Abstract
Tyrosine kinase inhibitors that target pro-angiogenic pathways improve progression-free and overall survival in patients with metastatic kidney cancer and were thus tested in the adjuvant setting in studies published this past year. 2016 also saw the emergence of new inhibitors of pro-angiogenic pathways that might represent the next step in kidney cancer therapy.
Kidney cancer has long been understood to be a disease of altered hypoxia signalling. Indeed, loss of Von Hippel–Lindau (VHL) —the most common genetic alteration in clear cell renal cell carcinoma (ccRCC) — leads to the accumulation of hypoxia inducible factor (HIF) transcription factors1, which activate crucial genes involved in the hypoxic response including those encoding pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF). Targeting the VEGF pathway has therefore been the molecular rationale for the development of multiple agents to treat renal cell carcinoma (RCC), including sorafenib, sunitinib, pazopanib, axitinib, and bevacizumab. In addition, regulatory approval was obtained in 2016 for lenvatinib2 and cabozantinib3, which target the VEGF receptor (VEGFR) and putative pro-angiogenic resistance mechanisms mediated by growth factors such as fibroblast growth factor receptor (FGFR) and hepatocyte growth factor receptor (cMET) (FIG. 1). In the metastatic setting, these drugs have demonstrated clear clinical benefits including improvements in progression-free survival and overall survival; however, their efficacy in earlier stages of disease remains unclear and they are therefore being tested as adjuvant therapies after surgery in patients who are at high risk of disease recurrence.
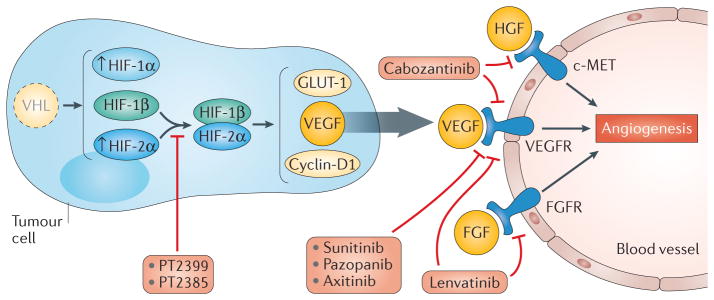
Figure 1. Current and future anti-angiogenic therapies.
Loss of VHL is a common genetic feature in clear cell renal cell carcinoma that leads to the accumulation of hypoxia inducible factor (HIF)-1α and HIF-2α, and expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), G1/S specific cyclin D1 and glucose transporter type 1 (GLUT 1). Current anti-angiogenic tyrosine kinase inhibitors (sunitinib, pazopanib, sorafenib, axitinib, bevacizumab) treat kidney cancer by targeting VEGF-dependent pathways at the tumour–endothelium interface. New agents including lenvatinib and cabozantinib also target other tyrosine kinases (VEGFR receptor (VEGFR), hepatocyte growth factor receptor (c-MET), fibroblast growth factor receptor (FGFR)) thought to be critical for resistance to VEGF-directed therapy. A novel class of agents that inhibit HIF-2α-dependent transcription (PT2399 and PT2385) has been developed. These molecules target more directly key pathways in kidney tumorigenesis, downstream of VHL loss. HGF, hepatocyte growth factor; FGF, fibroblast growth factor.
In 2016, two clinical trials — ASSURE4 and S-TRAC5 — tested the efficacy of VEGFR-directed tyrosine kinase inhibitors (TKIs) as an adjuvant treatment for RCC. The ASSURE phase III trial4 randomly assigned 1,943 patients with completely resected high-grade RCC (defined as Fuhrman grade 3 or grade 4 and at least stage pT1b according to the TMN classification system), to receive placebo, sunitinib (50 mg daily for 4 weeks followed by 2 weeks off), or sorafenib (400 mg twice daily). In the original trial design, patients were administered the typical starting dose for metastatic disease; however, the rates of drug discontinuation were high due to toxicity (44% with sunitinib and 45% with sorafenib). After 1,323 patients were enrolled, the clinical trial was amended and starting doses were reduced to 37.5 mg of sunitinib daily for 4 weeks followed by 2 weeks off, or 400 mg of sorafenib daily. With a median follow-up of 5.8 years, the primary end point of median disease-free survival was not significantly different between the groups (79.6 months with placebo, 73.4 months with sorafenib and 70 months with sunitinib; HR 0.97 for sorafenib versus placebo (97.5% CI 0.80–1.17, P = 0.7184) and HR 1.02 for sunitinib versus placebo (97.5% CI 0.85–1.23, P = 0.8038)). Overall survival at 5 years was also not different between the three groups (80.3% with placebo, 97.5% CI 76.6–84.0%; 80.5% with sorafenib, 97.5% CI 76.8–84.2%; 77.9% with sunitinib, 97.5% CI 74.1–81.9%). This study suggested that in the adjuvant setting, sunitinib and sorafenib were difficult to tolerate and that the use of sunitinib or sorafenib based on an intention-to-treat analysis was not beneficial.
The phase III S-TRAC trial5 randomly assigned 615 patients with completely resected ccRCC, who originally presented with local invasion (tumour stage 3 or greater) or regional lymph node metastasis, to placebo or sunitinib (50 mg daily for 4 weeks on followed by 2 weeks off). Dose reductions and treatment discontinuations in the sunitinib group were 34.3% and 28.1%, respectively, compared to 2% and 5.6% in the placebo group. After a median follow-up of 5.4 years, the primary end point of median disease-free survival on sunitinib was 6.8 years (95% CI 5.8–not reached) compared to 5.6 years (95% CI 3.8–6.6 years) in the placebo group (HR 0.76, 95% CI 0.59–0.98, P = 0.03). At the time of data analysis, overall survival was not significantly different between sunitinib (79.1%) and placebo (79.3%; HR 1.01 95% CI 0.72–1.44, P = 0.94). These findings suggest an improvement in disease-free survival with the use of sunitinib in the adjuvant setting.
On the basis of these conflicting findings regarding disease-free survival outcomes with sunitinib, the role of TKIs as an adjuvant therapy remains controversial. Whether the discordant outcomes are due to different trial populations or to differences in drug discontinuation rates and dosing, remains speculative. In both trials, median overall survival has not yet been reached, and additional follow-up will be helpful to see if TKIs have an effect on overall survival in the S-TRAC trial. The results of other adjuvant trials with axitinib and pazopanib (ATLAS6; PROTECT7) are pending analysis and might shed additional light on the role of TKIs (besides sunitinib) in this setting.
Although strategies that inhibit receptor tyrosine kinases have made substantial advances, direct targeting of HIF has been a long-standing challenge owing to the absence of a known catalytic domain. Loss of VHL can lead to the accumulation of HIF-2α, which dimerizes with aryl hydrocarbon receptor nuclear translocator (ARNT, also known as HIF-1β) and the dimerized complex binds to DNA to induce transcription of HIF-2α target genes. PT2399 is a small molecule that directly binds to HIF-2α and prevents it from dimerizing with ARNT and activating its transcriptional targets8 (FIG. 1). Use of PT2399 led to an >80% reduction in levels of VEGF, G1/S-specific cyclin-D1 (encoded by CCND1), and glucose transporter protein type 1 (GLUT-1) in HIF-2α-dependent RCC cell lines8. PT2399 also inhibited colony formation in soft agar, a measure of carcinogenic potential, and decreased the volume of tumours induced by orthotopically implanted RCC cell lines8. The decreased colony formation and tumour shrinkage seen in response to PT2399 correlated with baseline levels of HIF-2α protein, which suggests that a subset of tumours that depend on HIF2-associated transcription might be particularly sensitive to PT2399.
The efficacy of PT2399 was compared to that of sunitinib in 22 patient-derived xenografts9. This model system recapitulates clinical responses to TKIs such that decreased tumour growth in mice often correlates with tumour shrinkage or disease stabilization in patients. PT2399 decreased tumour growth in xenografts by 60% on average compared to 40% with sunitinib (P <0.0001). Almost half of the xenografts (10 of 22) were sensitive to PT2399 (>80% reduction in tumour volume compared to vehicle control), and these included sarcomatoid and rhabdoid histologic variants and tumours resistant to sunitinib. HIF-2α-dependent transcripts were down-regulated in sensitive xenografts, whereas they were unaffected in resistant xenografts (<40% reduction in tumour volume), despite dissociation of the HIF-2α–ARNT complex. Similar to findings in cell lines, the levels of HIF-2α correlated with the response to PT2399. Resistance to PT2399 was acquired with prolonged exposure to the agent, owing to mutations in either HIF-2α or ARNT. A patient from whom a PT2399-sensitive xenograft was generated was enrolled on a phase I trial10 to assess the efficacy of PT2385, a close analogue of PT2399. This patient had metastatic ccRCC and was heavily pretreated with high doses of IL-2, bevacizumab, sorafenib, everolimus, sunitinib, pazopanib, and axitinib. After 11 months of treatment, he remains on treatment and his disease is stable.
2016 was a year of rapid scientific advancement and improved understanding of the applications of anti-angiogenic therapies. With data from the adjuvant trials, we have begun to explore of the role of systemic TKI treatment for the management of micro-metastatic RCC and we await the results of ongoing and future trials to provide further clarity on the efficacy of TKIs as adjuvant treatment. We might also see the development of a novel class of agents that can target the known genetic defects in RCC more specifically than can current treatments. Further studies will be necessary to determine the efficacy and toxicity profile of these agents.
Key advances.
The ASSURE trial showed no significant differences in disease-free survival for adjuvant sorafenib or sunitinib treatments4
The S-TRAC trial showed improved disease-free survival with adjuvant sunitinib5
Overall survival data for adjuvant clinical trials remains pending4,5
Novel HIF-directed treatments (PT2399 and PT2385) decreased tumour volume in mouse models8,9 and stabilized kidney cancer in one patient10, and are worthy of further investigation
Footnotes
Competing interests statement
R.J.M. consults for Pfizer, Novartis, Eisai and Exelixis, and has received research funds for the institute for work with Pfizer, Genentech/Roche, Novartis, Eisai and Exelixis. C.H.L consults for Exelixis, and has received research funds for the institute for work with Eisai and Pfizer.
References
- 1.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1814–1823. doi: 10.1056/NEJMoa1510016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas NB, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet. 2016;387:2008–2016. doi: 10.1016/S0140-6736(16)00559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravaud A, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med. 2016;375:2246–2254. doi: 10.1056/NEJMoa1611406. [DOI] [PubMed] [Google Scholar]
- 6.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT01599754.
- 7.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT01235962.
- 8.Cho H, et al. On-target efficacy of a HIF2alpha antagonist in preclinical kidney cancer models. Nature. 2016;539:107–111. doi: 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature. 2016;539:112–117. doi: 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US National Library of Medicine. ClinicalTrials.gov. 2016 https://clinicaltrials.gov/ct2/show/NCT02293980.