Abstract
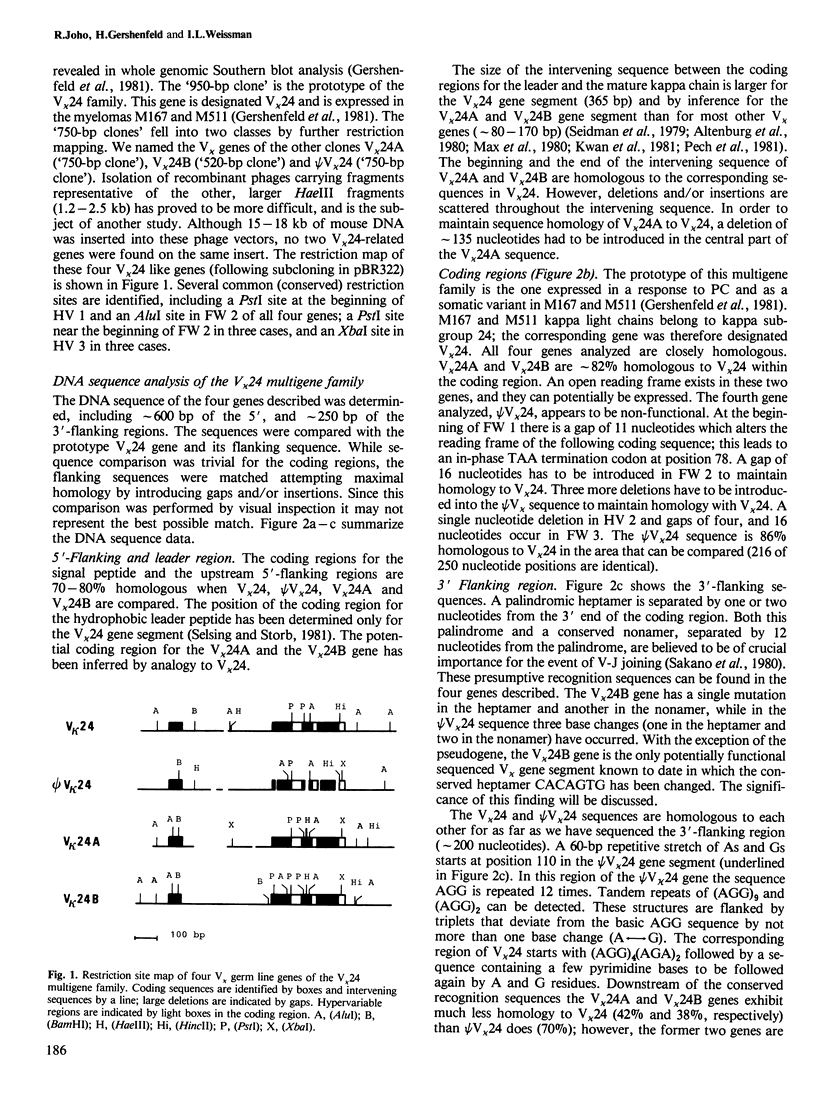
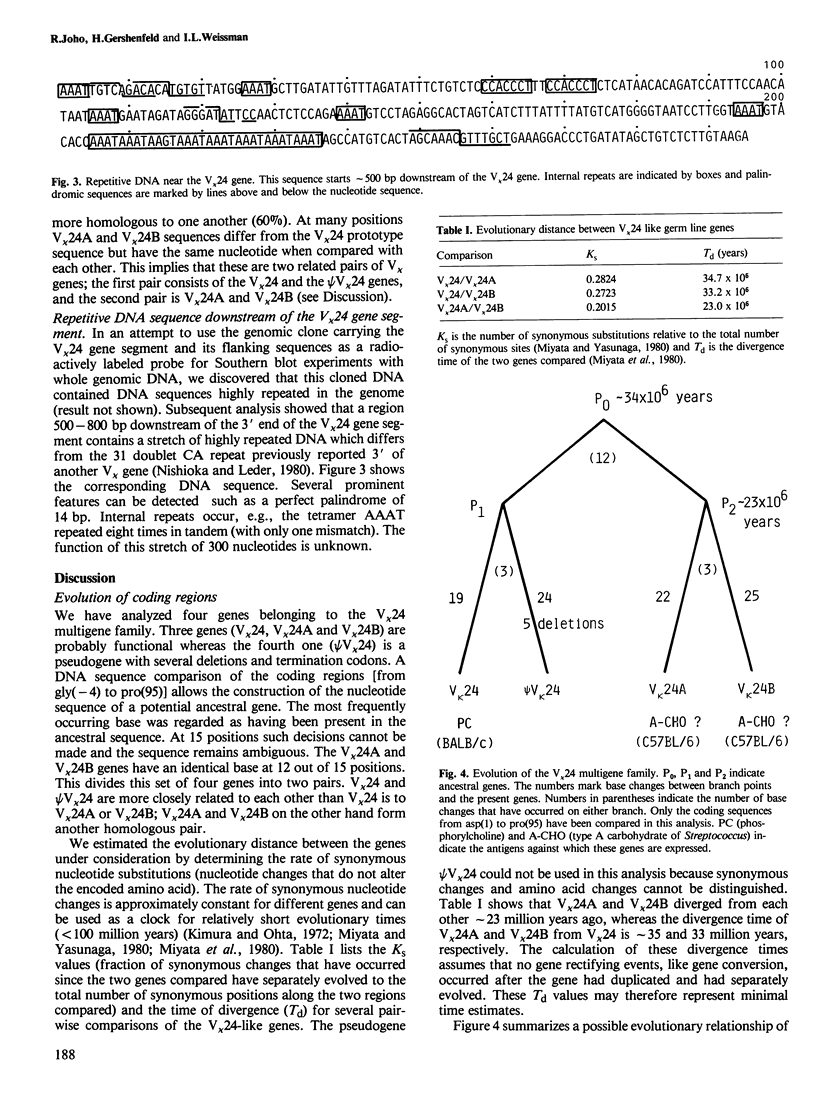
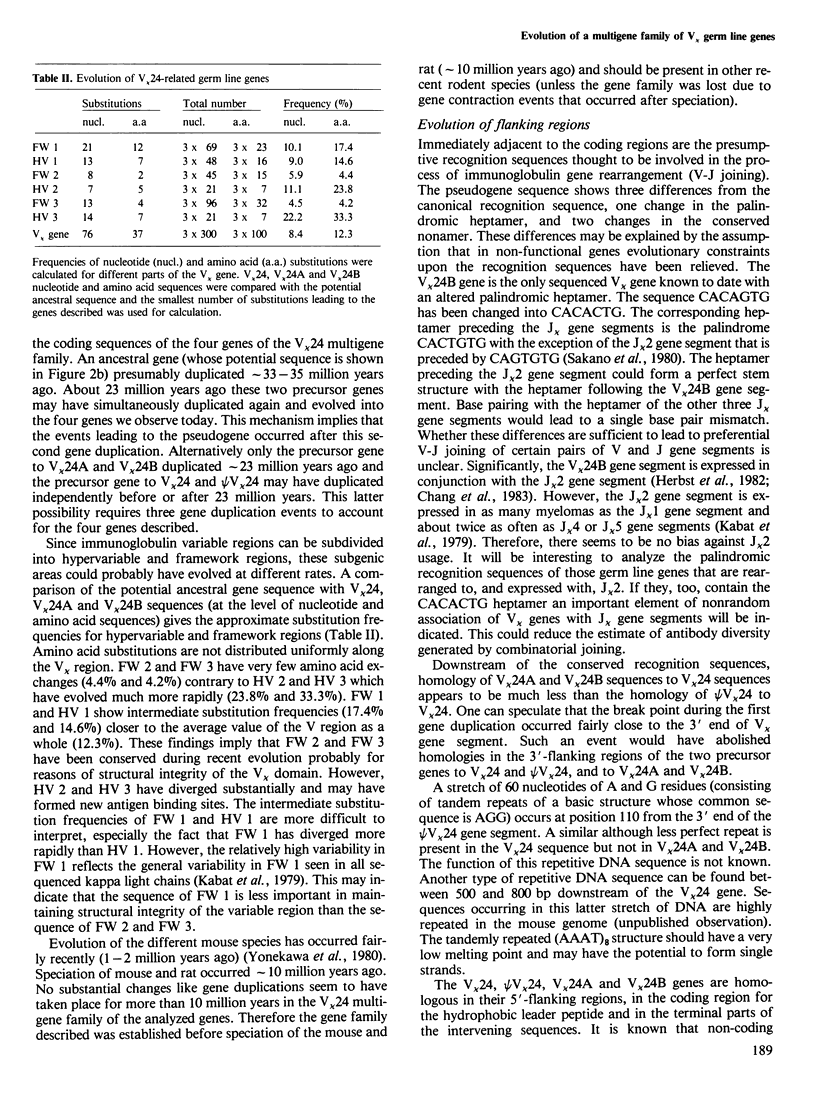
We have isolated a series of related V kappa germ line genes from a BALB/c sperm DNA library. DNA sequence analysis of four members of this V kappa 24 multigene family implies that three V kappa genes are functional whereas the fourth one (psi V kappa 24) is a pseudogene. The prototype gene (V kappa 24) encodes the variable region gene segment expressed in an immune response against phosphorylcholine. The other two functional genes (V kappa 24A and V kappa 24B) may be expressed against streptococcal group A carbohydrate. The time of divergence of the four genes was estimated by the rate of synonymous nucleotide changes. This implies that an ancestral gene has duplicated approximately 33-35 million years ago and a subsequent gene duplication event has occurred approximately 23 million years ago.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altenburger W., Steinmetz M., Zachau H. G. Functional and non-functional joining in immunoglobulin light chain genes of a mouse myeloma. Nature. 1980 Oct 16;287(5783):603–607. doi: 10.1038/287603a0. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Claflin J. L. Uniformity in the clonal repertoire for the immune response to phosphorylcholine in mice. Eur J Immunol. 1976 Oct;6(10):669–674. doi: 10.1002/eji.1830061002. [DOI] [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Calame K., Early P. W., Livant D. L., Joho R., Weissman I. L., Hood L. An immunoglobulin heavy-chain gene is formed by at least two recombinational events. Nature. 1980 Feb 21;283(5749):733–739. doi: 10.1038/283733a0. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Gershenfeld H. K., Tsukamoto A., Weissman I. L., Joho R. Somatic diversification is required to generate the V kappa genes of MOPC 511 and MOPC 167 myeloma proteins. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7674–7678. doi: 10.1073/pnas.78.12.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst H., Chang J. Y., Aebersold R., Braun D. G. Murine VK25 isotype sequence: monoclonal antibody 2S1.3 specific for the group A streptococcal polysaccharide. Hoppe Seylers Z Physiol Chem. 1982 Sep;363(9):1069–1076. doi: 10.1515/bchm2.1982.363.2.1069. [DOI] [PubMed] [Google Scholar]
- Joho R., Nottenburg C., Coffman R. L., Weissman I. L. Immunoglobulin gene rearrangement and expression during lymphocyte development. Curr Top Dev Biol. 1983;18:15–58. doi: 10.1016/s0070-2153(08)60578-5. [DOI] [PubMed] [Google Scholar]
- Joho R., Weissman I. L., Early P., Cole J., Hood L. Organization of kappa light chain genes in germ-line and somatic tissue. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1106–1110. doi: 10.1073/pnas.77.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabat E. A. The structural basis of antibody complementarity. Adv Protein Chem. 1978;32:1–75. [PubMed] [Google Scholar]
- Kimura M., Ota T. On the stochastic model for estimation of mutational distance between homologous proteins. J Mol Evol. 1972 Dec 29;2(1):87–90. doi: 10.1007/BF01653945. [DOI] [PubMed] [Google Scholar]
- Kwan S. P., Max E. E., Seidman J. G., Leder P., Scharff M. D. Two kappa immunoglobulin genes are expressed in the myeloma S107. Cell. 1981 Oct;26(1 Pt 1):57–66. doi: 10.1016/0092-8674(81)90033-7. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Miller H., Leder P. Variation in the crossover point of kappa immunoglobulin gene V-J recombination: evidence from a cryptic gene. Cell. 1980 Oct;21(3):793–799. doi: 10.1016/0092-8674(80)90442-0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T. Molecular evolution of mRNA: a method for estimating evolutionary rates of synonymous and amino acid substitutions from homologous nucleotide sequences and its application. J Mol Evol. 1980 Sep;16(1):23–36. doi: 10.1007/BF01732067. [DOI] [PubMed] [Google Scholar]
- Miyata T., Yasunaga T., Nishida T. Nucleotide sequence divergence and functional constraint in mRNA evolution. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7328–7332. doi: 10.1073/pnas.77.12.7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Varmus H. E. Form and function of retroviral proviruses. Science. 1982 May 21;216(4548):812–820. doi: 10.1126/science.6177038. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]



