Abstract
Background
Major organizations recommend cytology screening (Pap test) every 3 years for women aged 21–65; women aged 30 to 65 have the option of adding the HPV test (co-test) every 5 years. We examined national percentages of cervical cancer screening, and we examined use of co-testing as an option for screening.
Methods
We used 2015 U.S. National Health Interview Survey (NHIS) data to examine recent cervical cancer screening (Pap test within 3 years among women aged 21–65without a hysterectomy; N = 10,596) and co-testing (N = 9,125). We also conducted a multivariable analysis to determine odds of having had a Pap test or co-test by demographic variables. To evaluate changes in screening over time, we examined Pap testing during the years 2000, 2005, 2008, 2010, 2013 and 2015. Analysis completed in Atlanta, GA during 2016.
Results
Overall, 81.1% of eligible women reported having a Pap test within 3 years; percentages declined over time among all age groups. An estimated 14 million women aged 21–65 had not been screened within the past 3 years. Recent immigrants to the United States, women without insurance, and women without a usual source of healthcare had lower odds of being up to date with screening. About 1/3 of women up to date on Pap testing reported having a co-test with their most recent Pap test.
Conclusions
Declines in screening among women aged 21–65 are cause for concern. More research is needed on co-testing practices. Provider and patient education efforts may be needed to clarify recommended use of HPV tests.
Keywords: Pap test, HPV DNA test, Cervical cancer, Cancer screening, Cytology
1. Introduction
In the United States, cervical cancer screening has proven to be extremely successful, resulting in declining incidence and mortality rates, although recent statistics suggest declines in mortality have stabilized (Ryerson et al., 2016; Saraiya et al., 2013; Benard et al., 2014). In 2012, major organizations that issue guidelines on cervical cancer screening recommended cytology screening (Pap test) every 3 years for women aged 21–65;women aged 30 to 65 have the option of adding the HPV test (co-test) every 5 years (Centers for Disease Control and Prevention, 2013).
Healthy People provides national objectives for improving the health of all Americans. The Healthy People 2020 (HP2020) cervical cancer objective is to increase the proportion of women aged 21–65 who receive a screening based on the most recent guidelines to 93% (Healthy People 2020, 2016). Analyses of national data from 2013 showed that the percentage of recommended screening (every 3 years among women aged 21–65) had not yet attained this objective, and in fact were declining (Sabatino et al., 2015).
The purpose of this study was to examine the most recent national survey data (2015) on cervical cancer screening in accordance with current recommendations to assess progress toward HP2020 objectives, and to examine national data on the use of co-testing as an option for screening.
2. Methods
We used data from the 2015 U.S. National Health Interview Survey (NHIS) to examine recent cervical cancer screening. NHIS is a cross-sectional household survey conducted in person in English or Spanish and representative of the civilian, noninstitutionalized US population (National Center for Health Statistics, Centers for Disease Control and Prevention, 2016a). One sample adult aged ≥18 years and sample child (if present) in each family are randomly selected for additional detailed questions. We used the Sample Adult file, which had a response rate of 55.2% for 2015 (National Center for Health Statistics, Centers for Disease Control and Prevention, 2016b). We also used the Person and Imputed Income files for additional information. The overall proportions of persons screened were presented as crude percentages and age standardized to the 2000 U.S. standard population.
We considered having had a Papanicolaou (Pap) test within 3 years as being up to date with screening. Women age 18+ who reported ever having had a Pap test were asked the NHIS question: When did you have your MOST RECENT Pap test? In order to assess information on co-testing, these women were also asked: An HPV test is sometimes given with the Pap test for cervical cancer screening. Did you have an HPV test with your most recent Pap test? We limited our analysis to women recommended for screening: age 21–65 years, not having had a hysterectomy.
We examined screening by race/ethnicity (white, black, and Asian [all non-Hispanic], and Hispanic [regardless of race]), age group, U.S. residence, education level, family income (% of federal poverty threshold), usual source of health care, and health care insurance coverage. Insurance includes public or private health care coverage, but excludes Indian Health Service coverage or single service plans (i.e., that pay for only one type of service). We also examined the odds ratios of these variables in a multivariate analysis, to determine which of these factors may be most strongly associated with cervical cancer screening. NHIS data from2000, 2005, 2008, 2010, 2013, and 2015 were used to evaluate changes in cervical cancer screening percentages over time. We used two test timing recodes for NHIS data, depending on the year or years analyzed. Timing recode “A” was used for 2015 data (NHIS variable RPAP3A1) (National Center for Health Statistics, Centers for Disease Control and Prevention, 2016a). This recode is available for 2005 and forward data, and provides the most accurate estimates. The timing recode “B”, used for 2000–2015 trends, uses the year 2000 estimation method and assumptions for missing data (NHIS variable RPAP3B1). The “B” version results in slightly biased screening estimates, but allows for unbiased comparisons with the 2000 and 2003 data.
We used SAS-callable SUDAAN Version 9.3 for statistical analysis. Differences in demographic variables were considered statistically significant if 95% confidence intervals did not overlap. Percent change was calculated as the percentage receiving screening in 2015 subtracted from the percentage screened in 2000, divided by the percentage screened in 2000. Pearson Wald F tests were used to test for differences in rates across years. All statistics were weighted to account for unequal probability of selection and nonresponse.
3. Results
Overall, 81.1% of women aged 21–65 reported having a Pap test within 3 years, in accordance with recommendations (Table 1). Non-Hispanic Asian and Hispanic women had lower percentages of Pap test within 3 years (73.5% and 76.9, respectively) than non-Hispanic white and non-Hispanic black women (82.6% and 84.5%, respectively). Only 66.8% of women in the United States < 10 years reported a recent screening, compared to 77.0% of those in the united States > 10 years and 82.8% of US = born women. About 1/3 of women up to date on Pap testing reported having a co-test at their most recent screening. Co-testing percentages were highest among non-Hispanic black women and lowest among non-Hispanic Asian women (35.2% and 21.4%, respectively). Co-testing varied by age. Among women up to date with Pap testing, 30–39 year olds most commonly reported a co-test (41.0%), followed by 21–29 (38.2%) year olds.
Table 1.
| Pap test within 3 years | Co-test (Pap + HPV) within 3 years | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| N | % | 95% CI | N | % | 95% CI | |
| Overall (crude) AGE 21–65 ONLY | 10,596 | 81.1 | (80.1, 82.1) | 9125 | 32.0 | (30.6, 33.4) |
| Overall (age-adjusted to 2000 U.S. Standard Population) | 10,596 | 81.4 | (80.4, 82.4) | 9125 | 31.9 | (30.5, 33.3) |
| Race | ||||||
| Hispanic | 2121 | 76.9 | (74.4, 79.2) | 1861 | 30.5 | (27.6, 33.6) |
| Non-Hispanic White | 6062 | 82.6 | (81.3, 83.8) | 5174 | 33.0 | (31.1, 34.9) |
| Non-Hispanic Black | 1579 | 84.5 | (82.1, 86.6) | 1372 | 35.2 | (31.9, 38.6) |
| Non-Hispanic Asian | 684 | 73.5 | (69.3, 77.3) | 598 | 21.4 | (17.5, 25.8) |
| Non-Hispanic Other | 150 | 69.7 | (55.8, 80.8) | 120 | 27.5 | (18.1, 39.4) |
| Age in years | ||||||
| 21–29 | 2281 | 76.7 | (74.1, 79.1) | 2066 | 38.2 | (35.4, 41.0) |
| 30–39 | 2737 | 86.1 | (84.3, 87.7) | 2384 | 41.0 | (38.2, 43.8) |
| 40–49 | 2246 | 81.9 | (79.6, 84.1) | 1928 | 29.8 | (27.1, 32.8) |
| 50–65 | 3332 | 79.8 | (78.1, 81.4) | 2747 | 20.3 | (18.1, 22.8) |
| Period of U.S. residence | ||||||
| US-born | 8320 | 82.8 | (81.6, 83.9) | 7153 | 34.2 | (32.6, 35.8) |
| In United States <10 years | 470 | 66.8 | (61.7, 71.5) | 409 | 21.3 | (16.7, 26.9) |
| In United States ≥10 years | 1783 | 77.0 | (74.3, 79.5) | 1545 | 24.2 | (21.3, 27.3) |
| Education | ||||||
| Less than high school | 1230 | 69.5 | (65.9, 72.9) | 1068 | 20.8 | (17.6, 24.3) |
| High school graduate | 2161 | 74.7 | (72.0, 77.2) | 1889 | 26.7 | (23.7, 29.9) |
| Some college/associate degree | 3480 | 81.2 | (79.2, 83.1) | 3010 | 33.3 | (31.0, 35.7) |
| College graduate | 3698 | 87.8 | (86.5, 89.1) | 3132 | 37.0 | (34.8, 39.4) |
| % of federal poverty threshold | ||||||
| <139% | 2999 | 73.5 | (71.2, 75.7) | 2627 | 26.6 | (24.1, 29.2) |
| 139%–250% | 2100 | 76.4 | (73.7, 78.9) | 1844 | 29.4 | (26.7, 32.2) |
| 251%–400% | 1983 | 80.6 | (78.2, 82.8) | 1703 | 31.8 | (29.0, 34.6) |
| > 400% | 3514 | 87.9 | (86.4, 89.3) | 2951 | 36.6 | (34.3, 39.0) |
| Usual source of care | ||||||
| None or hospital emergency department | 702 | 74.2 | (69.4, 78.5) | 618 | 33.6 | (28.4, 39.2) |
| Has usual source | 8483 | 84.5 | (83.5, 85.4) | 7225 | 32.7 | (31.2, 34.3) |
| Health care coverage | ||||||
| Private | 6708 | 85.3 | (84.1, 86.3) | 5790 | 34.5 | (32.8, 36.3) |
| Medicaid and other public | 1852 | 77.9 | (75.1, 80.5) | 1614 | 29.9 | (26.6, 33.5) |
| Other coverage | 472 | 83.5 | (78.6, 87.5) | 408 | 33.9 | (28.5, 39.7) |
| Uninsured | 1334 | 61.2 | (57.7, 64.6) | 1212 | 21.4 | (18.4, 24.8) |
Abbreviations: CI = confidence interval; Pap = Papanicolaou.
NHIS question for Pap test: When did you have your MOST RECENT Pap test?
NHIS question for HPV test: An HPV test is sometimes given with the Pap test for cervical cancer screening. Did you have an HPV test with your most recent Pap test?
Data analysis completed in Atlanta, GA during 2016.
Percentages expressed are weighted. Overall percentages presented as crude and age–adjusted estimates. Other percentages are crude estimates.
Percentages for co-testing are calculated as a subset of those who reported their most recent Pap test within 3 years.
We conducted a multivariable analysis to calculate odds ratios of receipt of cervical cancer screening, adjusting for other variables (Table 2). Compared with non-Hispanic white women, non-Hispanic black women had higher odds of reporting up to date Pap tests (adjusted OR 1.44; 95% CI 1.15–1.81), while non-Hispanic Asian women had lower odds (OR 0.53, 95% CI 0.39–0.73). Women age 30–39 years had higher odds than women of other ages of being up to date with cervical cancer screening. Lower odds of reporting a Pap test was associated with being in the United States < 10 years, no health coverage, and no usual source of health care. Higher odds of reporting a Pap test were associated with having higher income and a college degree.
Table 2.
Adjusted odds ratios of Pap testing and co-testing within 3 years by demographic variables, United States, 2015.
| Pap test within 3 years |
Co-test (Pap + HPV) within 3 years |
|||
|---|---|---|---|---|
|
|
|
|||
| OR | 95% CI | OR | 95% CI | |
| Race/ethnicity | ||||
| Hispanic | 1.19 | (0.89, 1.60) | 1.31 | (1.03, 1.66) |
| Non-Hispanic White | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| Non-Hispanic Black | 1.44 | (1.15, 1.81) | 1.30 | (1.08, 1.56) |
| Non-Hispanic Asian | 0.53 | (0.39, 0.73) | 0.68 | (0.50, 0.94) |
| Non-Hispanic other | 0.68 | (0.34, 1.35) | 0.90 | (0.53, 1.53) |
| Age in years | ||||
| 21–29 | 0.55 | (0.43, 0.71) | 0.89 | (0.73, 1.08) |
| 30–39 | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| 40–49 | 0.69 | (0.54, 0.88) | 0.57 | (0.48, 0.69) |
| 50–65 | 0.57 | (0.45, 0.72) | 0.34 | (0.28, 0.41) |
| Period of U.S. residence | ||||
| US-born | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| In United States <10 years | 0.56 | (0.39, 0.80) | 0.48 | (0.32, 0.71) |
| In United States ≥10 years | 0.87 | (0.67, 1.13) | 0.73 | (0.56, 0.95) |
| Education | ||||
| Less than high school | 0.48 | (0.35, 0.67) | 0.67 | (0.50, 0.90) |
| High school graduate | 0.52 | (0.41, 0.65) | 0.77 | (0.62, 0.95) |
| Some college/associate degree | 0.65 | (0.53, 0.80) | 0.92 | (0.78, 1.09) |
| College graduate | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| % of federal poverty threshold | ||||
| <139% | 0.63 | (0.46, 0.85) | 0.66 | (0.52, 0.85) |
| 139%–250% | 0.64 | (0.49, 0.84) | 0.72 | (0.59, 0.88) |
| 251%–400% | 0.80 | (0.62, 1.03) | 0.79 | (0.64, 0.96) |
| >400% | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| Usual source of care | ||||
| None or hospital emergency department | 0.59 | (0.45, 0.77) | 0.99 | (0.76, 1.29) |
| Has usual source | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| Health care coverage | ||||
| Private | 1.00 | (1.00, 1.00) | 1.00 | (1.00, 1.00) |
| Medicaid and other public | 0.93 | (0.74, 1.17) | 0.95 | (0.76, 1.18) |
| Other coverage | 1.15 | (0.78, 1.70) | 1.26 | (0.94, 1.70) |
| Uninsured | 0.57 | (0.43, 0.76) | 0.72 | (0.54, 0.97) |
Abbreviations: CI = confidence interval; HPV = Human Papillomavirus; OR = odds ratio; Pap = Papanicolaou.
NHIS question for Pap test: When did you have your MOST RECENT Pap test?
NHIS question for HPV test: An HPV test is sometimes given with the Pap test for cervical cancer screening. Did you have an HPV test with your most recent Pap test?
Data analysis completed in Atlanta, GA during 2016.
Results for the multivariate analysis to determine odds of having had a co-test at the most recent screening were similar to those for being up to date with Pap test screening, with a few exceptions. Hispanic women, in addition to non-Hispanic black women, had higher odds of reporting a co-test than white women. Differences in co-testing among women aged 21–29 and women aged 30–39 were not statistically significant (OR 0.89; 95% CI 0.73, 1.08 compared to referent group). US-born women had higher odds of reporting co-testing than foreign-born women. As with recent Pap testing, co-testing generally appeared to increase with educational attainment in the adjusted analysis, and uninsured women had lower odds of co-testing than women with some type of insurance.
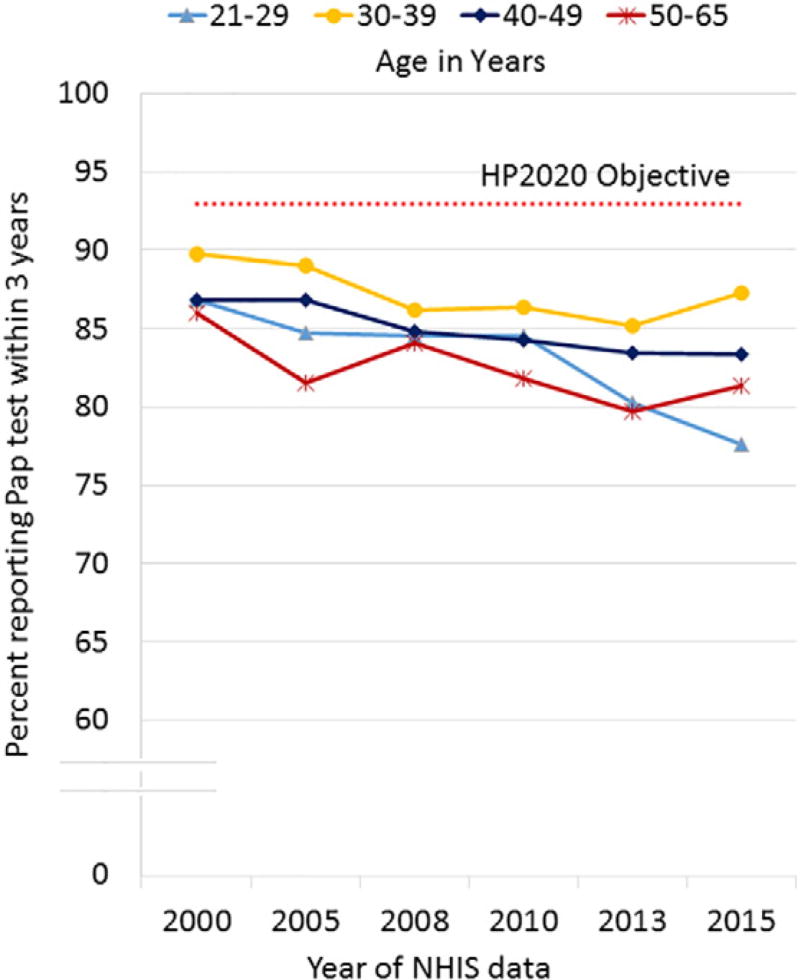
We observed small, though statistically significant, declines in Pap testing among women aged 21–65 from 2000 to 2015 (−5.8%, Pearson Wald F test for trend p < 0.001; Fig. 1). Screening percentages were lowest and declined the most (10.6%) among women aged 21–29, from 86.8% in 2000 to 77.6% in 2015 (test for trend p < 0.001). Women aged 30–39 had the highest screening percentages and the smallest declines over time.
Fig. 1.
Trends in cervical cancer screening (Pap test) within 3 years, United States, 2000–2015. All trends 2000–2015 statistically significant (p < 0.01). NHIS question for Pap test: When did you have your MOST RECENT Pap test? NHIS question for co-test: An HPV test is sometimes given with the Pap test for cervical cancer screening. Did you have an HPV test with your most recent Pap test? Data analysis completed in Atlanta, GA during 2016.
4. Discussion
Over 80% of women reported being screened with a Pap test in accordance with recommendations; however, declines are cause for concern, and trends are not approaching the HP2020 objective (93% of eligible women screened). However, the nearly 20% of women were not screened within the past 3 years translates to > 14 million women aged 21–65. No demographic group of women examined obtained the national objective, and consistent with previous research, some groups had markedly lower screening prevalence (Tsui et al., 2007). For example, non-Hispanic Asian women had lower screening percentages compared to non-Hispanic white and non-Hispanic black women, and this difference was statistically significant in the adjusted analysis. Also, only 66.8% of foreign-born women living in the United States for < 10 years were recently screened in accordance with recommendations. Previous studies have documented that foreign-born women are less likely to be screened, especially recent immigrants (Tsui et al., 2007; Tangka et al., 2015). Uninsured women were also less likely to be screened (61.2%), despite federal programs to provide screening services to these women (Tangka et al., 2015).
Nearly one-third of women who were up to date with Pap testing reported having had a co-test at their most recent screening. Overall patterns of co-testing mirrored those for Pap testing, with a few exceptions. Co-testing varied by age, with the highest percentages among those younger than age 40.
Among women aged 21–29 who were up to date with screening guidelines, 38% reported having had a co-test at their most recent screening. Co-testing is not recommended as part of screening for women younger than age 30. A small proportion of HPV tests among women aged 21–29 may have been conducted according to guidelines, because women of any age diagnosed with atypical squamous cells of undetermined significance (ASC-US) can be tested for HPV to determine next steps. Some prior recommendations recommended “reflex testing” of abnormal Pap test results to determine whether abnormal results were HPV-positive or not (Saraiya et al., 2013). Inaccurate self-report may also contribute to findings. Nearly 1 in 5 women overall (17%) reported not knowing whether or not they had had an HPV test at their most recent screening (data not shown). This percentage varied by age, with only 13% of women aged 20–29 reporting being unsure of whether they had been tested for HPV during their most recent screening, and higher proportions among women over age 40 (19%). Liquid-based Pap tests allow for the use of HPV tests without taking an additional sample (Committee on Practice Bulletins–Gynecology, 2016), and women may not be informed of co-test use except in the case of positive results. Potential overuse of HPV tests among women aged 21–29 and lack of information about whether HPV tests were used in screening highlight the need for increased provider and patient education regarding use of co-tests.
In 2014, the FDA approved use of one HPV test as primary screening for women aged 25 and older (U.S. Food and Drug Administration, 2014). Although current screening guidelines do not recommend primary screening via HPV test, representatives from several organizations including the American Cancer Society, the American Society for Colposcopy and Cervical Pathology, and the American Society for Clinical Pathology have issued interim guidance for clinicians wishing to use the HPV test as primary screening (Centers for Disease Control and Prevention, 2013; Huh et al., 2015). We were not able to identify women using the HPV test as primary screening, because this modality was not in use at the time of development of the NHIS questions.
In 2006, the first vaccine protecting against HPV infection was approved for use in the United States (Centers for Disease Control and Prevention, 2007). Soon after the vaccine approval, there was concern that women who had been vaccinated against HPV might be less likely to participate in cervical cancer screening (Kulasingam et al., 2007). Our preliminary analysis showed that on the contrary, vaccinated women of all ages, as well as those aged 21–29, were more likely to be screened than unvaccinated women. These findings are consistent with previous research (Chao et al., 2017). Associations between vaccine receipt and cervical cancer screening were not significant in the multivariate adjusted analysis. Socioeconomic factors such as education, income, and health insurance were important variables in our model, and are likely to be positively associated with both HPV vaccination and cervical screening. One modeling study suggests that HPV vaccination will reduce overall screening rates, but that current inequities will persist as long as some populations have low rates of vaccination and screening (Malagon et al., 2015). Future studies to determine factors in screening by HPV vaccination status may elucidate additional important findings.
While current screening guidelines remain consistent for vaccinated and unvaccinated women, recommendations for screening might differ by HPV vaccination status in the future, or screening intervals may lengthen for all women if the prevalence of high-risk HPV reaches low enough levels in the population. As vaccine rates increase the prevalence of abnormal lesions, especially high-grade, is expected to decrease, reducing the specificity of cytology (Rodriguez et al., 2013). Primary HPV testing with cytology used as a follow-up may be a more effective way to screen vaccinated women (El-Zein et al., 2016).
5. Limitations
To our knowledge, this is the first examination of nationally representative data examining HPV test use in cervical cancer screening. Our paper also provides updated information on cervical cancer screening, providing information with which to monitor success toward HP2020 objectives. Despite these strengths, we did identify some limitations. First, the NHIS question currently asks women if they were screened for HPV “with your most recent Pap test”. This wording does not allow for women to report whether they have had the HPV test as a primary test (separate from Pap test), or whether they have had an HPV test at another time other than at the last Pap test. Also, because this is the first use of HPV test questions, future versions of the survey may identify improvements on the question. Many women (17%) reported not knowing whether they had a HPV test or not. Because the NHIS uses self-report data, these data may be less accurate than medical records.
While many cancer screening questions have high validation scores when compared with medical data, cervical cancer screening is frequently over reported, and women frequently confuse Pap testing with pelvic exams for other reasons (Rauscher et al., 2008). Questions on HPV testing may be subject to similar concerns, especially since women aged 21–30 were most likely to report having had an HPV test despite the fact that HPV testing is not recommended for women younger than age 30. Studies using medical records to validate self-report of co-tests are needed to determine the accuracy of co-test data. Also, given variability of screening intervals as well as multiple options for test types, new methods may be needed to accurately assess cervical cancer screening. One method has been proposed to ask women first if they have ever been screened, the timing of the most recent test, and then the type of test used (Lowe et al., 2015).
We do not have longitudinal data over time from the same women enabling us to look at the frequency of screening. As guidelines have changed from one-year screening intervals to three- or five-year intervals, it would be helpful to better understand patterns of screening intervals. Also, we defined being up to date on screening as having had a Pap test within 3 years; it is possible that some women had co-tests and are extending intervals to 5 years. A related analysis found that including these women increased the age-adjusted percentage of women screened according to guidelines to 83.0% (compared to 81.4% in our analysis) (White et al., 2017). Finally, while this is a large and robust national survey, there is always the possibility of nonrandom survey sampling errors with survey data.
6. Conclusions
This study provides important new information on the use of HPV tests in screening. Provider and patient education may be needed to clarify when HPV tests are being administered, what they are for, and for which populations (women age 30 to 65). Declines in screening among women aged 21–65 are cause for concern. Certain populations, such as recent immigrants and uninsured women, have very low percentages of screening that need to be addressed. If declines in cervical cancer are to be continued, more efforts may be needed to reach rarely-or never-screened women.
Acknowledgments
Funding support
This research was supported in part by an appointment (Anatasha Crawford) to the Research Participation Program at the Centers for Disease Control and Prevention administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflict of interest disclosures
None.
References
- Committee on Practice Bulletins–Gynecology. Practice bulletin no. 168: cervical cancer screening and prevention. Obstet. Gynecol. 2016;128(4):e111–e130. doi: 10.1097/AOG.0000000000001708. [DOI] [PubMed] [Google Scholar]
- Benard VB, Thomas CC, King J, Massetti GM, Doria-Rose VP, Saraiya M, et al. Vital signs: cervical cancer incidence, mortality, and screening - United States, 2007–2012. MMWR Morb. Mortal. Wkly Rep. 2014;63(44):1004–1009. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 2007;56(rr02):1–24. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cervical Cancer Screening Guidelines for Average-risk Women. [Accessed on March 4 2016];2013 Available from:. http://www.cdc.gov/cancer/cervical/pdf/guidelines.pdf.
- Chao C, Silverberg MJ, Becerra TA, Corley DA, Jensen CD, Chen Q, et al. Human papillomavirus vaccination and subsequent cervical cancer screening in a large integrated healthcare system. Am. J. Obstet. Gynecol. 2017;216(2):151 e1–151 e9. doi: 10.1016/j.ajog.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Zein M, Richardson L, Franco EL. Cervical cancer screening of HPV vaccinated populations: cytology, molecular testing, both or none. J. Clin. Virol. 2016;76(Suppl. 1):S62–S68. doi: 10.1016/j.jcv.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healthy People 2020 [Internet] Office of Disease Prevention and Health Promotion. Washington, DC: U.S. Department of Health and Human Services; 2016. [cited August 4, 2016]. Available from. http://www.healthypeople.gov/2020/topics-objectives/topic/cancer/objectives. [Google Scholar]
- Huh WK, Ault KA, Chelmow D, Davey DD, Goulart RA, Garcia FA, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol. Oncol. 2015;136(2):178–182. doi: 10.1016/j.ygyno.2014.12.022. [DOI] [PubMed] [Google Scholar]
- Kulasingam SL, Pagliusi S, Myers E. Potential effects of decreased cervical cancer screening participation after HPV vaccination: an example from the U.S. Vaccine. 2007;25(48):8110–8113. doi: 10.1016/j.vaccine.2007.09.035. [DOI] [PubMed] [Google Scholar]
- Lowe A, Saraiya M, Joseph R. Development of standardized questions to monitor cervical cancer screening and treatment in population-based surveys. International Papillomavirus Conference; 2015 September 17–21, 2015; Lisbon, Portugal. 2015. [Google Scholar]
- Malagon T, Drolet M, Boily MC, Laprise JF, Brisson M. Changing inequalities in cervical cancer: modeling the impact of vaccine uptake, vaccine herd effects, and cervical cancer screening in the post-vaccination era. Cancer Epidemiol. Biomark. Prev. 2015;24(1):276–285. doi: 10.1158/1055-9965.EPI-14-1052. [DOI] [PubMed] [Google Scholar]
- National Center for Health Statistics, Centers for Disease Control and Prevention. National Health Interview Survey, 2015. [Accessed on November 30 2016];Public-use Data File and Documentation. 2016C Available from: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- National Center for Health Statistics, Centers for Disease Control and Prevention. Survey Description, National Health Interview Survey, 2015. [Accessed on November 30 2016];2016C Available from: http://www.cdc.gov/nchs/nhis/quest_data_related_1997_forward.htm.
- Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol. Biomark. Prev. 2008;17(4):748–757. doi: 10.1158/1055-9965.EPI-07-2629. [DOI] [PubMed] [Google Scholar]
- Rodriguez AC, Solomon D, Herrero R, Hildesheim A, Gonzalez P, Wacholder S, et al. Impact of human papillomavirus vaccination on cervical cytology screening, colposcopy, and treatment. Am. J. Epidemiol. 2013;178(5):752–760. doi: 10.1093/aje/kwt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryerson AB, Eheman CR, Altekruse SF, Ward JW, Jemal A, Sherman RL, et al. Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122(9):1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatino SA, White MC, Thompson TD, Klabunde CN. Cancer screening test use - United States, 2013. MMWR Morb. Mortal. Wkly Rep. 2015;64(17):464–468. [PMC free article] [PubMed] [Google Scholar]
- Saraiya M, Steben M, Watson M, Markowitz L. Evolution of cervical cancer screening and prevention in United States and Canada: implications for public health practitioners and clinicians. Prev. Med. 2013;57(5):426–433. doi: 10.1016/j.ypmed.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangka FK, Howard DH, Royalty J, Dalzell LP, Miller J, O'Hara BJ, et al. Cervical cancer screening of underserved women in the United States: results from the National Breast and Cervical Cancer Early Detection Program, 1997–2012. Cancer Causes Control. 2015;26(5):671–686. doi: 10.1007/s10552-015-0524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui J, Saraiya M, Thompson T, Dey A, Richardson L. Cervical cancer screening among foreign-born women by birthplace and duration in the United States. J. Women's Health. 2007;16(10):1447–1457. doi: 10.1089/jwh.2006.0279. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. FDA Approves First Human Papillomavirus Test for Primary Cervical Cancer Screening. 2014 Available from: http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm.
- White A, Thompson TD, White MC, Sabatino SA, de Moor J, Doria-Rose PV, et al. Cancer screening test use - United States, 2015. MMWR Morb. Mortal. Wkly Rep. 2017;66(8):201–206. doi: 10.15585/mmwr.mm6608a1. [DOI] [PMC free article] [PubMed] [Google Scholar]