Abstract
The unique amino acid hypusine is present in only two proteins in eukaryotic cells, eukaryotic translation initiation factor 5A-1 (eIF5A1), and eIF5A2, where it is covalently linked to the lysine-50 residue of these proteins via a post-translational modification coined hypusination. This unique modification is directed by two highly conserved and essential enzymes, deoxyhypusine synthase (DHPS), and deoxyhypusine hydroxylase (DOHH), which selectively use the polyamine spermidine as a substrate to generate hypusinated eIF5A. Notably, elevated levels of polyamines are a hallmark of most tumor types, and increased levels of polyamines can also be detected in the urine and blood of cancer patients. Further, in-clinic agents that block the function of key biosynthetic enzymes in the polyamine pathway markedly impair tumor progression and maintenance of the malignant state. Thus, the polyamine pathway is attractive as a prognostic, prevention and therapeutic target. As we review, recent advances in our understanding of the specific functions of hypusinated eIF5A and its role in tumorigenesis suggest that the polyamine-hypusine circuit is a high priority target for cancer therapeutics.
Keywords: Hypusine, Hypusination, eIF5A, DHPS, DOHH, GC7, Spermidine, Tumor, Metastasis
Introduction
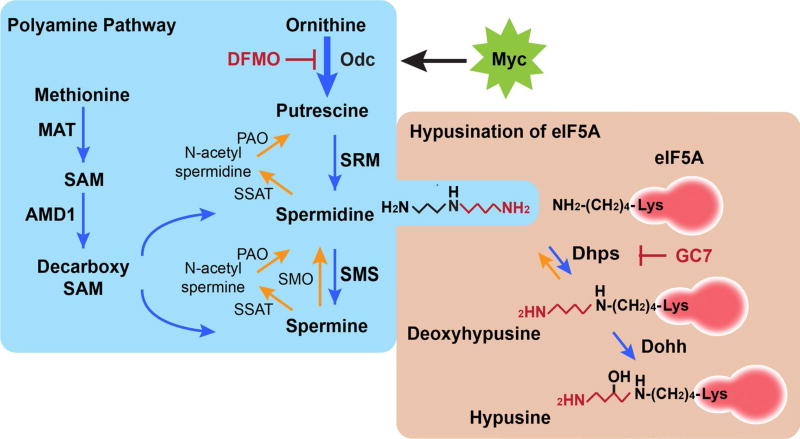
In humans, the two isoforms of eukaryotic translation initiation factor 5A (eIF5A), eIF5A1 and eIF5A2, are the only two eukaryotic proteins that undergo a post-translational modification coined hypusination. This unique modification involves the unusual amino acid hypusine, and is mediated by two highly conserved and essential enzymes, deoxyhypusine synthase (DHPS) and deoxyhypusine hydroxylase (DOHH) (Park et al. 1993a, b, 2010) (Fig. 1). First, the 4-aminobutyl moiety of the polyamine spermidine is transferred to the epsilon amino group of a specific eIF5A lysine residue (lysine-50 in human eIF5A) by DHPS to form deoxyhypusinated eIF5A. Second, deoxyhypusine is hydroxylated by DOHH to generate hypusinated eIF5A. Like DHPS and DOHH, eIF5A is also conserved in all eukaryotes, underscoring its importance for development, growth and survival. Polyamines are derived from the amino acids ornithine and methionine, and their cellular levels are tightly regulated through polyamine biosynthesis, catabolism and transport (Miller-Fleming et al. 2015). Notably, increased polyamine levels are observed in most tumor types, and elevated polyamines have also been reported in the urine and blood of cancer patients (Durie et al. 1977; Russell 1971).
Fig. 1.
The polyamine-hypusine pathway. The most common polyamines are putrescine, spermidine and spermine, which are predominantly derived from the amino acids ornithine and methionine. One of the polyamines, spermidine, is used as the substrate to synthesize a unique amino acid coined hypusine, which is covalently linked to the lysine-50 residue of eIF5A. In cancer cells, ornithine decarboxylase 1 (ODC1), the enzyme catalyzing the first step of polyamine biosynthesis, is often up-regulated, especially in Myc-driven malignant cancers, which in turn, increases levels of polyamines. As a result, the levels of polyamines are elevated in cancer cells. 2-difluoromethylornithine (DFMO) is suicide inhibitor of ODC1 and has shown impressive efficacy in several clinical cancer prevention trials, including colon and prostate cancer. Hypusination of eIF5A is mediated by two enzymes, DHPS and DOHH. Hypusination is conserved among all eukaryotes. MAT methionine adenosyltransferase, SAM S-adenosyl-l-methionine, AMD1 AdoMet decarboxylase, SRM spermidine synthase, SMS spermine synthase, SMO spermine oxidase, SSAT spermidine/ spermine N1-acetyltransferase, PAO polyamine oxidase
EIF5A1 and eIF5A2 proteins have 84 % homology, yet these isoforms have distinct patterns of expression, where eIF5A2 is expressed in select tissues, such as the brain and testis, while eIF5A1 is ubiquitously expressed. Furthermore, the eIF5A2 gene is located in chromosome 3q26.2, a region that is frequently amplified in lung squamous, ovarian, esophageal, gastric, bladder, colorectal, breast, pancreatic and liver cancers.
EIF5A was first identified as one of the general translation initiation factors (Benne et al. 1978), where it was thought to function in the formation of the first peptide bond. However, subsequent studies established that eIF5A functions as an elongation factor. Furthermore, even early work investigating eIF5A indicated that it was not absolutely required for general protein synthesis, but was rather necessary for the translation of select mRNA subsets (Kang and Hershey 1994). For example, a conditional eIF5A mutant yeast strain that had marked inhibition of growth had only mild effects on the overall protein synthesis. Two decades later, this early observation has now been explained by two recent breakthrough studies that established the bacteria ortholog of eIF5A, EF-P, is required for the efficient translation of mRNAs-containing poly-Proline (Pro) motifs (Doerfel et al. 2013; Ude et al. 2013). Specifically, although EF-P does not undergo hypusination, it is also post-translationally modified at a specific lysine residue (lysine-34 of E. coli EF-P) to generate β-lysyl hydroxylysine, and β-lysylation is required for EF-P function (Bullwinkle et al. 2013; Park et al. 2012). Notably, this function is conserved for eukaryotic eIF5A, where hypusination of eIF5A is necessary for the translation of mRNAs containing consecutive Pro codons (Gutierrez et al. 2013).
EIF5A is increasingly recognized as a critical regulator of tumor cell growth. Furthermore, some eIF5A translational targets appear to directly link eIF5A to tumor development and identifying such targets will advance our knowledge of the roles of hypusination in cancer. This Mini Review focuses on recent insights into the role of polyamine-hypusine circuit in tumorigenesis, with emphasis on its functions that are associated with the tumor progression. Furthermore, we discuss the possibility of targeting the hypusine circuit for cancer therapeutics.
Clinical correlations
The expression of eIF5A1 and eIF5A2 is highly correlated with patient prognosis in many tumor types (Table 1). For example, in a study comparing the expression of different genes from sporadic colorectal cancer (CRC) patients with first diagnosis less than 50 years of age vs. normal colon mucosa tissues (Tunca et al. 2013), eIF5A1 was one among the top four up-regulated genes in colon tumors. In addition, elevated levels of eIF5A1 were associated with poor prognosis in early onset CRC. Immunohistochemistry (IHC) analysis of human gastric cancer (GC) and its adjacent normal tissues revealed that elevated levels of eIF5A2 and its potential target metastasis-associated protein (MTA1) correlate with more advanced stages of disease, and with lymphovascular invasion (Meng et al. 2015). Furthermore, elevated levels of eIF5A2 are highly correlated with poor prognosis of GC patients and with advanced clinical stage in non-small cell lung carcinoma (He et al. 2011) and ovarian cancer (Yang et al. 2009). Therefore, eIF5A2 may serve as a prognostic marker of some tumor types. Finally, survival analysis of neuroblastoma has shown that high levels of DHPS expression correlates with poor prognosis (Bandino et al. 2014).
Table 1.
Roles of the hypusine pathway in various cancer types
| Biological function/ survival |
Target genes/proteins | Cancer type | Types of study | Cell line/mouse model |
References |
|---|---|---|---|---|---|
| Apoptosis | eIF5A (unhypusinated) | Colon cancer | Ex vivo | HT-29, RKO (CRL-2577) | Taylor et al. (2007) |
| Apoptosis | eIF5A (unhypusinated) | Cervical cancer | Ex vivo | HeLa | Sun et al. (2010) |
| Apoptosis | eIF5A (unhypusinated) | Lung cancer | Ex vivo | A549 | Taylor et al. (2013) |
| Apoptosis | eIF5A (unhypusinated) | Multiple myeloma | Ex vivo, in vivo | KAS-6/1 | Taylor et al. (2012) |
| Cell growth, cell proliferation | eIF5A, DHPS, DOHH | Glioblastoma (GBM) | Ex vivo, IHC | U87-MG, G55T2, 173 glioma samples | Preukschas et al. (2012) |
| Cell growth, cell proliferation, poor prognosis | DHPS | Neuroblastoma | Ex vivo, survival | BE(2)-C, 88 tumors in NB cohort Versteeg-88 | Bandino et al. (2014) |
| Cell growth, cell proliferation | eIF5A1 | Vulvar high grade intraepithelial neoplasia (VIN) | IHC | Biopsies VIN | Cracchiolo et al. (2004) |
| Cell growth, cell proliferation | eIF5A2, DHPS, DOHH | Hepatocellular carcinoma | Ex vivo, clinical | Primary HCC, metastatic HCC | Lee et al. (2010) |
| Cell growth, cell proliferation | eIF5A1 | Pancreatic ductal adenocarcinoma | Ex vivo | PANC1, FG | Fujimura et al. (2014) |
| Cell growth, cell proliferation | DOHH eIF5A1 | Cervical cancer | Ex vivo | HeLa, U2OS | Memin et al. (2014) |
| Cell growth, cell proliferation | DOHH | Prostate cancer | Ex vivo | DU145 | Epis et al. (2012) |
| Cell growth, cell proliferation | eIF5A2 | Ovarian cancer | Ex vivo, in vivo | UACC-1598 | Guan et al. (2004) |
| Cell proliferation, migration, invasion | eIF5A2 | Gastric cancer (GC) | Ex vivo, survival | HGC27, GC patients | Meng et al. (2015) |
| Tumor suppressor | eIF5A1, DHPS, SRM, AMD1 | Lymphoma | In vivo | Eµ-Myc lymphoma | Scuoppo et al. (2012) |
| Early onset/poor prognosis | eIF5A1 | Colorectal cancer (CRC) | Survival | CRC patients | Tunca t al. (2013) |
| Disease progression, poor prognosis | eIF5A2 | Gastric cancer (GC) | Survival, IHC | 160 GC patients | Meng et al. (2015) |
| Disease progression, poor prognosis | eIF5A2 | Ovarian | Survival, IHC | 170 epithelial ovarian tumors | Yang et al. (2009) |
| Disease progression, poor prognosis | eIF5A2 | Non-small cell lung carcinoma (NSCLC) | Survival | 248 NSCLC patients | He et al. (2011) |
| Disease progression, poor prognosis | eIF5A2 | Bladder caner (BC) | Ex vivo, in vivo, clinical | BC patients, T24, 5637, BIU-87 cells | Wei et al. (2014) |
| Disease progression |
Roles of hypusination in cell growth and tumor development
The generation of knockout mice of eIF5A1, Dhps or Dohh revealed that all three are essential for early embryogenesis, whereas heterozygous mice are viable and fertile (Nishimura et al. 2012; Pallmann et al. 2015; Sievert et al. 2014; Templin et al. 2011); thus, hypusination of eIF5A1 is essential for survival and development of mammals. Interestingly, homozygous eIF5A2 knockout mice are viable and fertile (Pallmann et al. 2015). Whether this is due to functional redundancy of eIF5A1 and eIF5A2 remains to be investigated.
Several studies have demonstrated that depletion of hypusinated eIF5A, via siRNA- or shRNA-directed eIF5A knockdown, or by inhibiting DHPS enzymatic activity using the competitive spermidine analog N1-guanyl-1,7-diamineheptane (GC7), inhibits tumor cell growth ex vivo and tumorigenic potential in mouse xenograft models (Table 1). Interestingly, silencing of eIF5A1 in colon adenocarcinoma cell lines (HT-29) has little effect on cell proliferation (BrdU assay) or cell growth (XTT assay) (Taylor et al. 2007), suggesting, perhaps, that expression of eIF5A2 serves essential roles in these cells. Indeed, elevated levels of eIF5A2 have been detected in several cancer types, including those bearing amplifications of chromosome 3q (where eIF5A2 resides), which are found in lung squamous cell carcinoma and ovarian, esophageal, breast and prostate cancers. In such tumor cells, eIF5A2 knockdown usually impairs cancer cell growth ex vivo and tumorigenic potential in vivo (Table 1). Furthermore, eIF5A2 behaves as an oncogene that induces morphological transformation and tumors when overexpressed in immortal hepatocytes (Guan et al. 2004).
DOHH is a dinuclear iron enzyme that catalyzes the second step of hypusination, hydroxylating the intermediate deoxyhypusine residue to form hypusine. The poly(rC)-binding proteins PCBP1 or PCBP2 serve as iron chaperones for DOHH and, accordingly, knockdown of PCBP1 and/or PCBP2 reduces levels of iron-bound DOHH and its enzymatic activity, and leads to reductions of hypusinated eIF5A1 (Frey et al. 2014). Thus, DOHH is inhibited by iron chelators, such as ciclopirox (CPX), an antifungal agent (Subissi et al. 2010), or deferiprone (DEF) (Abbruzzese et al. 1991), which is used to treat iron-overload thalassemia caused by repeated blood transfusions (Neufeld 2010). Both CPX and DEF inhibit the growth of cancer cell lines ex vivo (Clement et al. 2002; Eberhard et al. 2009; Luo et al. 2011; Zhou et al. 2010) and impair tumor growth in vivo (Kim et al. 2011). Finally, treatment of cancer cells with CPX or DEF inhibits hypusination of eIF5A1 and leads to the accumulation of the immature and inactive form of eIF5A containing deoxyhypusine (Memin et al. 2014).
MicroRNAs (miRNA), a class of small (~22 nucleotide) non-coding RNAs that direct the destruction or that impair translation of their target mRNAs, have also been shown to regulate the polyamine-hypusine circuit. Specifically, the miRNAs mir-331-3p and mir-642-5p control DOHH expression, and reduced expression of these two miRNAs in prostate cancer cells leads to elevated levels of DOHH expression. Furthermore, overexpression of miR-331-3p or miR-642-5p reduces DOHH mRNA levels and inhibits cancer cell proliferation (Epis et al. 2012), and combined inhibition of DOHH by these miRNAs and the iron chelator mimosine shows synergistic inhibitory effects on cell proliferation. Interestingly, miR-331-3p was previously identified as a tumor suppressor that regulates ERBB2 expression (Epis et al. 2009), and its control of DOHH suggests that hypusination also contributes to tumorigenesis.
Several independent studies have shown that eIF5A1 is required for cell proliferation and that eIF5A1 or eIF5A2 overexpression in cancer cells contributes to tumorigenic potential (Table 1). However, in stark contrast, a shRNA screen by Scuoppo et al. identified four genes of the polyamine-hypusine pathway, specifically eIF5A1, Dhps, spermidine synthase (SRM), and adenosylmethionine decarboxylase1 (AMD1), as tumor suppressors (Scuoppo et al. 2012). Specifically, knockdown of these genes in hematopoietic stem cells (HSC) from Eµ-Myc transgenic mice, a validated model of human B-cell lymphoma, and transplant studies, showed that knockdown of these polyamine regulators accelerated lymphoma development in recipient mice. Although these findings contrast with those of others, one possible explanation is that shRNAs that target these genes were expressed in all HSC-derived cells, which could affect immune surveillance.
Invasion and metastasis
Most cancer patients succumb to cancer due to metastases. To define differences between primary tumors and metastases, the Golub laboratory compared gene expression profiles of metastatic adenocarcinoma of several tumor types and unmatched primary adenocarcinomas (Ramaswamy et al. 2003). This study identified 17 genes that are associated with metastasis and showed that primary solid tumors with this gene expression signature were most likely to metastasize and have poor prognosis. Interestingly, DHPS was one of the eight genes that were up-regulated in the metastatic gene signature.
Supporting important roles in metastasis, eIF5A2 overexpression promotes epithelial-mesenchymal transition (EMT) and leads to metastasis in hepatocellular carcinoma (Tang et al. 2010), non-small cell lung cancer (NSCLC) (Xu et al. 2014), esophageal squamous cell carcinoma (Li et al. 2014) and bladder cancer (Wei et al. 2014). For example, inhibition of hypusination by GC7 treatment, or the depletion of eIF5A2 by siRNA or shRNA, provokes a mesenchymal to epithelial transition, where there are increased levels of the epithelial markers E-cadherin and β-catenin and decreased levels of the mesenchymal markers vimentin and fibronectin. Finally, inhibition of hypusination reduces cell migration and invasion in wound healing and matrigel assays, respectively (Xu et al. 2014). Similar findings have been reported in bladder cancer (Wei et al. 2014), where eIF5A2 depletion or overexpression in xenograft studies was shown to impair or augment metastasis, respectively (Wei et al. 2014). Finally, overexpression of eIF5A2 and its potential target MTA1 have been correlated with disease progression, and knockdown of eIF5A2 in human gastric cancer cells up-regulates E-cadherin and down-regulates vimentin, cyclin D1, cyclin D3, c-Myc and MTA1 (Meng et al. 2015).
Pancreatic ductal adenocarcinoma (PDAC) is one of most deadly cancers, and most PDAC have metastasized by the time of first diagnosis. Notably, eIF5A1 knockdown reduces PDAC cell migration, invasion and metastasis to the liver (Fujimura et al. 2014, 2015). Furthermore, these studies identified Pseudopodium-enriched atypical kinase 1 (PEAK1) (Fujimura et al. 2014) and RhoA/ROCK (Fujimura et al. 2015) as eIF5A1 downstream targets using bioinformatics analysis combined with proteomic profiling. Collectively, these findings, from several independent studies and laboratories, and on several cancer types, indicate that overexpression of eIF5A1 or eIF5A2 promotes tumor metastases.
Potential eIF5A translational targets
EIF5A and its bacterial ortholog EF-P are required for efficient translation of mRNAs containing consecutive Pro motifs (PPP or PPG) (Doerfel et al. 2013; Gutierrez et al. 2013; Ude et al. 2013). Genome-wide analyses of proteins-containing poly-Pro motifs have revealed that 23.7 or 19.9 % of human proteins contain one or more PPP or PPG motifs, respectively (Mandal et al. 2014). Functional classification of human proteins with PPP motifs showed that these proteins are involved in DNA binding and transcription (31 %), RNA processing, splicing and metabolism (17 %), the actin cytoskeleton (11 %), and signaling/ligand/receptor pathways (8 %) (Mandal et al. 2014). The next logical question would be which of these eIF5A translational targets contribute to tumor development. This question can be addressed by two approaches. One is a sequence-based manual search, where one could look for known oncogenes among the genes containing poly-Pro motifs. The second approach is an unbiased genome-wide search using ribosome profiling technology (Ingolia et al. 2009), which allows one to identify the regions of mRNA where ribosomes are stalled during active translation. Using this technique, one could evaluate if ribosomes are indeed stalling at poly-Pro codons when hypusination is inhibited.
The search for such eIF5A translational targets has just begun. One appears to be the aforementioned PEAK1, a non-receptor tyrosine kinase that promotes progression of pancreatic ductal adenocarcinoma (PDAC). PEAK1 contains poly-Pro motifs, and depletion of both eIF5A1 and eIF5A2 or inhibition of hypusination by treatment with GC7 or CPX results in reduced protein levels of PEAK1, suggesting that PEAK1 is one of the targets of eIF5A (Fujimura et al. 2014).
The polyamine-hypusine circuit as a therapeutic target in cancer
Agents that block the function of the key enzymes of the circuit, DHPS, and DOHH, as well as their substrates spermidine and eIF5A, markedly impair tumor cell growth, progression and maintenance of the malignant state. Hypusination of eIF5A is the sole role of DHPS and DOHH, and no other enzymatic targets have been found. Thus, the polyamine-hypusine pathway is attractive as a prognostic, prevention and therapeutic target.
SNS01-T is a polyethylenimine (PEI)-based nanoparticle containing both siRNA targeting eIF5A1 and an overexpression plasmid expressing the non-modifiable eIF5A-K50R mutant under the regulation of B-cell specific promoter (Francis et al. 2014). This nanoparticle was developed based on the finding that overexpression of unhypusinated eIF5A induces apoptosis (Sun et al. 2010). SNS01-T has been shown to impair tumor growth in xenograft models of B-cell cancers (multiple myeloma, mantle cell lymphoma, and diffuse large B-cell lymphoma) (Table 2), and is currently being tested in a Phase1b/2a clinical trial for these B-cell cancers. In addition, combinatorial treatment of SNS01-T with the current standards of adjuvant therapy, lenalidomide or bortezomib, have shown synergistic effects and enhanced survival relative to the treatment with either drug alone (Table 2).
Table 2.
Targeting the polyamine-hypusine pathway
| Cancer type | Target | Drug/agent | Combination therapy | Cell line | Inhibitory effect | References |
|
| ||||||
| Ex vivo | ||||||
| Leukemia | DHPS | GC7 | Imatinib | BCR-ABL+ | Synergistic effect | Balabanov et al. (2007) |
| Glioblastoma | DHPS | GC7 | Temozolomide (TMZ), Bischloroethyl-nitrosourea (BCNU) | U87-MG, G55T2 | Additive effect | Preukschas et al. (2012) |
| Lung (NSCLC) | DHPS | GC7 | Cisplatin | H1299, A549 | Increased sensitivity to cisplatin | Xu et al. (2014) |
| Lung (NSCLC) | eIF5A | siRNA | Cisplatin | H1299, A549 | Increased sensitivity to cisplatin | Xu et al. (2014) |
| Prostate cancer | DOHH | miRNA | Mimosine | DU145 | Synergistic effect | Epis et al. (2012) |
|
| ||||||
| Cancer type/other disease | Target | Drug/agent | Combination therapy | Mouse models | Inhibitory effect | References |
|
| ||||||
| In vivo | ||||||
| B-cell cancers (MM, MCL, and DLBCL) | eIF5A | SNS01 | – | Xenograft of B-cell cancers | Inhibited tumor growth | Francis et al. (2014) |
| Lenalidmide | Xenograft of B-cell cancers | Synergistic effect | Francis et al. (2014) | |||
| Bortezomib | Xenograft of B-cell cancers | Synergistic effect | Francis et al. (2014) | |||
| Islet beta cell inflammation/diabetes | DHPS | GC7 | – | Low-dose streptozotocin (STZ) model | Reduced inflammatory response | Maier et al. (2010) |
| Type 1 diabetes (T1D) | DHPS | GC7 | – | Humanized mouse model of T1D | Does not abrogate the development of T1D | Imam et al. (2014) |
| Severe sepsis | eIF5A | siRNA-liposome complexes | – | Mouse model of severe sepsis | Reduced inflammation and increased survival | Moore et al. (2008) |
MM multiple myeloma, MCL mantle cell lymphoma, DLBCL diffuse large B-cell lymphoma
As noted above, several studies have shown that the DHPS inhibitor GC7 inhibits the growth of many cancer types. Furthermore, a few combinatorial treatments with GC7 have been tested (Table 2). For example, patients with chronic myeloid leukemia (CML) are commonly treated with imatinib, which inhibits BCR-ABL tyrosine kinase. Interestingly, proteomics analyses of imatinib-treated BCR-ABL-positive leukemia cells (K562 cells) revealed the down-regulation of eIF5A1 (Balabanov et al. 2007). Furthermore, co-treatment of imatinib and GC7 or with siRNA targeting eIF5A1 showed synergistic effects in inhibiting leukemia cell proliferation.
Although GC7 is commonly used to inhibit DHPS enzymatic activity, its clinical utility is unclear given concerns regarding specificity of this agent. Specifically, GC7 is spermidine analog, and thus, it can potentially affect other key targets of spermidine, including SAT1 (spermidine/spermine N(1)-acetyltransferase), a key regulator of polyamine catabolism (Pegg 2008), inward rectifying Kir2.1 potassium channels (Liu et al. 2012), and flux through the autophagy pathway (Eisenberg et al. 2009). Indeed, GC7 has documented effects on autophagy when used at higher concentrations (200 µM) (Oliverio et al. 2014).
CPX and DEF inhibit DOHH, which, in turn, inhibits hypusination of eIF5A, and both drugs have anti-proliferative, anti-tumor (Zhou et al. 2010) and anti-angiogenic (Clement et al. 2002) effects. Specifically, CPX impairs lymphangiogenesis by inhibiting tube formation of lymphatic endothelial cells, possibly through suppression of a VEGFR3-mediated ERK signaling pathway (Luo et al. 2011). Based on these promising results, the iron chelators CPX and DEF are being considered as potential therapeutic agents for treating cancers. Indeed, CPX has been tested in the phase 1 clinical trial for individuals with relapsed or refractory hematologic malignancies, and these patients displayed some hematological improvements (Minden et al. 2014). However, as an iron chelator, CPX has many cellular targets, for example, iron-dependent enzymes, such as ribonucleotide reductase (Eberhard et al. 2009), and the Wnt signaling pathway (Song et al. 2011). Thus, the anti-tumor activity of CPX most likely reflects pleiotropic effects on diverse cellular pathways.
Hypusine and other diseases
Knockdown of eIF5A reduces inflammatory cytokines and increases survival in a mouse model of severe sepsis and acute lung injury induced by lipopolysaccharide (Moore et al. 2008). Furthermore, hypusinated eIF5A regulates the levels of inducible nitric oxide synthase and pharmacological inhibition of hypusination or depletion of eIF5A protects against glucose intolerance in inflammatory mouse models of diabetes (Maier et al. 2010; Robbins et al. 2010). Finally, Dhps heterozygosity attenuates acute cytokine signaling in mice (Templin et al. 2011). Thus, targeting hypusine pathway can also impair the inflammatory response, which is a known driver of tumor progression (Lasry et al. 2016).
Conclusions and perspectives
Great progress has been made towards understanding the role of hypusine in cancer, where there is growing evidence that inhibition of hypusination inhibits tumorigenesis. Indeed, the preponderance of studies indicate that hypusination of eIF5A is required for tumor maintenance and disease progression. However, important questions still remain regarding how hypusinated eIF5A contributes to tumorigenesis and how to selectively target tumors. First, there appears to be context specific effects of eIF5A on tumorigenesis, where overexpression of eIF5A2 can render the liver cell line LO2 tumorigenic, but not NIH3T3 fibroblast cells (Guan et al. 2004). Second, there may be deleterious, non-tumor cell autonomous effects of targeting hypusination, for example on immune surveillance, which might explain the paradoxical findings that shRNA targeting eIF5A1 or Dhps in HSCs can accelerate the development of Eµ-Myc lymphomas (Scuoppo et al. 2012). Alternatively, eIF5A1 may functions as both a tumor suppressor and oncogene and these roles may switch between tumor initiation and progression. To understand tumor development from initiation to progression, rigorous analyses of tumor cell intrinsic versus extrinsic effects of the hypusine circuit are clearly needed. Recently developed DHPS, DOHH, and eIF5A2 conditional knockout mice will be useful tools for such studies.
The recent discovery of the biochemical function of eIF5A in directing the efficient translation of a select cast of mRNAs having consecutive poly-Pro codons allows the field to identify eIF5A downstream targets. The challenge is that there are so many candidates. Indeed, 24 % of human proteins contain PPP motif and 19.9 % contain the PPG motif. Future work is needed to identify which of these eIF5A translational targets are directly associated with tumor initiation, maintenance and/or metastasis. Finally, recent studies suggest that not all poly-Pro containing proteins are eIF5A (or EF-P) dependent, and that some proteins lacking poly-Pro codons are independent of eIF5A (or EF-P) (Fujimura et al. 2015; Memin et al. 2014; Woolstenhulme et al. 2015).
Now that our knowledge of the anti-proliferative effects of hypusination inhibition on cancer cell growth is solidified by many independent studies, research in the field should move towards the development of therapeutic compounds targeting polyamine-hypusine circuit, focusing on future clinical application. In addition to screens for small molecules that selectively inhibit the function of DHPS, DOHH and eIF5A, structure-guided design will no doubt contribute to the generation of potent and selective inhibitors. Indeed, the structure of human DHPS bound with its cofactor NAD+ and the inhibitor GC7 revealed the mechanism of enzyme function (Umland et al. 2004) and provides a basis for inhibitor design. Furthermore, the cryo-electron microscopy structure of yeast eIF5A bound to 80S ribosome at 3.9 Å resolution supports a model where eIF5A facilitates the efficient transfer of the nascent polypeptide chain during synthesis of structurally restricting amino acid stretches, including polyprolines (Schmidt et al. 2016), and again this structure can be exploited in the design of small molecules that specifically inhibit eIF5A.
Of course, there are several roadblocks to generating small molecules that selectively disable the polyamine-hypusine circuit. First, such small molecules need to have proper pharmacokinetic and pharmacodynamic properties, and they will need to be unique chemical matter, so they are patentable and can move forward into the clinic. Second, the field needs to rigorously assess if there are possible risk factors of disabling hypusination, to confirm there is a suitable therapeutic window for any such agents that are developed. Third, specific biomarkers of the in vivo response to such agents need to be identified and validated. For example, a CLIA-certified assay that determines levels of hypusinated eIF5A in tumor biopsies (e.g., an ELISA assay or an IHC test) would be an important step to validate on-target effects of agents that ostensibly selectively inhibit DHPS, DOHH, and/or eIF5A. Finally, potential mechanisms of resistance to such agents need to be identified.
Despite these challenges, targeted drugs that disable hypusination of eIF5A by DHPS and DOHH can also be combined with the other therapies to effectively treat cancer patients. Furthermore, such treatments could be tailored to those tumors showing high levels of hypusinated eIF5A (personalized medicine), to improve the likelihood of an effective therapeutic response.
Acknowledgments
This study was supported by the NCI Comprehensive Cancer Center Grant P30-CA076292 to the H. Lee Moffitt Cancer Center and Research Institute.
Abbreviations
- eIF5A
Eukaryotic translation initiation factor 5A
- DHPS
Deoxyhypusine synthase
- DOHH
Deoxyhypusine hydroxylase
- GC7
N1-guanyl-1,7-diamineheptane
- CPX
Ciclopirox
- DEF
Deferiprone
Footnotes
Compliance with ethical standards
Research involving human participants and/or animals This article does not contain any studies with human participants or animals that were performed by any of the authors.
Conflict of interest The authors declare that they have no conflict of interest.
References
- Abbruzzese A, Hanauske-Abel HM, Park MH, Henke S, Folk JE. The active site of deoxyhypusyl hydroxylase: use of catecholpeptides and their component chelator and peptide moieties as molecular probes. Biochim Biophys Acta. 1991;1077:159–166. doi: 10.1016/0167-4838(91)90053-3. [DOI] [PubMed] [Google Scholar]
- Balabanov S, Gontarewicz A, Ziegler P, Hartmann U, Kammer W, Copland M, Brassat U, Priemer M, Hauber I, Wilhelm T, et al. Hypusination of eukaryotic initiation factor 5A (eIF5A): a novel therapeutic target in BCR-ABL-positive leukemias identified by a proteomics approach. Blood. 2007;109:1701–1711. doi: 10.1182/blood-2005-03-037648. [DOI] [PubMed] [Google Scholar]
- Bandino A, Geerts D, Koster J, Bachmann AS. Deoxyhypusine synthase (DHPS) inhibitor GC7 induces p21/Rb-mediated inhibition of tumor cell growth and DHPS expression correlates with poor prognosis in neuroblastoma patients. Cell Oncol. 2014;37:387–398. doi: 10.1007/s13402-014-0201-9. [DOI] [PubMed] [Google Scholar]
- Benne R, Brown-Luedi ML, Hershey JW. Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D, and eIF-5 from rabbit reticulocytes. J Biol Chem. 1978;253:3070–3077. [PubMed] [Google Scholar]
- Bullwinkle TJ, Zou SB, Rajkovic A, Hersch SJ, Elgamal S, Robinson N, Smil D, Bolshan Y, Navarre WW, Ibba M. (R)-beta-lysine-modified elongation factor P functions in translation elongation. J Biol Chem. 2013;288:4416–4423. doi: 10.1074/jbc.M112.438879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement PM, Hanauske-Abel HM, Wolff EC, Kleinman HK, Park MH. The antifungal drug ciclopirox inhibits deoxyhypusine and proline hydroxylation, endothelial cell growth and angiogenesis in vitro. Int J Cancer. 2002;100:491–498. doi: 10.1002/ijc.10515. [DOI] [PubMed] [Google Scholar]
- Cracchiolo BM, Heller DS, Clement PM, Wolff EC, Park MH, Hanauske-Abel HM. Eukaryotic initiation factor 5A-1 (eIF5A-1) as a diagnostic marker for aberrant proliferation in intraepithelial neoplasia of the vulva. Gynecol Oncol. 2004;94:217–222. doi: 10.1016/j.ygyno.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Doerfel LK, Wohlgemuth I, Kothe C, Peske F, Urlaub H, Rodnina MV. EF-P is essential for rapid synthesis of proteins containing consecutive proline residues. Science. 2013;339:85–88. doi: 10.1126/science.1229017. [DOI] [PubMed] [Google Scholar]
- Durie BG, Salmon SE, Russell DH. Polyamines as markers of response and disease activity in cancer chemotherapy. Cancer Res. 1977;37:214–221. [PubMed] [Google Scholar]
- Eberhard Y, McDermott SP, Wang X, Gronda M, Venugopal A, Wood TE, Hurren R, Datti A, Batey RA, Wrana J, et al. Chelation of intracellular iron with the antifungal agent ciclopirox olamine induces cell death in leukemia and myeloma cells. Blood. 2009;114:3064–3073. doi: 10.1182/blood-2009-03-209965. [DOI] [PubMed] [Google Scholar]
- Eisenberg T, Knauer H, Schauer A, Buttner S, Ruckenstuhl C, Carmona-Gutierrez D, Ring J, Schroeder S, Magnes C, Antonacci L, et al. Induction of autophagy by spermidine promotes longevity. Nature Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem. 2009;284:24696–24704. doi: 10.1074/jbc.M109.030098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epis MR, Giles KM, Kalinowski FC, Barker A, Cohen RJ, Leedman PJ. Regulation of expression of deoxyhypusine hydroxylase (DOHH), the enzyme that catalyzes the activation of eIF5A, by miR-331-3p and miR-642-5p in prostate cancer cells. J Biol Chem. 2012;287:35251–35259. doi: 10.1074/jbc.M112.374686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis SM, Taylor CA, Tang T, Liu Z, Zheng Q, Dondero R, Thompson JE. SNS01-T modulation of eIF5A inhibits B-cell cancer progression and synergizes with bortezomib and lenalidomide. J Am Soc Gene Ther. 2014;22:1643–1652. doi: 10.1038/mt.2014.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey AG, Nandal A, Park JH, Smith PM, Yabe T, Ryu MS, Ghosh MC, Lee J, Rouault TA, Park MH, et al. Iron chaperones PCBP1 and PCBP2 mediate the metallation of the dinuclear iron enzyme deoxyhypusine hydroxylase. Proc Natl Acad Sci USA. 2014;111:8031–8036. doi: 10.1073/pnas.1402732111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K, Wright T, Strnadel J, Kaushal S, Metildi C, Lowy AM, Bouvet M, Kelber JA, Klemke RL. A hypusine-eIF5APEAK1 switch regulates the pathogenesis of pancreatic cancer. Cancer Res. 2014;74:6671–6681. doi: 10.1158/0008-5472.CAN-14-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura K, Choi S, Wyse M, Strnadel J, Wright T, Klemke R. Eukaryotic translation initiation factor 5A (EIF5A) regulates pancreatic cancer metastasis by modulating RhoA and Rho-associated kinase (ROCK) protein expression levels. J Biol Chem. 2015;290:29907–29919. doi: 10.1074/jbc.M115.687418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Wu QL, Fang Y, et al. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res. 2004;64:4197–4200. doi: 10.1158/0008-5472.CAN-03-3747. [DOI] [PubMed] [Google Scholar]
- Gutierrez E, Shin BS, Woolstenhulme CJ, Kim JR, Saini P, Buskirk AR, Dever TE. eIF5A promotes translation of polyproline motifs. Mol Cell. 2013;51:35–45. doi: 10.1016/j.molcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LR, Zhao HY, Li BK, Liu YH, Liu MZ, Guan XY, Bian XW, Zeng YX, Xie D. Overexpression of eIF5A-2 is an adverse prognostic marker of survival in stage I non-small cell lung cancer patients. Int J Cancer. 2011;129:143–150. doi: 10.1002/ijc.25669. [DOI] [PubMed] [Google Scholar]
- Imam S, Mirmira RG, Jaume JC. Eukaryotic translation initiation factor 5A inhibition alters physiopathology and immune responses in a "humanized" transgenic mouse model of type 1 diabetes. Am J Physiol Endocrinol Metab. 2014;306:E791–E798. doi: 10.1152/ajpendo.00537.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, Weissman JS. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science. 2009;324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HA, Hershey JW. Effect of initiation factor eIF-5A depletion on protein synthesis and proliferation of Saccharomyces cerevisiae. J Biol Chem. 1994;269:3934–3940. [PubMed] [Google Scholar]
- Kim Y, Schmidt M, Endo T, Lu D, Carson D, Schmidt-Wolf IG. Targeting the Wnt/beta-catenin pathway with the antifungal agent ciclopirox olamine in a murine myeloma model. In Vivo. 2011;25:887–893. [PubMed] [Google Scholar]
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol. 2016;17:230–240. doi: 10.1038/ni.3384. [DOI] [PubMed] [Google Scholar]
- Lee NP, Tsang FH, Shek FH, Mao M, Dai H, Zhang C, Dong S, Guan XY, Poon RT, Luk JM. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127:968–976. doi: 10.1002/ijc.25100. [DOI] [PubMed] [Google Scholar]
- Li Y, Fu L, Li JB, Qin Y, Zeng TT, Zhou J, Zeng ZL, Chen J, Cao TT, Ban X, et al. Increased expression of EIF5A2, via hypoxia or gene amplification, contributes to metastasis and angiogenesis of esophageal squamous cell carcinoma. Gastroenterology. 2014;146(1701–1713):e1709. doi: 10.1053/j.gastro.2014.02.029. [DOI] [PubMed] [Google Scholar]
- Liu TA, Chang HK, Shieh RC. Revisiting inward rectification: K ions permeate through Kir2.1 channels during high-affinity block by spermidine. J Gen Physiol. 2012;139:245–259. doi: 10.1085/jgp.201110736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Zhou H, Liu L, Shen T, Chen W, Xu B, Han X, Zhang F, Scott RS, Alexander JS, et al. The fungicide ciclopirox inhibits lymphatic endothelial cell tube formation by suppressing VEGFR-3-mediated ERK signaling pathway. Oncogene. 2011;30:2098–2107. doi: 10.1038/onc.2010.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, Nunemaker CS, Stull ND, Taylor CA, Thompson JE, et al. The unique hypusine modification of eIF5A promotes islet beta cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–2170. doi: 10.1172/JCI38924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal A, Mandal S, Park MH. Genome-wide analyses and functional classification of proline repeat-rich proteins: potential role of eIF5A in eukaryotic evolution. PLoS One. 2014;9:e111800. doi: 10.1371/journal.pone.0111800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memin E, Hoque M, Jain MR, Heller DS, Li H, Cracchiolo B, Hanauske-Abel HM, Pe’ery T, Mathews MB. Blocking eIF5A modification in cervical cancer cells alters the expression of cancer-related genes and suppresses cell proliferation. Cancer Res. 2014;74:552–562. doi: 10.1158/0008-5472.CAN-13-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng QB, Kang WM, Yu JC, Liu YQ, Ma ZQ, Zhou L, Cui QC, Zhou WX. Overexpression of eukaryotic translation initiation factor 5A2 (EIF5A2) correlates with cell aggressiveness and poor survival in gastric cancer. PLoS One. 2015;10:e0119229. doi: 10.1371/journal.pone.0119229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Fleming L, Olin-Sandoval V, Campbell K, Ralser M. Remaining mysteries of molecular biology: the role of polyamines in the cell. J Mol Biol. 2015;427:3389–3406. doi: 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- Minden MD, Hogge DE, Weir SJ, Kasper J, Webster DA, Patton L, Jitkova Y, Hurren R, Gronda M, Goard CA, et al. Oral ciclopirox olamine displays biological activity in a phase I study in patients with advanced hematologic malignancies. Am J Hematol. 2014;89:363–368. doi: 10.1002/ajh.23640. [DOI] [PubMed] [Google Scholar]
- Moore CC, Martin EN, Lee G, Taylor C, Dondero R, Reznikov LL, Dinarello C, Thompson J, Scheld WM. Eukaryotic translation initiation factor 5A small interference RNA-liposome complexes reduce inflammation and increase survival in murine models of severe sepsis and acute lung injury. J Infect Dis. 2008;198:1407–1414. doi: 10.1086/592222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EJ. Update on iron chelators in thalassemia. Hematology/the Education Program of the American Society of Hematology American Society of Hematology Education Program 2010. 2010:451–455. doi: 10.1182/asheducation-2010.1.451. [DOI] [PubMed] [Google Scholar]
- Nishimura K, Lee SB, Park JH, Park MH. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 2012;42:703–710. doi: 10.1007/s00726-011-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliverio S, Corazzari M, Sestito C, Piredda L, Ippolito G, Piacentini M. The spermidine analogue GC7 (N1-guanyl-1,7-diamineoheptane) induces autophagy through a mechanism not involving the hypusination of eIF5A. Amino Acids. 2014;46:2767–2776. doi: 10.1007/s00726-014-1821-0. [DOI] [PubMed] [Google Scholar]
- Pallmann N, Braig M, Sievert H, Preukschas M, Hermans-Borgmeyer I, Schweizer M, Nagel CH, Neumann M, Wild P, Haralambieva E, et al. Biological relevance and therapeutic potential of the hypusine modification system. J Biol Chem. 2015;290:18343–18360. doi: 10.1074/jbc.M115.664490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Wolff EC, Folk JE. Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation. BioFactors. 1993a;4:95–104. [PubMed] [Google Scholar]
- Park MH, Wolff EC, Folk JE. Is hypusine essential for eukaryotic cell proliferation? TIBS. 1993b;18:475–479. doi: 10.1016/0968-0004(93)90010-k. [DOI] [PubMed] [Google Scholar]
- Park MH, Nishimura K, Zanelli CF, Valentini SR. Functional significance of eIF5A and its hypusine modification in eukaryotes. Amino Acids. 2010;38:491–500. doi: 10.1007/s00726-009-0408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Johansson HE, Aoki H, Huang BX, Kim HY, Ganoza MC, Park MH. Post-translational modification by beta-lysylation is required for activity of Escherichia coli elongation factor P (EF-P) J Biol Chem. 2012;287:2579–2590. doi: 10.1074/jbc.M111.309633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE. Spermidine/spermine-N(1)-acetyltransferase: a key metabolic regulator. Am J Physiol Endocrinol Metab. 2008;294:995–1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- Preukschas M, Hagel C, Schulte A, Weber K, Lamszus K, Sievert H, Pallmann N, Bokemeyer C, Hauber J, Braig M. Expression of eukaryotic initiation factor 5A and hypusine forming enzymes in glioblastoma patient samples: implications for new targeted therapies. PloS one. 2012;7:e43468. doi: 10.1371/journal.pone.0043468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Ross KN, Lander ES, Golub TR. A molecular signature of metastasis in primary solid tumors. Nat Gen. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- Robbins RD, Tersey SA, Ogihara T, Gupta D, Farb TB, Ficorilli J, Bokvist K, Maier B, Mirmira RG. Inhibition of deoxyhypusine synthase enhances islet beta cell function and survival in the setting of endoplasmic reticulum stress and type 2 diabetes. J Biol Chem. 2010;285:39943–39952. doi: 10.1074/jbc.M110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DH. Increased polyamine concentrations in the urine of human cancer patients. Nat New Biol. 1971;233:144–145. doi: 10.1038/newbio233144a0. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Becker T, Heuer A, Braunger K, Shanmuganathan V, Pech M, Berninghausen O, Wilson DN, Beckmann R. Structure of the hypusinylated eukaryotic translation factor eIF-5A bound to the ribosome. Nucleic Acids Res. 2016;44:1944–1951. doi: 10.1093/nar/gkv1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuoppo C, Miething C, Lindqvist L, Reyes J, Ruse C, Appelmann I, Yoon S, Krasnitz A, Teruya-Feldstein J, Pappin D, et al. A tumour suppressor network relying on the polyamine-hypusine axis. Nature. 2012;487:244–248. doi: 10.1038/nature11126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievert H, Pallmann N, Miller KK, Hermans-Borgmeyer I, Venz S, Sendoel A, Preukschas M, Schweizer M, Boettcher S, Janiesch PC, et al. A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis Models Mech. 2014;7:963–976. doi: 10.1242/dmm.014449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Christova T, Perusini S, Alizadeh S, Bao RY, Miller BW, Hurren R, Jitkova Y, Gronda M, Isaac M, et al. Wnt inhibitor screen reveals iron dependence of beta-catenin signaling in cancers. Cancer Res. 2011;71:7628–7639. doi: 10.1158/0008-5472.CAN-11-2745. [DOI] [PubMed] [Google Scholar]
- Subissi A, Monti D, Togni G, Mailland F. Ciclopirox: recent nonclinical and clinical data relevant to its use as a topical antimycotic agent. Drugs. 2010;70:2133–2152. doi: 10.2165/11538110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Sun Z, Cheng Z, Taylor CA, McConkey BJ, Thompson JE. Apoptosis induction by eIF5A1 involves activation of the intrinsic mitochondrial pathway. J Cell Physiol. 2010;223:798–809. doi: 10.1002/jcp.22100. [DOI] [PubMed] [Google Scholar]
- Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51:1255–1263. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Sun Z, Cliche DO, Ming H, Eshaque B, Jin S, Hopkins MT, Thai B, Thompson JE. Eukaryotic translation initiation factor 5A induces apoptosis in colon cancer cells and associates with the nucleus in response to tumour necrosis factor alpha signalling. Exp Cell Res. 2007;313:437–449. doi: 10.1016/j.yexcr.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Taylor CA, Liu Z, Tang TC, Zheng Q, Francis S, Wang TW, Ye B, Lust JA, Dondero R, Thompson JE. Modulation of eIF5A expression using SNS01 nanoparticles inhibits NF-kappaB activity and tumor growth in murine models of multiple myeloma. J Am Soc Gene Ther. 2012;20:1305–1314. doi: 10.1038/mt.2012.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CA, Zheng Q, Liu Z, Thompson JE. Role of p38 and JNK MAPK signaling pathways and tumor suppressor p53 on induction of apoptosis in response to Ad-eIF5A1 in A549 lung cancer cells. Mol Cancer. 2013;12:35. doi: 10.1186/1476-4598-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templin AT, Maier B, Nishiki Y, Tersey SA, Mirmira RG. Deoxyhypusine synthase haploinsufficiency attenuates acute cytokine signaling. Cell Cycle. 2011;10:1043–1049. doi: 10.4161/cc.10.7.15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunca B, Tezcan G, Cecener G, Egeli U, Zorluoglu A, Yilmazlar T, Ak S, Yerci O, Ozturk E, Umut G, et al. Overexpression of CK20, MAP3K8 and EIF5A correlates with poor prognosis in early-onset colorectal cancer patients. J Cancer Res Clin Oncol. 2013;139:691–702. doi: 10.1007/s00432-013-1372-x. [DOI] [PubMed] [Google Scholar]
- Ude S, Lassak J, Starosta AL, Kraxenberger T, Wilson DN, Jung K. Translation elongation factor EF-P alleviates ribosome stalling at polyproline stretches. Science. 2013;339:82–85. doi: 10.1126/science.1228985. [DOI] [PubMed] [Google Scholar]
- Umland TC, Wolff EC, Park MH, Davies DR. A new crystal structure of deoxyhypusine synthase reveals the configuration of the active enzyme and of an enzyme·NAD·inhibitor ternary complex. J Biol Chem. 2004;279:28697–28705. doi: 10.1074/jbc.M404095200. [DOI] [PubMed] [Google Scholar]
- Wei JH, Cao JZ, Zhang D, Liao B, Zhong WM, Lu J, Zhao HW, Zhang JX, Tong ZT, Fan S, et al. EIF5A2 predicts outcome in localised invasive bladder cancer and promotes bladder cancer cell aggressiveness in vitro and in vivo. Br J Cancer. 2014;110:1767–1777. doi: 10.1038/bjc.2014.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolstenhulme CJ, Guydosh NR, Green R, Buskirk AR. High-precision analysis of translational pausing by ribosome profiling in bacteria lacking EFP. Cell Rep. 2015;11:13–21. doi: 10.1016/j.celrep.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Yu H, Shi X, Sun L, Zhou Q, Zheng D, Shi H, Li N, Zhang X, Shao G. Cisplatin sensitivity is enhanced in non-small cell lung cancer cells by regulating epithelial-mesenchymal transition through inhibition of eukaryotic translation initiation factor 5A2. BMC Pulm Med. 2014;14:174. doi: 10.1186/1471-2466-14-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang GF, Xie D, Liu JH, Luo JH, Li LJ, Hua WF, Wu HM, Kung HF, Zeng YX, Guan XY. Expression and amplification of eIF-5A2 in human epithelial ovarian tumors and overexpression of EIF-5A2 is a new independent predictor of outcome in patients with ovarian carcinoma. Gynecol Oncol. 2009;112:314–318. doi: 10.1016/j.ygyno.2008.10.024. [DOI] [PubMed] [Google Scholar]
- Zhou H, Shen T, Luo Y, Liu L, Chen W, Xu B, Han X, Pang J, Rivera CA, Huang S. The antitumor activity of the fungicide ciclopirox. Int J Cancer. 2010;127:2467–2477. doi: 10.1002/ijc.25255. [DOI] [PMC free article] [PubMed] [Google Scholar]