Abstract
The brain is the central organ of stress and adaptation to stress because it perceives and determines what is threatening, as well as the behavioral and physiological responses to the stressor, which promote adaptation (“allostasis”) but also contribute to pathophysiology (“allostatic load/overload”) when overused and dysregulated. The adult as well as developing brain possesses a remarkable ability to show structural and functional plasticity in response to stressful and other experiences, including neuronal replacement, dendritic remodeling and synapse turnover. Stress can cause an imbalance of neural circuitry subserving cognition, decision making, anxiety and mood that can increase or decrease expression of those behaviors and behavioral states. This imbalance, in turn, affects systemic physiology via neuroendocrine, autonomic, immune and metabolic mediators. In the short term, these changes may be adaptive; but, if the threat passes and the behavioral state persists along with the changes in neural circuitry, such maladaptation requires intervention with a combination of pharmacological and behavioral therapies. There are important sex differences in how the brain responds to stressors. Moreover, adverse early life experience, interacting with alleles of certain genes, produces lasting effects on brain and body via epigenetic mechanisms. While prevention is key, the plasticity of the brain gives hope for therapies that utilize brain–body interactions. Policies of government and the private sector are important to promote health and increase “healthspan.”
Keywords: hippocampus, amygdala, prefrontal cortex, glucocorticoids, glutamate, epigenetics, lifecourse, adverse childhood experiences, sex differences, policy
Introduction
We use the word “stress” frequently in everyday discourse, and yet the meaning is ambiguous, since the word refers to many experiences in life that are sometimes beneficial or other times negative and even traumatic, but often reflect the daily “grind” of our lives as in feeling “stressed out.” The common definition of “stress” focuses on acute challenges as in the fight-or-flight response and generally mentions mediators of only two of the interacting systems that are involved, namely, adrenalin and cortisol. And it does not mention other mediators or the role of the brain nor does it make note of the health-damaging “health” behaviors that may result from the circumstances of a person's life.
This review has several goals: first, to include the many body systems affected by “stress,” particularly, the brain, and the multiple mediators involved and to do so in a broader discussion, centering around the concepts of allostasis and allostatic load and overload; second to discuss key mechanisms, namely, structural plasticity and remodeling of brain architecture and the role of glucocorticoids and excitatory amino acids along with other mediators; third, to put this in the context of events over the entire life course and the role of “epigenetics” which provides a basis for how the social and physical environment influences the life trajectory toward physical and mental health or disease and how interventions may redirect those trajectories in a more positive direction.
What Do We Mean by “Stress?”
What do we mean when we use the word “stress? One way of classifying it is as “good stress, tolerable stress or toxic stress” (see http://developingchild.harvard.edu/library/reports_and_working_papers/policy_framework/).
“Good stress” refers to the experience of rising to a challenge, taking a risk and feeling rewarded by an often positive outcome. A related term is “eustress.” Good self-esteem and good impulse control and decision making capability, all functions of a healthy architecture of the brain, are important! Even adverse outcomes can be “growth experiences” for individuals with such positive, adaptive characteristics that promote resilience in the face of adversity.
“Tolerable stress” refers to those situations where bad things happen, but the individual with healthy brain architecture is able to cope, often with the support of family, friends and other individuals. These adverse outcomes can be “growth experiences” for individuals with such positive, adaptive characteristics and support systems that promote resilience. Here, “distress” refers to the uncomfortable feeling related to the nature of the stressor and the degree to which the individual feels a lack of ability to influence or control the stressor.1–3
Finally, “toxic stress” refers to the situation in which bad things happen to an individual who has limited support and who may also have brain architecture that reflects effects of adverse early life events that have impaired the development of good impulse control and judgment and adequate self esteem. Here, the degree and/or duration of “distress” may be greater. With toxic stress, the inability to cope is likely to have adverse effects on behavior and physiology, and this will result in a higher degree of allostatic overload, as will now be explained.
Definition of Allostasis and Allostatic Load
In a changing social and physical environment, the brain and body respond physiologically and behaviorally in order to adapt. Physiologically, the sympathetic and parasympathetic systems, hypothalamic–pituitary–adrenal (HPA) axis, immune system and metabolic hormones and molecular processes within all organs, including the brain, operate non-linearly and promote adaptation via “allostasis” (achieving stability via activation of these systems). But the same mediators have biphasic effects and can also promote pathophysiology when overused or when their activity is out of balance with each other (allostatic load or overload). Adaptation and protection via allostasis and wear-and-tear on the body and brain via allostatic load/overload are the two contrasting sides of the physiology involved in responses of the individual during the challenges of daily life.
A good example of the biphasic actions of stress, i.e., “protection vs. damage,” is in the immune system, in which an acute stressor activates an acquired immune response via mediation by catecholamines and glucocorticoids and locally produced immune mediators; and, yet, a chronic exposure to the same stressor over several weeks has the opposite effect and results in immune suppression.4,5 Acute stress-induced immune enhancement is good for enhancing immunization, fighting an infection or repairing a wound, but it is deleterious to health for an autoimmune condition such as psoriasis or Krohn's disease. On the other hand, immune suppression is good in the case of an autoimmune disorder and deleterious for fighting an infection or repairing a wound. In an immune sensitive skin cancer, acute stress is effective in inhibiting tumor progression while chronic stress exacerbates progression.6,7
Health-Promoting and Health Damaging Behaviors
The three-part terminology for “stress” described above is helpful because it recognizes that the sense of control and mindset8 determines whether or not the response to experiences can have a successful outcome or may lead to allostatic overload, but the terminology ignores health damaging versus health promoting behaviors that people adopt in a stressful lifestyle, as well as factors like circadian disruption, loneliness, noise, pollution, lack of green space and crowding. Indeed, the most common stressors are ones that operate chronically, often at a low level, and that cause us to behave in certain ways. For example, being “stressed out” may cause us to be anxious and or depressed, to lose sleep at night, to eat comfort foods and take in more calories than our bodies need, and to smoke or drink alcohol excessively. Being “stressed out” may also cause us to neglect seeing friends, or to take time off from our work, or reduce our engagement in regular physical activity as we, for example, sit at a computer and try to get out from under the burden of too much to do. Often, we are tempted to take medications—anxiolytics, sleep promoting agents—to help us cope, and, with time, our bodies may increase in weight and develop other symptoms of an unhealthy lifestyle. In all of this, our brains play a central role.
Central Role of the Brain
The brain is the organ that determines what is novel and possibly threatening and therefore “stressful” and it orchestrates the behavioral and physiological responses, whether health promoting or health damaging. And the brain is a biological organ that changes in its architecture and its molecular profile and its neurochemistry under acute and chronic stress and directs many systems of the body—metabolic, cardiovascular and immune—that are involved in the short- and long-term consequences of being “stressed out” and the consequent health-damaging behaviors.
The neural circuits in a healthy brain are remodeled by experiences to enable behavioral responses that are appropriate to what the individual is experiencing, e.g., being more vigilant and anxious in a potentially dangerous environment.9 The healthy brain is resilient and neural circuitry adapts to a new situation along with underlying changes in gene expression.10 The unhealthy brain may not be so plastic, or it may have maladaptive circuitry or plasticity and, as a result, is less able to adapt appropriately or likely to “get stuck.” In these cases, there need be external intervention involving pharmacological agents and behavioral modification. With persistence of this condition, involving excessive activation of excitatory amino acids, potentiated by glucocorticoids, irreversible damage occurs; this is postulated to be a key step in the irreversible activation of the cascade leading to Alzheimer's disease involving inactivation of the adaptive insulin receptor mechanism.11 In contrast, normal brain aging involves potentially reversible loss of resilience, which, for example, can be counteracted by regular physical activity.12 In order to appreciate this, we now consider the diverse role of adrenal steroids and excitatory amino acids working in concert with other mediators.
Adrenal Steroid Receptors in Hippocampus
With the discovery of glucocorticoid and mineralocorticoid receptors in the hippocampal formation,13,14 a brain region involved in episodic and spatial memory and mood regulation, the hippocampus became the gateway to understanding how systemic hormones affect higher brain functions. In the hippocampus, stress and glucocorticoids were first shown to cause dendritic shrinkage and loss of spines.15 The rediscovery of neurogenesis in the dentate gyrus16 galvanized the widespread interest in the functional role of neuronal replacement in the adult brain. It was in the hippocampus that the role of excitatory amino acids in stress effects was first recognized.17 Effects of acute and chronic stress on the amygdala differ from those in the hippocampus. Acute traumatic stressors were found to cause increased spine density on basolateral amygdale (BlA) neurons and chronic stress leads to the expansion of BlA dendrites.18 Yet, the medial amygdala shows a chronic stress-induced loss of spines19 and shrinkage of dendrites.20 These alterations are implicated in increased anxiety and in posttraumatic stress disorder (PTSD)-like behaviors18,21 as well as social avoidance as in social defeat.20,22 Within the prefrontal cortex (PFC), chronic stress causes medial PFC (mPFC) neurons to show debranching and shrinkage of dendrites, whereas orbitofrontal cortical neurons expand dendrites that may be related to increased vigilance, and dendritic shrinkage is associated with cognitive rigidity.23,24 The PFC under stress has provided important clues to age-related loss of resilience and impaired memory as well as effects of circadian disruption and extinction of fear memory.9 These brain regions have contributed to our knowledge of cellular and molecular mechanisms and cellular processes that are now described, revealing the complex interactions among mediators, brain region specializations as well as common mediators and mechanisms.
Glucocorticoids produce effects in the brain both genomically and non-genomically via multiple sites and pathways, and they have biphasic effects in which the timing and the level of glucocorticoid response (GR) expression are critical.25,26 Glucocorticoid actions via the genome involve both direct interactions with GR elements and indirect actions via tethering to other transcription factors.27 Glucocorticoids also directly stimulate release of excitatory amino acids via membrane-associated receptors and they indirectly regulate both glutamate and GABA release via their ability to induce local synthesis of endocannabinoids.28 Glucocorticoids also translocate GR to mitochondria where they promote Ca++ sequestration and regulate mitochondrial gene expression; these effects are biphasic and high glucocorticoid levels cause a failure of this mechanism and lead to increase free-radical formation.29
The level of expression of glucocorticoid receptors is very important. Genetically induced overexpression of GR in forebrain leads to increased ability of mood-related behaviors and yet also confers greater responsiveness to antidepressant drugs.30 Genetically induced underexpression of GR has the opposite effect.31 Likewise the increased CpG methylation within the GR promotor is associated with a sluggish HPA stress response and is associated with poor maternal care in rodents and early life abuse in human suicide victims.32,33
Glucocorticoid actions are biphasic, as illustrated above for mitochondria,29 and timing is important. For example, in several animal models of traumatic stress-induced PTSD-like delayed anxiety and (in one model) traumatic stress induced spine synapse formation in BlA, a timed elevation of glucocorticoids prevents these effect.21 Data on human PTSD support a protective role for adequate glucocorticoid levels at time of trauma.34,35 Yet, repeated high dose glucocorticoids treatment mimics chronic stress and induces dendritic lengthening in BlA.36
In contrast, the natural ultradian fluctuations of glucocorticoids mediate turnover of a subset of synapses in cerebral cortex and inhibiting the fluctuations with a tiny dose of dexamethasone impairs spine turnover.37 Moreover, circadian changes in spine formation and removal are important for motor learning.38 Finally, glucocorticoids are able to program some of the cellular circadian clocks in brain39 as well as in liver40 leading to dissonance between brain regions and also contributing to obesity and metabolic syndrome that is produced by chronic glucocorticoid administration.41
Key Role of Excitatory Amino Acids
Excitatory amino acids, particularly glutamate, play a key role in structural as well as functional changes in the brain since glutamate is the major excitatory transmitter, while, at the same time, excess glutamate causes damage and inflammation.17 Initial studies of restraint stress which, when chronic, causes shrinkage of apical dendrites of hippocampal CA3 neurons,15 showed that acute restraint stress elevates extracellular glutamate levels via a process that is blocked in adrenalectomized animals, implicating the adrenal cortex.42 Indeed, corticosterone acts directly via membrane associated mineralocorticoid receptors (MR) and GR to cause glutamate release.26,43,44 Blocking N-methyl-D-aspartate (NMDA) receptors and interfering with excitatory stimulation of ion channels blocks stress-induced dendritic remodeling, as also does blockade of adrenal steroid synthesis.45,46 A stress-induced NMDA-dependent dendritic remodeling has been reported in mPFC neurons.47 It is important to note that many factors are involved in dendritic remodeling including cytoskeletal depolymerization48 and at least one cell nuclear pore protein49 that suggests that gene expression may be involved in maintaining the dendritic tree. Excess glutamatergic activity, without adequate reuptake in the aftermath of trauma from seizures, ischemia and head trauma, leads to permanent neuronal loss by a process that is exacerbated by glucorticoids.50 These relationships can be summarized in an inverted U-shaped dose and time response curve.
An unregulated overflow of glutamate appears to play a role in depressive-like behavior in animal models in which shrinkage of dendrites in the hippocampus and suppression of neurogenesis occurs that can be prevented by upregulation of the metabotrophic glutamate receptor, mGlu2, by agents such as acetyl-L-carnitine as well as histone deacetylase inhibitors, which act in a few days, and by selective serotonin re-uptake inhibitors which act more slowly.51,52 Chronic stress causes dendritic shrinkage not only in CA3 and dentate gyrus neurons of hippocampus but also in medial amygdala and mPFC, while dendrites in BlA and orbitofrontal cortex expand with chronic stress.9,17,18,20,24,53
Unregulated glutamate overflow is also implicated in aging and dementia. During aging in the rat, treatment with riluzole, which is known to retard glutamate release and promote glutamate reuptake by astrocytes, retards aging in the hippocampus as measured by preservation of spatial memory and thin spines that are found in young hippocampal neurons.54 Moreover, assessing gene expression changes associated with aging in rodents using RNA-sequencing (RNA-seq), riluzole prevented many of the hippocampal age-related gene expression changes. Moreover, a comparison of the effects of riluzole in rats against human AD data sets revealed that many of the gene changes in Alzheimer's Disease (AD) are reversed by riluzole.55
Insulin Resistance in the Brain and Excitatory Amino Acids
The brain is, indeed, a major target of insulin as well as other metabolic hormones.56 In middle-aged adults, insulin resistance is associated with disrupted memory and executive function, and corresponding metabolic decline in the mPFC, reductions in hippocampal volumes and aberrant intrinsic connectivity between the hippocampus and mPFC.57–59 These findings are supported by recent work in animal models, in which antisense inactivation of the insulin receptor in hippocampus leads to cognitive impairment without systemic consequences,60 whereas antisense inactivation of the hypothalamic insulin receptor creates systemic insulin resistance and dyslipidemia and also insulin resistance in the hippocampus along with depressive-like behavior and cognitive impairment.61 Remarkably, these changes induced in hypothalamus are reversed by dietary restriction62,63 indicating that the brain can be resilient.
Yet, there is at some point, a “switch” that triggers irreversible changes that lead toward amyloid beta (Abeta) toxicity and dementia.11 Before this switch is triggered, synaptic NMDA receptor activation normally has an antioxidant role by suppressing FOXO1 transcription factor in hippocampus, but abnormal and excessive NMDA activation in the insulin resistant state appears to enable FOXO1 translocation to the cell nucleus leading to the generation of reactive oxygen species and possibly also activation of stress kinases, which further impairs insulin signaling.11 Moreover, Abeta production is accelerated and Abeta oligomers enter into a vicious cycle leading to further damage,11 and mitochondrial function declines under these conditions and contributes to the positive feedback cycle of toxicity.64
With possible therapeutic potential, glucagon-like peptide (GLP-1) has insulinotrophic actions and promotes weight loss and has been shown to exert neuroprotective and anti-apoptotic effects, to reduce Abeta plaque accumulation, to modulate long-term potentiation and synaptic plasticity, and to promote differentiation of neuronal progenitor cells.65,66 Behaviorally, in animal models, treatment with GLP-1 receptor agonists improve learning and memory, as well as reduce depressive-like behaviors.66 Another potential intervention with a natural molecule, based on animal models, is acetyl-L-carnitine (LAC) which has not only rapid anti-depresssant-like effects, as noted above, but also has metabolic functions that rapidly reverses hyperinsulinemia and hyperglycemia in the FS, rat which is deficient in LAC.52,67
Life course and the Epigenetics of Individual Differences
Gene-environment interactions are key to how the brain develops and changes with experience, and “epigenetics” now refers to the important role of the social and physical environment in shaping the brain and body over the lifecourse.68 Mechanistically, “epigenetics” refers to events “above the genome” that regulate expression of genetic information without altering the DNA sequence. Besides the CpG methylation described above, other mechanisms include histone modifications that repress or activate chromatin unfolding69 and the actions of non-coding RNA's,70 as well as transposons and retrotransposons71 and RNA editing.72 Much of what is described earlier in this review involves epigenetic mechanisms at a cellular and molecular level. Now we turn to a more integrative view of epigenetics in the animal and human world that lead to trajectories of experience-dependent adaptation or maladaptation, which then will lead to a discussion of possible interventions.
The individual traits that allow these adaptive or maladaptive outcomes depend upon the unique neurological capacity of each individual, which is built upon experiences in the life course, particularly those early in life.68 These influences can result in healthy or unhealthy brain architecture and in epigenetic regulation that either promotes or fails to promote gene expression responses to new challenges. Genetically similar or identical individuals differ in many ways ranging from length of dendrites in the prefrontal cortex73 to differences in MR levels in hippocampus,74 locomotor activity and neurogenesis rates,75 and the influences that lead to those differences begin early in life. For example, identical twins diverge over the life course in patterns of CpG methylation of their DNA reflecting the influence of “non-shared” experiences.76
Early-life events related to maternal care in animals, as well as parental care in humans, play a powerful role in later mental and physical health, as demonstrated by the adverse childhood experiences (ACE) studies,77 and recent work that will be noted below. Animal models have contributed enormously to our understanding of how the brain and body are affected, starting with the “neonatal handling” studies of Levine and Denenberg78 and the more recent, elegant work of Meaney, Syzf and colleagues79 involving methylation of CpG residues in DNA. Such epigenetic, transgenerational effects transmitted by maternal care are central to these findings and may underlie the finding that an anxiety-like phenotype detected in adolescence predicts not only a consistent anxiety phenotype but also a shorter lifespan.80,81
Besides the amount of maternal care, the consistency over time of that care and the exposure to novelty are also very important not only in rodents82,83 but also in monkey models.84 Prenatal stress impairs hippocampal development in rats, as does stress in adolescence.85 Insufficient maternal care in rodents (e.g., Rice et al.86) and the surprising attachment shown by infant rats to their less-attentive mothers appears to involve an immature amygdale,87 activation of which by glucocorticoids causes an aversive conditioning response to emerge. Maternal anxiety in the variable foraging demand model in rhesus monkeys leads to chronic anxiety in the offspring, as well as signs of metabolic syndrome.88,89
Besides the important role of the social and physical environment and experiences of individuals in the health outcomes, genetic factors also play an important role. Different alleles of commonly occurring genes determine how individuals will respond to experiences. For example, the short form of the serotonin transporter is associated with a number of conditions such as alcoholism, and individuals who have this allele are more vulnerable to respond to stressful experiences by developing depressive illness.90,91 In childhood, individuals with an allele of the monoamine oxidase A gene are more vulnerable to abuse in childhood and more likely themselves to become abusers and to show antisocial behaviors compared to individuals with another commonly occurring allele.92 Nevertheless, in a positive, nurturing environment, as formulated by Suomi and by Tom Boyce and colleagues,93–95 these same alleles may lead to successful outcomes, which has led them to be called “reactive or context-sensitive alleles” rather than “bad genes.”
Sex Differences in the Brain
Female rodents do not show the same pattern of neural remodeling after chronic stress as do males. The first realization of this was for the hippocampus, in which the remodeling of CA3 dendrites did not occur in females after Chronic Restraint Stress (CRS), even though all the measures of stress hormones indicated that the females were experiencing that aspect of stress as much as males.96 Females and males also differ in the cognitive consequences of repeated stress, with males showing impairment of hippocampal dependent memory, whereas females do not.97–99 In contrast, acute tail shock stress during classical eyeblink conditioning improves performance in males, but suppresses it in females100 by mechanisms influenced by gonadal hormones in development and in adult life.101,102 However, giving male and female rats control over the shock abolishes both the stress effects and the sex differences.103 These findings suggest that sex differences involve brain systems that mediate how males and females interpret stressful stimuli and that a sense of control is paramount to coping with those stimuli.
Female rats fail to show the mPFC dendritic remodeling seen in males after CRS in those neurons that do not project to amygdala. Instead, they show an expansion of the dendritic tree in the subset of neurons that project to the basolateral amygala.104 Moreover, ovariectomy prevented these CRS effects on dendritic length and branching. Furthermore, estradiol treatment of ovariectomized (OVX) females increased spine density in mPFC neurons, irrespective of where they were projecting.104
Taken together with the fact that estrogen, as well as androgen, effects are widespread in the central nervous system, these findings indicate that there are likely to be many more examples of sex × stress interactions related to many brain regions and multiple functions, as well as developmentally programmed sex differences that affect how the brain responds to stress, e.g., in the locus ceruleus.105,106 Clearly, the impact of sex and sex differences has undergone a revolution and much more is to come,107–111 including insights into X and Y chromosome contributions to brain sex differences.112 In men and women, neural activation patterns to the same tasks are quite different between the sexes even when performance is similar.113 This leads to the concept that men and women often use different strategies to approach and deal with issues in their daily lives, in part because of the subtle differences in brain architecture. Nevertheless, from the standpoint of gene expression and epigenetic effects, the principles of what we have learned in animal models regarding plasticity, damage and resilience, are likely to apply to both males and females.
When Things Go Wrong Over the Life Course
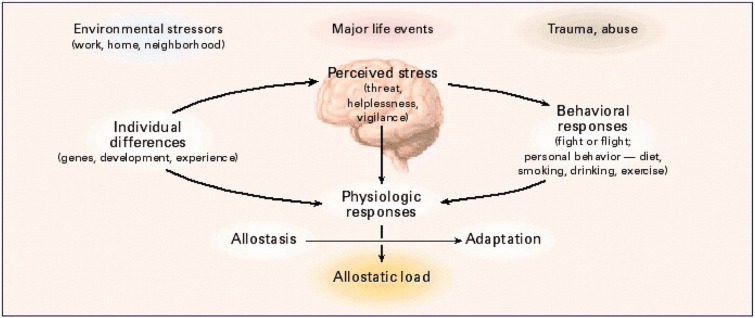
ACE have a disproportionately powerful effect on life long trajectories of health and disease,68 and poverty contributes in its own way as well as creating circumstances for ACE,114 which, however, occurs across all levels of socioeconomic status (SES).77 In studies of ACE,77 there are reports of increased inflammatory tone, not only in children but also in young adults related to early life abuse, that includes chronic harsh language, as well as physical and sexual abuse115,116 (see Figure 1).
Figure 1.
Central role of the brain in allostasis and the behavioral and physiological response to stressors. With permission from McEwen.117
It should be noted that the ACE study was carried out in a middle class population,118 indicating that poverty and low SES are not the only source of early life stressors. Nevertheless, low SES does increase the likelihood of stressors in the home and neighborhood, including also exposure to toxic chemical agents such as lead and air pollution,119 and chaos in the home is associated with development of poor self-regulatory behaviors, as well as obesity.120 Moreover, low SES children are found to be more likely to be deficient in language skills, as well as self-regulatory behaviors and also in certain types of memory that are likely to be reflections of impaired development of parasylvian gyrus language centers, prefrontal cortical systems and temporal lobe memory systems.121,122 Low SES is reported to correlate with smaller hippocampal volumes,123 and lower subjective SES, an important index of objective SES, is associated with reduction in prefrontal cortical gray matter.124 Moreover, having grown up in lower SES environment is accompanied by greater amygdala reactivity to angry and sad faces,125 which, as noted above, may be a predisposing factor for early cardiovascular disease that is known to be more prevalent at lower SES levels.126 Finally, depression is often associated with low SES, and children of depressed mothers, followed longitudinally, have shown increased amygdala volume while hippocampal volume was not affected.127
On the positive side, there are the “reactive alleles.” Genes that in nurturing environments facilitate beneficial outcomes when compared to less reactive alleles, even though those same alleles can enhance adverse outcomes in a stressful early life environment.90,93–95 Regarding adverse outcomes and good and bad “environments,” it must be recognized that allostatic processes are adjusted via epigenetic influences to optimize the individuals adaptation to, and resulting fitness for, a particular environment, whether more or less threatening or nurturing.128 Yet, there are “trade-offs” in terms of physical and mental health that, on one hand, may increase the likelihood of passing on one's genes by improving coping with adversity and enhancing mental health and overall reproductive success, but, on the other hand, may impair later health, e.g., by eating of “comfort foods” (e.g., Jackson et al.129).
Relevance to the RDoc Framework
Six of the units of analysis of the RDoc framework (genes, molecules, cells circuits, physiology and behavior; https://www.nimh.nih.gov/research-priorities/rdoc/units/index.shtml) are represented in this review, which focuses on how experiences over the life course can alter the circuitry that underlies the RDoc domains (negative and positive valence systems, cognitive systems, social processes and arousal and regulatory systems) and influence their function and the balance among them as they affect physiology and behavior. One deficiency of the RDoc framework, as presently formulated, is that it does not fully recognize the continuous reciprocal interactions between hormonal, metabolic and immune activity and the RDoc domains in the brain at the level of the units of analysis, particularly circuitry via cellular and molecular mechanisms. Systems biology and brain–body interactions should in the future be given a greater emphasis in RDoc, given the concepts of allostasis and allostatic load/overload and their implications for the multimorbidity of mood disorders with systemic disorders.130 Type 2 diabetes and its now recognized relationship to depression and dementia131 is an important example.
Conclusion: So What Can Be Done About Being “Stressed Out?”
The social and physical environments “get under the skin” and shape the brain and body. Adverse experiences and environments cause problems over the life course in which there is no such thing as “reversibility” (i.e., “rolling the clock back”) but rather a change in trajectory10 in keeping with the original definition of epigenetics132 as the emergence of characteristics not previously evident or even predictable from an earlier developmental stage. By the same token, we mean “redirection” instead of “reversibility”—in that changes in the social and physical environment on both a societal and a personal level can alter a negative trajectory in a more positive direction.68 The challenge is how to do this!
The Acheson report in the United Kingdom pointed out that virtually all policies of government (as well as the private sector) are ultimately health policies—whether it is housing, education, taxation, environmental health, health care or others.133 From the standpoint of policy, a major goal should be to create incentives at home and in work situations and in building community services that encourage individuals to learn tools that help them develop beneficial individual lifestyle practices. Education is essential as is providing some sense of economic security via a social safety net.
From the standpoint of the individual, a major goal should be to try to improve sleep quality and quantity, improve social support and promote a positive outlook on life, maintain a healthy diet, avoid smoking and have regular moderate physical activity. Concerning physical activity, it is not necessary to become an extreme athlete, and moderate physical activity has benefits for the brain and the body.
In order to change trajectories of mental and physical health, it is important to focus upon the use of targeted behavioral therapies along with treatments, including pharmaceutical agents, that “open up windows of plasticity” in the brain and facilitate the efficacy of the behavioral interventions.134 Indeed, a major challenge throughout the life course is to find ways of redirecting future behavior and physiology in more positive and healthy directions.68 This is more easily said than done, and it represents an important challenge for the future to increase “healthspan” and promote full enjoyment of life and also to reduce the financial burden of disease and disability on the individual and on society.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Supported by NIH grants MH41256, MH 58911
References
- 1.Lazarus RS, Folkman S. Stress, appraisal and coping, New York: Springer Verlag, 1984. [Google Scholar]
- 2.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci 2010; 1186: 125–145. [DOI] [PubMed] [Google Scholar]
- 3.Theall KP, Brett ZH, Shirtcliff EA, et al. Neighborhood disorder and telomeres: connecting children's exposure to community level stress and cellular response. Soc Sci Med 2013; 85: 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009; 16: 300–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhabhar FS, Malarkey WB, Neri E, et al. Stress-induced redistribution of immune cells – from barracks to boulevards to battlefields: a tale of three hormones – Curt Richter Award winner. Psychoneuroendocrinology 2012; 37: 1345–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dhabhar FS, Saul AN, Daugherty C, et al. Short-term stress enhances cellular immunity and increases early resistance to squamous cell carcinoma. Brain Behav Immun 2010; 24: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saul AN, Oberyszyn TM, Daugherty C, et al. Chronic stress and susceptibility to skin cancer. J Natl Cancer Inst 2005; 97: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crum AJ, Salovey P, Achor S. Rethinking stress: the role of mindsets in determining the stress response. J Pers Soc Psychol 2013; 104: 716–733. [DOI] [PubMed] [Google Scholar]
- 9.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013; 79: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray JD, Rubin TG, Hunter RG, et al. Hippocampal gene expression changes underlying stress sensitization and recovery. Mol Psychiatry 2014; 19: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Felice FG, Lourenco MV, Ferreira ST. How does brain insulin resistance develop in Alzheimer's disease? Alzheimers Dement 2014; 10(1 Suppl): S26–S32. [DOI] [PubMed] [Google Scholar]
- 12.Erickson KI, Voss MW, Prakash RS, et al. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci USA 2011; 108: 3017–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwen BS, Weiss J, Schwartz L. Selective retention of corticosterone by limbic structures in rat brain. Nature 1968; 220: 911–912. [DOI] [PubMed] [Google Scholar]
- 14.Reul JM, DeKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 1985; 117: 2505–2511. [DOI] [PubMed] [Google Scholar]
- 15.McEwen BS. Stress-induced remodeling of hippocampal CA3 pyramidal neurons. Brain Res 2016; 1645: 50–54. [DOI] [PubMed] [Google Scholar]
- 16.Cameron HA, Gould E. The control of neuronal birth and survival. In: Shaw CA. (ed). Receptor dynamics in neural development. Pharmacology and toxicology: basic and clinical aspects, 1st New York: CRC Press, 1996, pp. 141–157. [Google Scholar]
- 17.McEwen BS. Stress and hippocampal plasticity. Ann Rev Neurosci 1999; 22: 105–122. [DOI] [PubMed] [Google Scholar]
- 18.Vyas A, Mitra R, Rao BSS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci 2002; 22: 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennur S, Shankaranarayana Rao BS, Pawlak R, et al. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience 2007; 144: 8–16. [DOI] [PubMed] [Google Scholar]
- 20.Lau T, Bigio B, Zelli D, et al. Stress-induced structural plasticity of medial amygdala stellate neurons and rapid prevention by a candidate antidepressant. Mol Psychiatry 2017; 22: 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao RP, Anilkumar S, McEwen BS, et al. Glucocorticoids protect against the delayed behavioral and cellular effects of acute stress on the amygdala. Biol Psychiatry 2012; 72: 466–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krishnan V, Han M-H, Graham DL, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 2007; 131: 391–404. [DOI] [PubMed] [Google Scholar]
- 23.Radley JJ, Sisti HM, Hao J, et al. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience 2004; 125: 1–6. [DOI] [PubMed] [Google Scholar]
- 24.Liston C, Miller MM, Goldwater DS, et al. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci 2006; 26: 7870–7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joels M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol Sci 2006; 27: 244–250. [DOI] [PubMed] [Google Scholar]
- 26.Popoli M, Yan Z, McEwen BS, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2012; 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci 2009; 1179: 167–178. [DOI] [PubMed] [Google Scholar]
- 28.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuro-Psychopharm Biol Psychiat 2010; 34: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du J, Wang Y, Hunter R, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA 2009; 106: 3543–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Q, Lu X-Y, Liu L, et al. Glucocorticoid receptor overexpression in forebrain: a mouse model of increased emotional lability. Proc Natl Acad Sci USA 2004; 101: 11851–11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jacobson L. Forebrain glucocorticoid receptor gene deletion attenuates behavioral changes and antidepressant responsiveness during chronic stress. Brain Res 2014; 1583: 109–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szyf M, Weaver ICG, Champagne FA, et al. Maternal programming of steroid receptor expression and phenotype through DNA methylation in the rat. Front Neuroendocrin 2005; 26: 139–162. [DOI] [PubMed] [Google Scholar]
- 33.McGowan PO, Sasaki A, D'Alessio AC, et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neurosci 2009; 12: 241–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zohar J, Yahalom H, Kozlovsky N, et al. High dose hydrocortisone immediately after trauma may alter the trajectory of PTSD: interplay between clinical and animal studies. Eur Neuropsychopharmacol 2011; 21: 796–809. [DOI] [PubMed] [Google Scholar]
- 35.Schelling G, Roozendaal B, De Quervain DJ-F. Can posttraumatic stress disorder be prevented with glucocorticoids? Ann NY Acad Sci 2004; 1032: 158–166. [DOI] [PubMed] [Google Scholar]
- 36.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proc Natl Acad Sci USA 2008; 105: 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liston C, Gan WB. Glucocorticoids are critical regulators of dendritic spine development and plasticity in vivo. Proc Natl Acad Sci USA 2011; 108: 16074–16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liston C, Cichon JM, Jeanneteau F, et al. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci 2013; 16: 698–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lamont EW, Robinson B, Stewart J, et al. The central and basolateral nuclei of the amygdala exhibit opposite diurnal rhythms of expression of the clock protein Period2. Proc Natl Acad Sci USA 2005; 102: 4180–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy AB, Maywood ES, Karp NA, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 2007; 45: 1478–1488. [DOI] [PubMed] [Google Scholar]
- 41.Karatsoreos IN, Bhagat SM, Bowles NP, et al. Endocrine and physiological changes in response to chronic corticosterone: a potential model of the metabolic syndrome in mouse. Endocrinology 2010; 151: 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem 1993; 61: 1957–1960. [DOI] [PubMed] [Google Scholar]
- 43.Karst H, Berger S, Turiault M, et al. Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 2005; 102: 19204–19207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treccani G, Musazzi L, Perego C, et al. Stress and corticosterone increase the readily releasable pool of glutamate vesicles in synaptic terminals of prefrontal and frontal cortex. Mol Psychiatry 2014; 19: 433–443. [DOI] [PubMed] [Google Scholar]
- 45.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience 1995; 69: 89–98. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampus CA3 pyramidal neurons. Brain Res 1992; 588: 341–344. [DOI] [PubMed] [Google Scholar]
- 47.Martin KP, Wellman CL. NMDA receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb Cortex 2011; 21: 2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arendt T, Stieler J, Strijkstra AM, et al. Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J Neurosci 2003; 23: 6972–6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinoshita Y, Hunter RG, Gray JD, et al. Role for NUP62 depletion and PYK2 redistribution in dendritic retraction resulting from chronic stress. Proc Natl Acad Sci USA 2014; 111: 16130–16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sapolsky RM, Krey LC, McEwen BS. The Neuroendocrinology of Stress and Aging: The Glucocorticoid Cascade Hypothesis. EndocrRev 1986; 7: 284–301. [DOI] [PubMed] [Google Scholar]
- 51.Nasca C, Zelli D, Bigio B, et al. Stress dynamically regulates behavior and glutamatergic gene expression in hippocampus by opening a window of epigenetic plasticity. Proc Natl Acad Sci USA 2015; 112: 14960–14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasca C, Xenos D, Barone Y, et al. L-acetylcarnitine causes rapid antidepressant effects through the epigenetic induction of mGlu2 receptors. Proc Natl Acad Sci USA 2013; 110: 4804–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chattarji S, Tomar A, Suvrathan A, et al. Neighborhood matters: divergent patterns of stress-induced plasticity across the brain. Nat Neurosci 2015; 18: 1364–1375. [DOI] [PubMed] [Google Scholar]
- 54.Pereira AC, Lambert HK, Grossman YS, et al. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci USA 2014; 111: 18733–18738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pereira AC, Gray JD, Kogan JF, et al. Age and Alzheimer's disease gene expression profiles reversed by the glutamate modulator riluzole. Mol Psychiatry 2017; 22: 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev 2007; 87: 873–904. [DOI] [PubMed] [Google Scholar]
- 57.Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and hippocampal volume in women at risk for Alzheimer's disease. Neurobiol Aging 2011; 32: 1942–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasgon NL, Kenna HA, Wroolie TE, et al. Insulin resistance and medial prefrontal gyrus metabolism in women receiving hormone therapy. Psychiatry Res 2014; 223: 28–36. [DOI] [PubMed] [Google Scholar]
- 59.Kenna H, Hoeft F, Kelley R, et al. Fasting plasma insulin and the default mode network in women at risk for Alzheimer's disease. Neurobiol Aging 2013; 34: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grillo CA, Piroli GG, Lawrence RC, et al. Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 2015; 64: 3927–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grillo CA, Piroli GG, Kaigler KF, et al. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav Brain Res 2011; 222: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grillo CA, Piroli GG, Evans AN, et al. Obesity/hyperleptinemic phenotype adversely affects hippocampal plasticity: effects of dietary restriction. Physiol Behav 2011; 104: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grillo CA, Mulder P, Macht VA, et al. Dietary restriction reverses obesity-induced anhedonia. Physiol Behav 2014; 128: 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yao J, Irwin RW, Zhao L, et al. Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proc Natl Acad Sci USA 2009; 106: 14670–14675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.D'Alessio D. Is GLP-1 a hormone: whether and when?. J Diabetes Investig 2016; 7(Suppl 1): 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McIntyre RS, Powell AM, Kaidanovich-Beilin O, et al. The neuroprotective effects of GLP-1: possible treatments for cognitive deficits in individuals with mood disorders. Behav Brain Res 2013; 237: 164–171. [DOI] [PubMed] [Google Scholar]
- 67.Bigio B, Mathe AA, Sousa VC, et al. Epigenetics and energetics in ventral hippocampus mediate rapid antidepressant action: implications for treatment resistance. Proc Natl Acad Sci USA 2016; 113: 7906–7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Halfon N, Larson K, Lu M, et al. Lifecourse health development: past, present and future. Matern Child Health J 2014; 18: 344–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Allfrey VG. Changes in chromosomal proteins at times of gene activation. Fed Proc 1970; 29: 1447–1460. [PubMed] [Google Scholar]
- 70.Mehler MF. Epigenetic principles and mechanisms underlying nervous system functions in health and disease. Prog Neurobiol 2008; 86: 305–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Griffiths BB, Hunter RG. Neuroepigenetics of stress. Neuroscience 2014; 275: 420–435. [DOI] [PubMed] [Google Scholar]
- 72.Mehler MF, Mattick JS. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol Rev 2007; 87: 799–823. [DOI] [PubMed] [Google Scholar]
- 73.Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res 2012; 229: 280–288. [DOI] [PubMed] [Google Scholar]
- 74.Nasca C, Bigio B, Zelli D, et al. Mind the gap: glucocorticoids modulate hippocampal glutamate tone underlying individual differences in stress susceptibility. Mol Psychiatry 2015; 20: 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freund J, Brandmaier AM, Lewejohann L, et al. Emergence of individuality in genetically identical mice. Science 2013; 340: 756–759. [DOI] [PubMed] [Google Scholar]
- 76.Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci USA 2005; 102: 10604–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. Am J Prev Med 1998; 14: 245–258. [DOI] [PubMed] [Google Scholar]
- 78.Levine S, Haltmeyer G, Kara G, et al. Physiological and behavioral effects of infantile stimulation. Physiol Behav 1967; 2: 55–59. [Google Scholar]
- 79.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci 2005; 7: 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc Natl Acad Sci USA 2003; 100: 16131–16136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cavigelli SA, Yee JR, McClintock MK. Infant temperament predicts life span in female rats that develop spontaneous tumors. Horm Behav 2006; 50: 454–462. [DOI] [PubMed] [Google Scholar]
- 82.Akers KG, Yang Z, DelVecchio DP, et al. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS One 2008; 3: e2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tang AC, Akers KG, Reeb BC, et al. Programming social, cognitive, and neuroendocrine development by early exposure to novelty. Proc Natl Acad Sci USA 2006; 103: 15716–15721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parker KJ, Buckmaster CL, Sundlass K, et al. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA 2006; 103: 3000–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Isgor C, Kabbaj M, Akil H, et al. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus 2004; 14: 636–648. [DOI] [PubMed] [Google Scholar]
- 86.Rice CJ, Sandman CA, Lenjavi MR, et al. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008; 149: 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci 2006; 8: 1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kaufman D, Smith ELP, Gohil BC, et al. Early appearance of the metabolic syndrome in socially reared bonnet macaques. J Clin Endocrin & Metab 2005; 90: 404–408. [DOI] [PubMed] [Google Scholar]
- 89.Coplan JD, Smith ELP, Altemus M, et al. Variable foraging demand rearing: Sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiat 2001; 50: 200–204. [DOI] [PubMed] [Google Scholar]
- 90.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301: 386–389. [DOI] [PubMed] [Google Scholar]
- 91.Spinelli S, Schwandt ML, Lindell SG, et al. The serotonin transporter gene linked polymorphic region is associated with the behavioral response to repeated stress exposure in infant rhesus macaques. Dev Psychopathol 2012; 24: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science 2002; 297: 851–854. [DOI] [PubMed] [Google Scholar]
- 93.Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann NY Acad Sci 2006; 1094: 52–62. [DOI] [PubMed] [Google Scholar]
- 94.Obradovic J, Bush NR, Stamperdahl J, et al. Biological sensitivity to context: the interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Dev 2010; 81: 270–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol 2005; 17: 271–301. [DOI] [PubMed] [Google Scholar]
- 96.Galea LAM, McEwen BS, Tanapat P, et al. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 1997; 81: 689–697. [DOI] [PubMed] [Google Scholar]
- 97.Luine V, Villegas M, Martinez C, et al. Repeated stress causes reversible impairments of spatial memory performance. Brain Res 1994; 639: 167–170. [DOI] [PubMed] [Google Scholar]
- 98.Luine VN, Beck KD, Bowman RE, et al. Chronic stress and neural function: accounting for sex and age. J Neuroendocrinology 2007; 19: 743–751. [DOI] [PubMed] [Google Scholar]
- 99.Bowman RE, Zrull MC, Luine VN. Chronic restraint stress enhances radial arm maze performance in female rats. Brain Res 2001; 904: 279–289. [DOI] [PubMed] [Google Scholar]
- 100.Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci USA 1998; 95: 4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wood GE, Shors TJ, Beylin AV. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci 2001; 115: 175–187. [DOI] [PubMed] [Google Scholar]
- 102.Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci USA 2002; 99: 13955–13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leuner B, Mendolia-loffredo S, Shors TJ. Males and females respond differently to controllability and antidepressant treatment. Biol Psychiat 2004; 56: 964–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shansky RM, Hamo C, Hof PR, et al. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex 2010; 20: 2560–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bangasser DA, Curtis A, Reyes BA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 2010; 15: 877, 896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bangasser DA, Zhang X, Garachh V, et al. Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 2011; 103: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci 2006; 7: 477–484. [DOI] [PubMed] [Google Scholar]
- 108.McEwen BS, Lasley EN. The end of sex as we know it. Cerebrum The Dana Forum on Brain Science 2005; Vol. 7, New York, NY: Dana Press. [Google Scholar]
- 109.McEwen BS. Introduction: the end of sex as we once knew it. Physiol Behav 2009; 97: 143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laje G, Paddock S, Manji H, et al. Genetic markers of suicidal ideation emerging during citalopram treatment of major depression. Am J Psychiatry 2007; 164: 1530–1538. [DOI] [PubMed] [Google Scholar]
- 111.Meites J. Short history of neuroendocrinology and the international society of neuroendocrinology. Neuroendocrinology 1992; 56: 1–10. [DOI] [PubMed] [Google Scholar]
- 112.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nature Neurosci 2002; 5: 933–934. [DOI] [PubMed] [Google Scholar]
- 113.Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology 2010; 35: 67–82. [DOI] [PubMed] [Google Scholar]
- 114.McEwen BS, McEwen CA. Social, psychological, and physiological reactions to stress, New York: John Wiley & Sons, Inc, 2015. [Google Scholar]
- 115.Danese A, Moffitt TE, Harrington H, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pediatr Adolesc Med 2009; 163: 1135–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci 2010; 21: 848–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McEwen BS. Protective and damaging effects of stress mediators. New Engl J Med 1998; 338: 171–179. [DOI] [PubMed] [Google Scholar]
- 118.Anda RF, Butchart A, Felitti VJ, et al. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med 2010; 39: 93–98. [DOI] [PubMed] [Google Scholar]
- 119.McEwen BS, Tucker P. Critical biological pathways for chronic psychosocial stress and research opportunities to advance the consideration of stress in chemical risk assessment. Am J Public Health 2011; 101(Suppl 1): S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evans GW, Gonnella C, Marcynyszyn LA, et al. The role of chaos in poverty and children's socioemotional adjustment. Psychol Sci 2005; 16: 560–565. [DOI] [PubMed] [Google Scholar]
- 121.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res 2006; 1110: 166–174. [DOI] [PubMed] [Google Scholar]
- 122.Hart B, Risley TR. Meaningful differences in the everyday experience of young American Children, Baltimore, MD: Brookes Publishing Company, 1995, pp. 304. [Google Scholar]
- 123.Hanson JL, Chandra A, Wolfe BL, et al. Association between income and the hippocampus. PLoS One 2011; 6: e18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Soc Cogn Affect Neurosci 2007; 2: 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gianaros PJ, Horenstein JA, Hariri AR, et al. Potential neural embedding of parental social standing. Soc Cogn Affect Neurosci 2008; 3: 91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adler NE, Boyce TW, Chesney MA, et al. Socioeconomic Inequalities in Health. JAMA 1993; 269: 3140–3145. [PubMed] [Google Scholar]
- 127.Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci USA 2011; 108: 14324–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Del Giudice M, Ellis BJ, Shirtcliff EA. The Adaptive Calibration Model of stress responsivity. Neurosci Biobehav Rev 2011; 35: 1562–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jackson JS, Knight KM, Rafferty JA. Race and unhealthy behaviors: chronic stress, the HPA axis, and physical and mental health disparities over the life course. Am J Public Health 2010; 100: 933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tomasdottir MO, Sigurdsson JA, Petursson H, et al. Self reported childhood difficulties, adult multimorbidity and allostatic load. A cross-sectional analysis of the Norwegian HUNT study. PLoS One 2015; 10: e0130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rasgon NL, McEwen BS. Insulin resistance-a missing link no more. Mol Psychiatry 2016; 21: 1648–1652. [DOI] [PubMed] [Google Scholar]
- 132.Waddington CH. The epigenotype. Endeavoour 1942; 1: 18–20. [Google Scholar]
- 133.Acheson SD. Independent inquiry into inequalities in health report, London: The Stationary Office, 1998. [Google Scholar]
- 134.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci USA 2012; 109(Suppl 2): 17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]