex), it is striking that sex effects were incorporated relatively infrequently in models predicting outcomes among patients with existing CVD conditions, including stroke, CHF and CAD. The relative scarcity of sex effects in these models is consistent with current secondary prevention guidelines, which are largely the same for men and women.18–21 One notable exception is the inclusion of sex in the CHA2DS2-VASc score,22 which supports (appropriate) differential treatment of men versus women in the setting of a similar burden of stroke risk factors. Nonetheless the relative infrequency of sex effects in most of these models may be the result of weaker sex effects in these settings. For example, among patients with acute coronary syndromes, age and markers of clinical severity such as ST-segment deviation, serum cardiac biomarker elevation and shock are likely to be the strongest prognostic indicators for death and subsequent events.23,24 Similarly, the relative infrequency of sex effects in models predicting outcomes following CVD may be understood as arising from index event bias, which occurs here since the selection of patients with a first CVD event influences the correlation of (both measured and unmeasured) CVD risk factors with sex in ways that could obscure the effects of sex on subsequent outcome risk.25
CPMs hold great promise as clinical and methodological tools for guiding appropriate, sex-specific care, and estimating sex-based disparities in CVD clinical care. First, where sex is a risk factor for the outcome of interest, and risk modifies treatment effect (either on the absolute or relative risk scale), sex might be an important determinant of the benefit of medical interventions. The clinical utility of sex in CVD-related CPMs is illustrated in the case of lipid-lowering therapy for primary prevention. Using the latest risk-based guidelines for lipid-lowering therapy,12 a male could qualify for statin therapy, whereas a woman with identical risk factors (but non-identical risk, because of the protective effect of female sex itself) would not. Currently, the most common approach to isolating shortfalls in appropriate care involves accounting for eligibility criteria and adjustment by all other baseline risk factors. A recent framework on estimating disparities – or inequities – acknowledged the importance of accounting for these differences, as well as patient preferences.26 Where there is sexual or racial/ethnic dimorphism in risk, studies that endeavor to quantify inappropriate variations in care should account for known differences in outcome risk or therapy response, in addition to baseline patient factors and preferences. We recently proposed that risk models be used to take into account appropriate differences in clinical decision making due to intrinsic differences in risk between men and women not due to the distribution of identifiable, conventional risk factors.27
While the relationships between sex and risk of developing CVD, response to therapies, and prognosis have been examined4–8, the directionality and the clinical significance of sex differences remains elusive. Women have been shown to have a lower incidence of CVD and later age of onset,13 and female sex also seems protective for cardiovascular outcomes among patients at high risk for developing CVD.28 For patients with CHF, previous studies suggest that women have better survival (and are less likely to experience sudden cardiac death) when compared to men.29 However, because of historical under-representation of women in clinical trials for CHF,30 there have been few opportunities to replicate these observations, and occasional disagreements (where a survival advantage among women was limited only to non-ischemic heart failure patients).31 Although we reviewed CPMs, and not all types of studies examining the role of sex and gender in CVD (such as those endeavoring to estimate causal relationships), our results support and strengthen the claim that for patients with prevalent CHF, female sex is protective against subsequent morbidity and mortality. A similarly complex (and unclear) story has emerged for patients undergoing revascularization procedures, where some reports suggest worse hospital and post-discharge outcomes for women,32,33 while others indicate no long-term sex differences.34 While technologic innovations and the revascularization approach (CABG versus PCI) are likely important, we observed a remarkably consistent association between female sex and higher mortality among patients undergoing revascularization (though sex effects were included in only half of these models). Our inconsistent results across CPMs predicting outcomes following ACS likely relate to the clinical heterogeneity of these syndromes, the variety of approaches to management, and the burden of other risk factors.
Insufficient representation of women in CVD research, and inadequate conduct of sex- and gender-based analyses have been recognized as barriers to gender equity in CVD care for decades.35–37 While the NIH Revitalization Act led to an increase in the proportion of women enrolled in clinical trials, women are still underrepresented in trial cohorts30,38–40. We observed that approximately 35% of model development cohorts were comprised of women, which is consistent with the proportion of women (41%) enrolled in clinical trials for CVD referenced in 2007 AHA prevention guidelines for women.38 Furthermore, published results of CVD clinical trials do not consistently present results stratified by sex.41 In our review of CPMs for CVD, the majority of models that did not incorporate sex effects in a final model reported sex was a candidate for inclusion based on statistical or biological criteria, indicating that a sex-based analysis was at least considered most of the time. We found that increasing proportions of women enrolled in study cohorts was significantly associated with greater odds of incorporation of sex effects in CPMs, although the reasons for this are not entirely clear. These examples reinforce the recommendation that sex-based analyses must be prospectively planned and adequately powered if they are to identify differences in risks.42
While calendar time does not appear to have an important influence on the proportion of models including sex, the proliferation of CPMs (and of sex-including CPMs) over time15 points to broader changes in the conception of gender equity. While gender equity might simplistically be taken to imply identical (gender-neutral) care for men and women, newer (more sophisticated) concepts of gender equity can accommodate the broad influence of gender in all aspects of health care. Indeed, incorporating sex into risk models discriminates (literally) between males and females, but is necessary for better individualized (and gender-appropriate) care. Kassirer and Pauker defined personalized medicine as “the practice of clinical decision making such that the decisions made maximize the outcomes that the patient most cares about and minimizes those that the patient fears the most, on the basis of as much knowledge about the individual’s state as is available” (emphasis added).43 If patients are to receive the best, most individualized care, it may not be reasonable to anticipate fully gender-neutral decisions and outcomes. This evolution in the conception of gender equity in health care parallels a similar shift in the broader culture. While risk models can be helpful, when relevant determinants of care and of outcomes are themselves intrinsic properties of sex/gender, there may be theoretical limits on our ability to disentangle appropriate from inappropriate gender differences.
This review of the literature has several limitations. Our results reflect CVD-related CPMs published from 1990–2012, the date of the initial systematic review conducted to create the Tufts PACE CPM Registry. An update of this database is currently underway to include CPMs published through 2015, and it is likely that the number of models in the published literature has continued to proliferate. It is also possible that how sex is considered in CPMs has changed in the last several years given increasing attention to sex- and gender-based analysis by various national agencies and associations. Several recently published models predicting outcomes among patients with heart failure notably all incorporated gender44–46. We did not quantitatively synthesize the directionality of the predictive effect of sex on outcome risk for the most frequently occurring models because some CPMs were excluded (those that presented only point scores or nomograms without regression coefficients), and significant clinical heterogeneity (in terms of treatments and patient characteristics) remained even among models within the same index condition-outcome category. Moreover, as this was a review of clinical prediction models, and not all studies examining the role of sex and gender in CVD (such as those attempting to estimate causal or etiologic relationships between sex and outcomes, while adjusting for possible confounders), causal effects of sex on CVD outcomes may be obscured in the present studies by various biases or model building procedures.
Our field synopsis revealed that sex effects were included in approximately one third of CVD-related CPMs, but more commonly in models predicting a first occurrence of CVD in a general population sample, indicating that sex-specific risk assessment may be particularly well-established in a primary prevention setting. Nonetheless, we found that there were 5–15 models containing sex for many common CVD conditions, with more models likely published since 2012. We also observed that increasing numbers of outcome events were associated with a higher likelihood of including sex effects in CPMs, implying that model development from cohorts including adequate numbers of outcomes may uncover additional or more consistent sex effects. This observation is consistent with prior recommendations that a priori planning of sex- and gender-based analyses in cardiovascular disease is essential to level sex-based disparities in outcomes2,38,41,42. As the CPM literature continues to grow, these models may offer a rich source of information on sex effects that can be used to individualize CVD care, and as tools for sex and gender-based CVD research.
Supplementary Material
What is Known
Several CVD-related clinical prediction models (CPMs) that are widely used in clinical practice incorporate sex-specific risk algorithms.
While there is an abundance of CPMs for CVD outcomes in the literature, there has not been a systematic review of how sex is incorporated in these algorithms, and the potential of these models to support sex-specific care remains unexplored.
What the Study Adds
Sex is included in only one third of CVD prediction models.
Sex is more frequently included in models predicting a first occurrence of CVD in a general population (versus models predicting morbidity and/or mortality in patients with existing CVD), indicating that sex-specific risk assessment is relatively well-established in a primary prevention setting.
Increasing numbers of outcome events were associated with a higher likelihood of including sex effects in CPMs, implying that model development from cohorts including adequate numbers of outcomes may uncover additional or more consistent sex effects.
Acknowledgments
Sources of Funding
Supported by the National Institutes of Health (NIH) Administrative Supplements for Research on Sex/Gender Differences Grant (U01NS086294), as well as a Patient-Centered Outcomes Research Institute (PCORI) Pilot Project Program Award (IP2PI000722), and the Predictive Analytics and Comparative Effectiveness (PACE) Center at the Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston.
Footnotes
Disclosures
None.
References
- 1.Collins FS, Varmus H. A New Initiative on Precision Medicine. N Engl J Med. 2015;372:793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redberg RF. - Don’t assume women are the same as men: include them in the trial. Arch Intern Med. 2012;172:921. doi: 10.1001/archinternmed.2012.2407. [DOI] [PubMed] [Google Scholar]
- 3.Kent DM, Hayward RA. Limitations of applying summary results of clinical trials to individual patients: the need for risk stratification. JAMA. 2007;298:1209–1212. doi: 10.1001/jama.298.10.1209. [DOI] [PubMed] [Google Scholar]
- 4.Mosca L, Barrett-Connor E, Kass Wenger N. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ Cardiovasc Qual Outcomes. 2010;3:111–5. doi: 10.1161/CIRCOUTCOMES.109.925313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haast RAM, Gustafson DR, Kiliaan AJ. Sex differences in stroke. J Cereb Blood Flow Metab. 2012;32:2100–7. doi: 10.1038/jcbfm.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bushnell CD. Stroke and the female brain. Nat Clin Pract Neurol. 2008;4:22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RE, Coffman KE, Miller VM. Women-specific factors to consider in risk, diagnosis and treatment of cardiovascular disease. Womens Health (Lond Engl) 2015;11:239–57. doi: 10.2217/whe.14.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes aS, Gracia CR, Haan CK, Jackson Ea, Judelson DR, Kelepouris E, Lavie CJ, Moore a, Nussmeier Na, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC, Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V, Wenger NK. Effectiveness-Based Guidelines for the Prevention of Cardiovascular Disease in Women--2011 Update: A Guideline From the American Heart Association. Circulation. 2011;123:1243–1262. doi: 10.1161/CIR.0b013e31820faaf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger J, Roncaglioni M. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. Jama. 2006;295:306–13. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 11.Moons KGM, Altman DG, Vergouwe Y, Royston P. Prognosis and prognostic research: application and impact of prognostic models in clinical practice. BMJ. 2009;338:b606. doi: 10.1136/bmj.b606. [DOI] [PubMed] [Google Scholar]
- 12.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Sorlie P, Stone NJ, Wilson PWF. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 13.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: The Framingham heart study. Circulation. 2008;117:743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication. The Framingham Study. Stroke. 1994;25:40–43. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 15.Wessler BS, Lai Yh L, Kramer W, Cangelosi M, Raman G, Lutz JS, Kent DM. Clinical Prediction Models for Cardiovascular Disease: Tufts Predictive Analytics and Comparative Effectiveness Clinical Prediction Model Database. Circ Cardiovasc Qual Outcomes. 2015;8:368–75. doi: 10.1161/CIRCOUTCOMES.115.001693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lloyd-jones DM, Leip EP, Larson MG, Agostino RBD, Beiser A, Wilson PWF, Wolf PA, Levy D. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–8. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 17.Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, Dolor RJ, Ganiats TG, Gomes AS, Gornik HL, Gracia C, Gulati M, Haan CK, Judelson DR, Keenan N, Kelepouris E, Michos ED, Newby LK, Oparil S, Ouyang P, Oz MC, Petitti D, Pinn VW, Redberg RF, Scott R, Sherif K, Smith SC, Sopko G, Steinhorn RH, Stone NJ, Taubert KA, Todd BA, Urbina E, Wenger NK Expert Panel/Writing Group, American Heart Association, American Academy of Family Physicians, American College of Obstetricians and Gynecologists, American College of Cardiology Foundation, Society of Thoracic Surgeons, American Medical Women’s Association, Centers for Disease Control and PreventionOffice of Research on Women’s Health, Association of Black Cardiologists, American College of Physicians, World Heart Federation, National Heart, Lung and BI, American College of Nurse Practitioners. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–501. [Google Scholar]
- 18.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 19.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the Worl. J Am Coll Cardiol. 2011;58:2432–46. doi: 10.1016/j.jacc.2011.10.824. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ ACC/AHA Task Force Members, Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–94. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 21.Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, Fonarow GC, Lange RA, Levine GN, Maddox TM, Naidu SS, Ohman EM, Smith PK. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, a. J Am Coll Cardiol. 2014;64:1929–49. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 23.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 24.Pieper KS, Gore JM, FitzGerald G, Granger CB, Goldberg RJ, Steg G, Eagle KA, Anderson FA, Budaj A, Fox KAA Global Registry of Acute Coronary Events (GRACE) Investigators. Validity of a risk-prediction tool for hospital mortality: the Global Registry of Acute Coronary Events. Am Heart J. 2009;157:1097–105. doi: 10.1016/j.ahj.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Dahabreh IJ, Kent DM. Index event bias as an explanation for the paradoxes of recurrence risk research. JAMA. 2011;305:822–823. doi: 10.1001/jama.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathore SS, Krumholz HM. Differences, disparities, and biases: clarifying racial variations in health care use. Ann Intern Med. 2004;141:635–638. doi: 10.7326/0003-4819-141-8-200410190-00011. [DOI] [PubMed] [Google Scholar]
- 27.Paulus JK, Shah ND, Kent DM. All else being equal, men and women are still not the same: using risk models to understand gender disparities in care. Circ Cardiovasc Qual Outcomes. 2015;8:317–320. doi: 10.1161/CIRCOUTCOMES.115.001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kappert K, Böhm M, Schmieder R, Schumacher H, Teo K, Yusuf S, Sleight P, Unger T. Impact of sex on cardiovascular outcome in patients at high cardiovascular risk: analysis of the Telmisartan Randomized Assessment Study in ACE-Intolerant Subjects With Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan Alone and in Combinatio. Circulation. 2012;126:934–41. doi: 10.1161/CIRCULATIONAHA.111.086660. [DOI] [PubMed] [Google Scholar]
- 29.Rho RW, Patton KK, Poole JE, Cleland JG, Shadman R, Anand I, Maggioni A Pietro, Carson PE, Swedberg K, Levy WC. Important differences in mode of death between men and women with heart failure who would qualify for a primary prevention implantable cardioverter-defibrillator. Circulation. 2012;126:2402–7. doi: 10.1161/CIRCULATIONAHA.111.069245. [DOI] [PubMed] [Google Scholar]
- 30.Heiat A, Gross CP, Krumholz HM. Representation of the Elderly, Women, and Minorities in Heart Failure Clinical Trials. Arch Intern Med. 2002;162:1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 31.Ghali JK, Krause-Steinrauf HJ, Adams KF, Khan SS, Rosenberg YD, Yancy CW, Young JB, Goldman S, Peberdy MA, Lindenfeld J. Gender Differences in Advanced Heart Failure: Insights from the BEST Study. J Am Coll Cardiol. 2003;42:2128–2134. doi: 10.1016/j.jacc.2003.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Guru V, Fremes SE, Austin PC, Blackstone EH, Tu JV. Gender differences in outcomes after hospital discharge from coronary artery bypass grafting. Circulation. 2006;113:507–516. doi: 10.1161/CIRCULATIONAHA.105.576652. [DOI] [PubMed] [Google Scholar]
- 33.Lempereur M, Magne J, Cornelis K, Hanet C, Taeymans Y, Vrolix M, Legrand V. Impact of gender difference in hospital outcomes following percutaneous coronary intervention. Results of the Belgian Working Group on Interventional Cardiology (BWGIC) registry. EuroIntervention. 2014;10:1–8. doi: 10.4244/EIJY14M12_11. [DOI] [PubMed] [Google Scholar]
- 34.Onuma Y, Kukreja N, Daemen J, Garcia-Garcia HM, Gonzalo N, Cheng JM, van Twisk PH, van Domburg R, Serruys PW. Impact of Sex on 3-Year Outcome After Percutaneous Coronary Intervention Using Bare-Metal and Drug-Eluting Stents in Previously Untreated Coronary Artery Disease. Insights From the RESEARCH (Rapamycin-Eluting Stent Evaluated at Rotterdam Cardiology Hospit. JACC Cardiovasc Interv. 2009;2:603–610. doi: 10.1016/j.jcin.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Eaker ED, Packard B, Wenger NK, Clarkson TB, Tyroler HA. Coronary heart disease in women: proceedings of an NIH workshop. New York: Haymarket Doyma; 1987. [Google Scholar]
- 36.Women’s health. Report of the Public Health Service Task Force on Women’s Health Issues. Public Health Rep. 1985;100:73–106. [PMC free article] [PubMed] [Google Scholar]
- 37.Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. N Engl J Med. 1993;329:247–56. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- 38.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3:135–42. doi: 10.1161/CIRCOUTCOMES.110.868307. [DOI] [PubMed] [Google Scholar]
- 39.Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014;174:1868–70. doi: 10.1001/jamainternmed.2014.4758. [DOI] [PubMed] [Google Scholar]
- 40.Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med. 2008;168:136–140. doi: 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 41.Doull M, Runnels VE, Tudiver S, Boscoe M. Appraising the Evidence: Applying Sex- and Gender-Based Analysis (SGBA) to Cochrane Systematic Reviews on Cardiovascular Diseases. J Women’s Heal. 2010;19:997–1003. doi: 10.1089/jwh.2009.1626. [DOI] [PubMed] [Google Scholar]
- 42.Bucholz EM, Krumholz HM. Women in Clinical Research: What We Need for Progress. Circ Cardiovasc Qual Outcomes. 2015;8:S1–S3. doi: 10.1161/CIRCOUTCOMES.115.001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauker SG, Kassirer JP. Decision analysis. N Engl J Med. 1987;316:250–8. doi: 10.1056/NEJM198701293160505. [DOI] [PubMed] [Google Scholar]
- 44.Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–13. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 45.Adabag S, Rector TS, Anand IS, McMurray JJ, Zile M, Komajda M, McKelvie RS, Massie B, Carson PE. A prediction model for sudden cardiac death in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2014;16:1175–82. doi: 10.1002/ejhf.172. [DOI] [PubMed] [Google Scholar]
- 46.Chyu J, Fonarow GC, Tseng CH, Horwich TB. Four-variable risk model in men and women with heart failure. Circ Heart Fail. 2014;7:88–95. doi: 10.1161/CIRCHEARTFAILURE.113.000404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.