SECTION 1
An 87-year-old man with diastolic heart failure, coronary artery disease, TIAs, chronic obstructive pulmonary disease (COPD), hypothyroidism, chronic kidney disease, aplastic anemia, and benign prostatic hypertrophy with chronic indwelling catheter was transferred to the neurocritical care unit for acute encephalopathy. The patient initially presented to an outside facility with progressive lethargy evolving over 12 hours from a fully functional baseline. At the outside hospital, he was febrile (103.7°F) and hypotensive (62/40 mm Hg), without leukocytosis (white count 5.6 K/μL) but with urinalysis demonstrating evidence of infection. Empiric IV vancomycin and cefepime were started. CT scan of the chest, abdomen, and pelvis was unrevealing of an infectious source. MRI of the brain was also unremarkable. His mental status declined further over the following 4 days, prompting an EEG that revealed generalized periodic discharges (GPDs). The patient was treated with levetiracetam and transferred to our hospital out of concern for nonconvulsive status epilepticus (NCSE). Upon arrival to the neurocritical care unit, physical examination revealed persistent hypotension (79/44 mm Hg) that responded well to IV fluids without vasopressors and a Glasgow Coma Scale score of 8 with eyes opening to noxious stimulus only. He was mumbling unintelligible words and withdrew all 4 limbs antigravity to pain.
Questions for consideration:
What is the differential diagnosis at this time?
What diagnostic tests or additional history would be the most helpful?
SECTION 2
The differential diagnosis for our patient with subacute encephalopathy and unremarkable MRI included NCSE, sepsis, infectious or autoimmune encephalitis, metabolic derangement, and drug-related (cefepime) toxicity. To investigate these possibilities, video-EEG monitoring was performed, and MRI of the brain and CT of the chest, abdomen, and pelvis were reviewed and were unrevealing. Broad-spectrum antibiotics were continued. Admission laboratory studies revealed thrombocytopenia, anemia, a normal leukocyte count (5.5 K/μL) with 72% neutrophils, 16% lymphocytes, and 9% eosinophils (previously normal at 4% in 2013), baseline creatinine of 2.49 mg/dL, sodium of 138 mEq/L, potassium of 4 mEq/L, glucose of 59 mg/dL, normal lactate, troponin I of 0.06, and brain natriuretic peptide of >10,000 pg/mL. Thyroid-stimulating hormone, thiamine, ammonia, and vitamin B12 were normal. Continuous EEG monitoring demonstrated a poorly organized morphology without evidence of a waking posterior dominant rhythm, a background consisting of bifrontally predominant bilateral theta/delta, and GPDs with triphasic morphology, consistent with a toxic-metabolic encephalopathy (figure 1). Urine cultures grew pan-susceptible Klebsiella oxytoca. Blood cultures were negative. Cefepime was streamlined to ceftriaxone 48 hours after admission (5 days after presenting to the outside facility), and a 10-day course of antibiotics was completed to treat sepsis secondary to urinary tract infection (UTI).
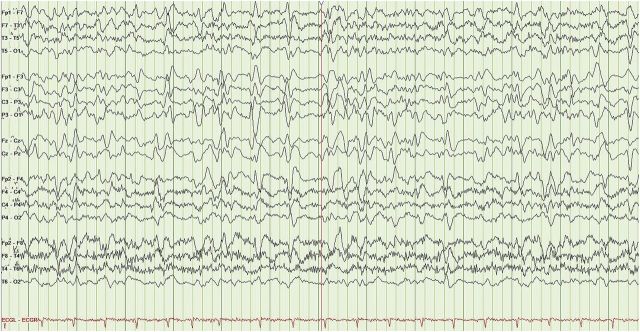
Figure 1. EEG performed prior to stress dose steroids.
(LFF 1 Hz, HFF 30 Hz.) The EEG reveals a poorly organized record with no evidence of a waking posterior dominant rhythm and a background consisting of bilateral theta/delta slowing, with shifting predominance and occasional runs of fluctuating 0.5–1 Hz, bifrontally predominant, generalized periodic discharges with triphasic morphology.
Questions for consideration:
How has the differential diagnosis changed?
What is the next diagnostic study that should be obtained?
SECTION 3
In the absence of focal pathology seen on imaging, unremarkable toxic-metabolic workup, nonfocal EEG, and persistent obtundation in spite of appropriately treated sepsis, lumbar puncture should be considered to rule out CNS infection. Prior to proceeding with this, however, further history revealed that the patient had been hospitalized at least twice yearly for COPD exacerbations, during which he was treated with high-dose prednisone. He had a remote history of chronic daily prednisone use that was discontinued more than 10 years prior to admission, but he continued to use a combination budesonide-formoterol inhaler daily. Morning cortisol was checked and was low at 3.3 μg/dL (normal 6.2–19.4 μg/dL). Standard dose adrenocorticotropic hormone (ACTH) stimulation test resulted in a cortisol level of 9.2 μg/dL at 60 minutes.
Questions for consideration:
What is the final diagnosis?
How would you treat this patient?
The patient's persistent encephalopathy in the setting of appropriately treated sepsis, hypotension, hypoglycemia, peripheral eosinophilia, low morning cortisol with inappropriate response to ACTH stimulation, and EEG semiology consistent with toxic-metabolic encephalopathy (TME) is consistent with the diagnosis of adrenal insufficiency (AI).
AI can be characterized as primary (affecting the adrenal glands), secondary (affecting the pituitary gland), or tertiary (affecting the hypothalamus).1 In our patient, a combined secondary and tertiary AI was most likely caused by chronic exogenous glucocorticoid administration, the most common cause of AI in adults.1 Common clinical features of adrenal crisis include nausea, vomiting, abdominal pain, refractory hypotension, and lethargy, with rare progression to confusion or coma. Most crises are precipitated by infection.1,2 The presentation of our patient, marked by severe encephalopathy with hypotension that responded well to fluid resuscitation, is thus unique. Another characteristic feature of adrenal crisis is the rapid reversal of symptoms in the setting of glucocorticoid administration. While clinical improvement is often demonstrated within 24 hours, full recovery has been reported to take up to 1 week.2 Diagnostic workup includes morning serum cortisol levels and ACTH stimulation testing. Serum cortisol typically peaks between 6:00 and 8:00 am, and a value less than 3 μg/dL suggests AI with a nearly 100% specificity but poor sensitivity (∼50%), while a value greater than 15 μg/dL predicts normal adrenal function.1,3 ACTH stimulation testing should then be performed to distinguish primary from secondary and tertiary AI. Checking ACTH levels is also of value, as elevated levels are seen in primary AI and low levels are seen in secondary and tertiary AI. The EEG findings of TME are nonspecific for AI and are expected to resolve upon treatment (figure 2); however, there are reports of bifrontal predominance of rhythmic high-amplitude sharp and slow wave complexes in the setting of hypocortisolism, as depicted in our patient's EEG.4
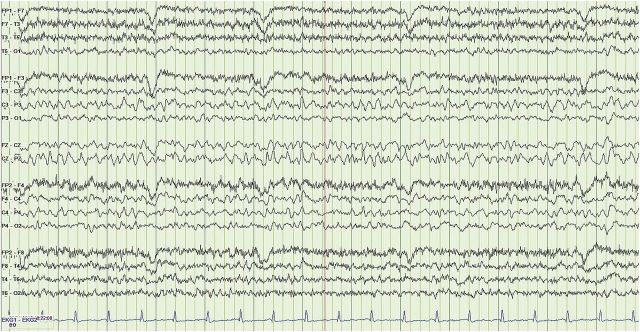
Figure 2. EEG performed after stress dose steroids.
(LFF 1 Hz, HFF 70 Hz.) There is improvement with the emergence of an awake and fairly well-organized background rhythm consisting of a 5- to 7-Hz theta rhythm with frequent superimposed bilateral delta slowing. The generalized periodic discharges have now resolved, though the record still remains abnormal due to the bilateral cerebral slowing.
Treatment for secondary and tertiary AI consists of glucocorticoid administration, while primary AI requires glucocorticoid and mineralocorticoid supplementation. Our patient was given IV hydrocortisone (100 mg/24 hours in divided doses), and improvement in the patient's mental status was noted within 2 hours of administration. By 24 hours, our patient was conversational and following commands. At follow-up 1 month later, the patient was back to baseline, living independently on physiologic doses of prednisone. Outpatient ACTH stimulation confirmed AI, and repeat laboratory studies revealed resolution of his eosinophilia.
DISCUSSION
Our patient's lack of recent oral steroid therapy lowered the clinical suspicion for adrenal crisis; however, the hypothalamus-pituitary-adrenal (HPA) axis can be suppressed by chronic, daily inhaled corticosteroid administration, with inhaled fluticasone being the most commonly implicated glucocorticoid.5,6 A retrospective analysis identified that the greatest risk factor for adrenal crisis was more than 4 years' duration of steroid therapy, as seen in our patient.7 Although he had been without systemic steroid supplementation for 10 years, he had previously been maintained on oral prednisone for 20 years, likely resulting in adrenal atrophy.7
There are little data available documenting the initial presentation of adrenal crisis as obtundation in the setting of fluid-responsive hypotension without immediate steroid supplementation. While our patient did exhibit persistent hypotension at the outside facility, his blood pressure responded well to fluid resuscitation in our critical care unit, thus obscuring the possibility of AI initially. This likely resulted from early antibiotic treatment of the patient's Klebsiella UTI. Adrenal encephalopathy is often described as somnolence and fatigue in the setting of hemodynamic stability, while confusion or coma is most often described in the setting of septic shock.2 Though hyponatremia and hypokalemia can be seen in adrenal crisis, their absence can be falsely reassuring, as these electrolyte abnormalities inconsistently contribute high diagnostic yield.2 In the case of secondary and tertiary AI, this can be explained by the functional preservation of the zona glomerulosa—the synthesizer of mineralocorticoids—as this portion of the adrenal gland is independent of ACTH and is regulated by the renin-angiotensin system.1 This may account for the less dramatic hemodynamic instability seen in these patients as compared to those with primary AI, in which both glucocorticoid and mineralocorticoid deficiencies are present. Peripheral eosinophilia is a frequently overlooked finding that can be helpful in diagnosing AI. A total of 20% to 25% of critical care patients with a relative peripheral eosinophilia (>3%) have an abnormal ACTH stimulation test, 70%–100% of whom are diagnosed with AI.8,9
While cefepime encephalopathy was considered, as it often manifests in patients with renal insufficiency, the timing is not consistent with this diagnosis. Cefepime encephalopathy typically occurs between days 3 and 5 of administration, and our patient's mental status was impaired prior to antibiotic administration. Moreover, cefepime toxicity tends to arise at a much higher doses (>4 g/d) than our patient received (1 g q24 h).10 Most notably, his mental status improved within 2 hours of hydrocortisone supplementation, arguing in favor of AI.
This case highlights an unusual presentation of AI and the difficulties inherent in pursuing this diagnosis in a patient with multiple comorbidities. The diagnosis was not initially evident given the absence of classic findings and a remote history of oral steroid use with subsequent maintenance on only a daily inhaled corticosteroid. Important clues suggesting adrenalopathy were that of persistent encephalopathy in spite of sepsis treatment, mild hypoglycemia, toxic-metabolic EEG semiology, and peripheral eosinophilia. This case demonstrates that adrenal crisis should be considered in critically ill patients with persistent encephalopathy in spite of standard medical intervention, as delay in treatment can be fatal.
AUTHOR CONTRIBUTIONS
Dr. Spera: case report data review, analysis, and interpretation. Drs. Rubin, Gupta, Fantaneanu, and Henderson: critical revision of the manuscript for important intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Charmandari E, Nicolaides NC, Chrousos GP. Adrenal insufficiency. Lancet 2014;383:2152–2167. [DOI] [PubMed] [Google Scholar]
- 2.Allolio B. Extensive expertise in endocrinology: adrenal crisis. Eur J Endocrinol 2015;172:R115–R124. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt IL, Lahner H, Mann K, Petersenn S. Diagnosis of adrenal insufficiency: evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. J Clin Endocrinol Metab 2003;88:4193–4198. [DOI] [PubMed] [Google Scholar]
- 4.Faigle R, Sutter R, Kaplan P. Electroencephalography of encephalopathy in patients with endocrine and metabolic disorders. J Clin Neurophysiol 2013;30:505–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todd GRG, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of adrenal crisis associated with inhaled corticosteroids in the United Kingdom. Arch Dis Child 2002;87:457–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dene C, Vogl-Voswinckel AE, Gruebl A, Burdach S. Adrenal Crisis caused by inhaled fluticasone in an adolescent with cystic fibrosis and advanced hepatopathy: a case report. Case Rep Pulmonol 2012;2012:913574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omori K, Nomura K, Shimizu S, Omori N, Takano K. Risk factors for adrenal crisis in patients with adrenal insufficiency. Endocr J 2003;50:745–752. [DOI] [PubMed] [Google Scholar]
- 8.Angelis M, Yu M, Takanishi D, Hasaniya NW, Brown MR. Eosinophilia as a marker of adrenal insufficiency in the surgical intensive care unit. J Am Coll Surg 1996;183:589–596. [PubMed] [Google Scholar]
- 9.Beishuizen A, Vermes I, Hylkema BS, Haanen C. Relative eosinophilia and functional adrenal insufficiency in critically ill patients. Lancet 1999;353:1675–1676. [DOI] [PubMed] [Google Scholar]
- 10.Sonck J, Laureys G, Verbeelen D. The neurotoxicity and safety of treatment with cefepime in patients with renal failure. Nephrol Dial Transplant 2008;23:966–970. [DOI] [PubMed] [Google Scholar]