Abstract
Monoclonal gammopathies consist of a spectrum of clonal plasma cell disorders that includes monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM) and Waldenstrom Macroglobulinemia (WM). In this review, we outline the epidemiology, etiology, classification, diagnosis, and treatment of monoclonal gammopathy associated peripheral neuropathy. Monoclonal gammopathy of undetermined significance (MGUS) is relatively common in the general population, with a prevalence of 3–4% among those over the age of 50. Therefore, the presence of M protein in a patient with neuropathy does not automatically indicate a causal relationship. Monoclonal gammopathy associated peripheral neuropathy is often a difficult diagnosis with limited treatment options. Studies addressing the optimal approach to diagnosis and management of this entity are limited. In addition to a review of the literature, we present a diagnostic approach to patients with monoclonal gammopathy associated peripheral neuropathy and discuss available data and options for treatment.
INTRODUCTION
Monoclonal gammopathies consist of a spectrum of clonal plasma cell disorders that includes monoclonal gammopathy of undetermined significance (MGUS), multiple myeloma (MM) and Waldenstrom Macroglobulinemia (WM). The hallmark of these disorders is the secretion of a monoclonal immunoglobulin, referred to as a monoclonal (M) protein. Peripheral neuropathy is a well-recognized complication of monoclonal gammopathies, and a difficult clinical problem in terms of diagnosis and treatment (Table 1). Since 3–4% of the general population over the age of 50 has a monoclonal gammopathy, it is very common to encounter patients with peripheral neuropathy in whom further work up reveals a monoclonal (M) protein. The vast majority of such patients does not have any evidence of overt malignancy such as MM or WM, but rather are at the premalignant MGUS stage in terms of plasma cell biology. Distinguishing patients in whom the M protein is causally related to the peripheral neuropathy from patients in whom the presence of an M protein is incidental and unrelated to the neuropathy is difficult. Further, despite the frequency of this condition, and the diagnostic difficulties, there are very limited data to guide management. In this paper, we review the epidemiology, etiology, classification, diagnosis, and treatment of monoclonal gammopathy associated peripheral neuropathy.
TABLE 1.
Clinical Presentation of Peripheral Neuropathy in Plasma Cell Disorders
|
Ig, immunoglobulin; POEMS, polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes.
Monoclonal gammopathy associated peripheral neuropathy must be differentiated from two other plasma cell disorders that cause neuropathy that have been well characterized and have strict diagnostic criteria, namely immunoglobulin light chain (AL) amyloidosis, and neuropathy associated with osteosclerotic myeloma (POEMS syndrome) (Table 1).1 In AL amyloidosis neuropathy and in POEMS syndrome, the causal relationship between the neurologic process and the underlying M protein is not in question, and therapy is aimed at the underlying disorder. These entities are reviewed in detail elsewhere.2–4
EPIDEMIOLOGY
Peripheral neuropathy can occur in patients across the spectrum of plasma cell disorders from the premalignant MGUS stage to the overt malignant stages of MM and WM. In order to understand the epidemiology of monoclonal gammopathy associated peripheral neuropathy, one needs to appreciate that MGUS is relatively common in the general population, and that the mere presence of an M protein in a patient with neuropathy does not mean that a causal relationship exists. In fact, more often than not, the association is probably coincidental, simply reflecting the relatively high prevalence of these two disorders in the population.
MGUS is present in over 3–4% of the population older than age 50.5 It is a premalignant precursor of multiple myeloma. There are three major types of MGUS depending on the type of M protein secreted: IgM MGUS, Non-IgM MGUS (includes IgG MGUS and IgA MGUS), and light-chain MGUS. Progression to malignancy is the main clinical consequence of MGUS, and occurs at a rate of 1% per year.6 IgM MGUS is associated with a risk of progression to Waldenstrom macroglobulinemia, while non-IgM MGUS carries a risk of progression to MM. Light-chain MGUS is a newly discovered entity that is associated with a risk of progression to light-chain type of MM. All forms of MGUS can progress to AL amyloidosis. The other main consequence of MGUS is the ability to cause organ damage due to the immunogenic properties of the M protein including peripheral neuropathy, membranoproliferative glomerulonephritis (MPGN), and necrobiotic xanthogranuloma. The association of MGUS with neuropathy has been confirmed in a population-based screening study of 17,398 persons living in Olmsted county, 605 with MGUS and 16,793 negative controls.7 With a mean follow up of 24 years, totaling 14,373 person-years, there was a significantly higher risk of chronic inflammatory demyelinating polyradiculoneuropathy in persons with MGUS, relative risk, 5.9 (95% CI 1.2–28.4). The risk of autonomic neuropathy was increased as well, relative risk, 3.2 (95% CI 1.3–8.3). However, this report did not analyze the underlying type of M protein, and the risk of chronic inflammatory demyelinating polyradiculoneuropathy may be partially accounted for by patient’s with POEMS syndrome and autonomic neuropathy may be partially accounted for by the increased risk of AL amyloidosis in this population. Importantly in this study, there was no significant association detected between monoclonal gammopathy and neurologic disorders such as cerebellar ataxia, demyelinating diseases of the central nervous system, and motor neuron disease.
Given the prevalence of MGUS, neuropathy associated with an M protein is frequently encountered in clinical practice. In a series of 132 patients with monoclonal gammopathy, 4 (3%) had peripheral neuropathy.8 In another study, Isobe and colleagues found peripheral neuropathy in approximately 5% of patients with a plasma cell dyscrasia of unknown significance.9 Other studies have found a statistically significant increase in the prevalence of M proteins in patients with peripheral neuropathy compared with normal populations in Minnesota, France and Sweden.10 Overall, these studies suggest that an M protein can be detected in approximately 3–5% of all cases of peripheral neuropathy, especially in patients referred to tertiary care centers with such symptoms. Among patients with peripheral neuropathy in whom no cause is apparent, the prevalence of an M protein may be as high as 10%.10 Although some associations are pathophysiologically related, since MGUS is prevalent in 2–3% of the normal adult population over 50 years of age,5 some of these associations are likely coincidental.
An even greater number of patients with MM or WM may have symptoms of peripheral neuropathy that is present at diagnosis or occurs during the course of the disease. As with MGUS, some of these associations are likely coincidental, while others may be causally related to the underlying M protein. Further, in patients with MM and WM, another factor that needs to be considered is that drugs used to treat these disorders may be neurotoxic and may be the true cause of the symptoms.
The type of M protein in monoclonal gammopathy associated peripheral neuropathy is most commonly IgM, while IgG, or IgA neuropathies are less common.11 In one study, approximately 60% of neuropathies associated with monoclonal gammopathy were IgM, 30% were IgG, and 10% were IgA.12 IgM M protein appears to be associated with a higher predilection for causing peripheral neuropathy compared to IgG or IgA MGUS combined.13 In a study of 74 patients with MGUS, 8 of 26 IgM MGUS patients (31%) had neuropathy, compared to 2 of 34 IgG MGUS patients (6%) and 2 of 14 IgA MGUS patients (14%).13
PATHOPHYSIOLOGY
The pathophysiology of monoclonal gammopathy associated peripheral neuropathy is not well understood.14 Pathological studies in WM and MGUS associated neuropathy have shown demyelination and widened myelin lamellae.11,15 Monoclonal IgM deposits can be detected in the widened lamellae of myelin fibers, and in myelin debris contained in Schwann cells and macrophages.16 Demyelination was also noted in a study of 5 patients with IgG MGUS associated peripheral neuropathy; in 3 patients there was “onion bulb” formation which is caused by concentric layers of Schwann cell cytoplasm and connective tissue around an axon felt to be causes by repeated demyelination and remyelination.17
The reactivity of M proteins in peripheral neuropathy has been best studied in IgM disorders. In about 40–50% of patients with IgM monoclonal gammopathy associated neuropathies, the M protein binds to myelin-associated glycoprotein (MAG).11,18,19 In the subset of patients with IgM monoclonal gammopathy associated peripheral neuropathy who do not have anti-MAG antibodies, a recent cohort study of 54 patients found anti-ganglioside antibodies in 35% of patients, and antibodies against ganglioside complexes among in 9% of patients.18 Most of the patients with anti-ganglioside or anti asialo-GM1 antibodies had sensory and motor symptoms, but no clear relationship was found between the antibody specificity and clinical characteristics.18 No significant differences have been found comparing the clinical presentation of IgM related neuropathies based on the antibody specificity (i.e. anti-MAG, anti-ganglioside or anti asialo-GM1).
The pathogenesis is thought to be a direct effect of M proteins (which are immunoglobulins) on the peripheral nerve, resulting in a demyelinating process. A study of 25 patients with WM and neuropathy found that among all the cases where the IgM had activity against myelin, patients experienced neuropathy symptoms at presentation.20 Among the 7 of the 15 patients in whom the IgM M protein did not have reactivity against myelin, neuropathic symptoms developed 1 to 5 years after the initial diagnosis of WM. In another study of 24 patients with IgM MGUS who were asymptomatic at initial evaluation, higher titers of anti-MAG antibodies were associated with the development of neuropathy symptoms.21 These studies suggest a likely causal relationship between in the M protein and peripheral neuropathy exists in at least a subset of cases. However, anti-MAG antibodies are not specific, and have also been detected in patients with IgM AL amyloidosis and neuropathy.22 Further, reduction in anti-MAG antibodies with rituximab therapy have not correlated with clinical improvement, so more research is needed on the mechanisms of nerve damage.
CLINICAL PRESENTATION
Peripheral neuropathy is much more commonly associated with IgM M proteins than with IgG or IgA M proteins. In fact, it is not clear whether there is a true causal relationship between non-IgM M proteins and peripheral neuropathy, except in cases with POEMS syndrome or AL amyloidosis. Additional studies in this regard are needed. Clinically, it is hard to distinguish monoclonal gammopathy associated peripheral neuropathy from other causes of neuropathy. There are differences in the clinical presentation of neuropathy associated IgM M proteins compared with that reported to be associated with IgG or IgA M proteins 23.
In general, IgM monoclonal gammopathy associated peripheral neuropathy presents as distal, acquired, demyelinating, symmetric neuropathy with M protein (DADS-M). It is considered a variant of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP).24,25 It typically affects men in their sixth to ninth decade as a distal, symmetric neuropathy that affects primarily large sensory nerve fibers causing sensory ataxia.26 Motor involvement can occur and is typically mild and distal. Cranial nerve involvement is rare, but can occur. Anti-MAG antibodies are present in approximately 50% of patients; however there is no difference in the severity or type of neuropathy with or without anti-MAG antibodies.27
In contrast, patients with non IgM monoclonal proteins can be seen in the full spectrum of neuropathy phenotypes such as the more common length-dependent sensorimotor axonal peripheral neuropathy to classic chronic inflammatory demyelinating polyneuropathy (CIDP) 28. A Mayo Clinic study of 65 patients with MGUS and peripheral neuropathy diagnoses found no significant clinical differences between the patients with IgG MGUS (n=24) and those with IgA MGUS (n=10).27 Patients with IgG MGUS can have antibodies against neural antigens, even in the absence of clinical neuropathy.28–30 Furthermore in patients with CIDP, those with and without a paraprotein respond similarly to treatment.31 For all of these reasons, the finding of an IgG or IgA monoclonal gammopathy, unless found in conjunction with POEMS syndrome or AL amyloidosis, may be coincidental, and less likely to be causally related to peripheral neuropathy.
Electrophysiology
There are common electrophysiological features in DADS-M that suggest demyelination and include slowed motor conduction velocities, markedly prolonged distal latencies and low terminal latency indices all implying involvement of the terminal nerves. The sensory responses are typically reduced or absent.27,32 In direct comparison to IgG patients, these findings are all worse in IgM and are suggestive of demyelination, whereas the IgG group typically showed axonal findings but rare patients showed findings seen in classic CIDP and motor neuropathy with conduction block.33
DIAGNOSIS
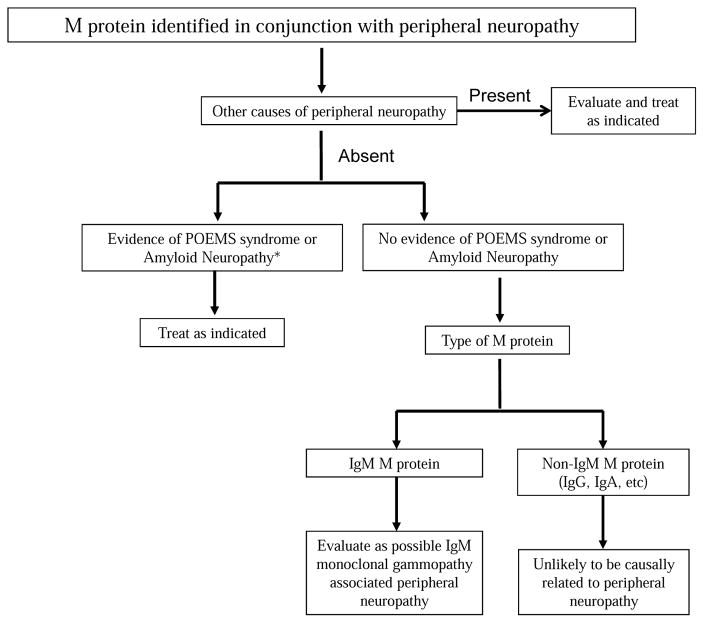
Monoclonal gammopathy associated peripheral neuropathy should be considered when an M protein is detected during work up of unexplained neuropathy. The first step in evaluation is to determine if the monoclonal gammopathy is the likely cause of peripheral neuropathy or if it is a coincidental finding related to the frequency with which M proteins are seen in the general population (Figure 1). Other explanations and causes of peripheral neuropathy such as genetic, diabetes mellitus, alcoholism, drugs etc, must first be considered and excluded as much as possible. There are no specific tests that can be done to distinguish between a true causal association and an incidental one. In general, the younger the patient, the more likely than the association is causal, since the prevalence of M proteins in persons less than 50 years of age is less than 1.5%. In contrast, the prevalence of M proteins is as high as 7% in persons over the age of 70 years of age, and thus many associations are likely coincidental. Similarly, the likelihood of a causal relationship is far higher with IgM M proteins than with IgG or IgA M proteins. Other laboratory studies are not as useful. Anti MAG antibodies can be detected in approximately 50% of patients with IgM M protein related peripheral neuropathy. Although this may suggest a causal relationship, the specificity is low.
Figure 1.
Approach to Evaluation of a Patient with a Monoclonal Protein identified in conjunction with Peripheral Neuropathy
*Based on history, physical examination, and if appropriate, laboratory and radiographic testing
†This group includes patients with multiple myeloma who have concurrent peripheral neuropathy. These patients need therapy to eradicate the neoplastic clone due to the nature of the malignancy in contrast to patients with monoclonal gammopathy of undetermined significance in whom establishing a causal relationship between neuropathy and the monoclonal protein is critical for therapeutic purposes.
POEMS, polyneuropathy, osteosclerotic myeloma, endocrinopathy, monoclonal protein, skin changes.
The next step in the evaluation is to differentiate monoclonal gammopathy associated peripheral neuropathy from specific plasma cell disorders that are known to have a definite causal relationship namely POEMS syndrome and amyloid neuropathy. In patients with POEMS syndrome and AL amyloidosis, the overall approach and nature of treatment is different.
Next, the nature of the underlying monoclonal gammopathy must be determined. Most patients with an M protein will have MGUS, but some will have MM or WM and these disorders need to be excluded by bone marrow studies to determine the proportion of clonal cells, and imaging studies to rule out osteolytic bone lesions, lymphadenopathy, or organomegaly.1,34 Patients diagnosed as having WM or MM need systemic therapy for the underlying malignancy regardless of the severity of the neuropathy. In these patients if there is improvement in neuropathy as the malignancy is treated, it can be assumed that a causal relationship is likely.
Finally, one must assess and document the severity of the neuropathy. The severity of the neuropathy, and progression over time, are major determinants on whether therapeutic intervention targeting the M protein is needed. This is mainly done based on history and physical examination. Nerve conduction and electromyographic (EMG) studies can be particularly helpful if they demonstrate the typical findings in DADS-M as well as providing information on severity of the neuropathy and serving as a baseline for future comparison, especially if treatment is considered.
DIFFERENTIAL DIAGNOSIS
When M proteins are found in the setting of peripheral neuropathy, POEMS syndrome and AL amyloid neuropathy must be considered in the differential diagnosis because the pathogenesis and treatment is different.
POEMS Syndrome
POEMS syndrome is a clonal plasma cell disorder described by the acronym in its name, polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes.4 POEMS syndrome is characterized by a progressive chronic demyelinating sensorimotor polyneuropathy that is either length-dependent or a polyradiculoneuropathy, and is often mistaken for CIDP. Organomegaly (hepatomegaly or splenomegaly) and skin changes (eg., hyperpigmentation) can be seen. Other characteristics include edema, pleural effusions, ascites, and papilledema.4 The bone lesions in POEMS syndrome are sclerotic (osteosclerotic myeloma) in contrast to MM where bone lesions are osteolytic. Many patients with POEMS may present mainly with neuropathy and a lambda type M protein, with the other features not being prominent. Careful evaluation with history and examination is needed, and if POEMS is suspected, radiographic (skeletal survey or computed tomographic scans) studies looking for one or more osteosclerotic bone lesions should be performed.
The peripheral neuropathy is motor predominant but with associated sensory symptoms and often pain at initial presentation.35,36 The histological pattern usually shows axonal degeneration along with demyelination. EMG usually shows a demyelinating pattern with more severe axonal loss (reduction of motor amplitudes and increased fibrillation potentials) than seen in CIDP or DADS-M. The M protein in POEMS syndrome is usually IgG or IgA. The underlying cause of the neuropathy remains unknown, although vascular endothelial growth factor may play a role in pathogenesis.37 Treatment of POEMS syndrome involves radiation and chemotherapy for the sclerotic plasmacytomas 11. This usually results in significant clinical improvement of the neuropathy as well as the other systemic features.38
AL Amyloid Neuropathy
Peripheral neuropathy occurs in 15–20% of patients with AL amyloidosis; however neuropathy is the dominant presentation of the disease among only 25% of such patients.3 The possible mechanisms of neuropathy in AL amyloidosis are direct effect of amyloid deposition in the nerves, nerve compression, or ischemia.39 The neuropathy is often length-dependent and associated with burning, pain and numbness. Upper limb involvement is common, often due to superimposed carpal tunnel syndrome. The neuropathy in AL amyloidosis often has an axonal pattern whereas monoclonal gammopathy (IgM) associated peripheral neuropathy is primarily demyelinating in nature. Autonomic function also tends to be impaired in neuropathy associated with AL amyloidosis. Compared with MGUS associated neuropathies, which can have an extended stable period, in patients with dominant AL neuropathy, the clinical course has a more progressive debilitating course.3
PROGNOSIS
Approximately 25–30% of patients with IgM monoclonal gammopathy associated peripheral neuropathy have moderate disability at 10 years.40 In a study of 26 patients with high titer anti-MAG IgM neuropathy, a favorable prognosis was seen in most patients after a mean follow up of over 8 years..41 The disability rates at 5, 10 and 15 years from onset of neuropathy was 16%, 24%, and 50%, respectively. It is more difficult to estimate the prognosis of non IgM related neuropathy since in most studies it is difficult to ascertain whether the monoclonal gammopathy was causal or coincidental.
TREATMENT
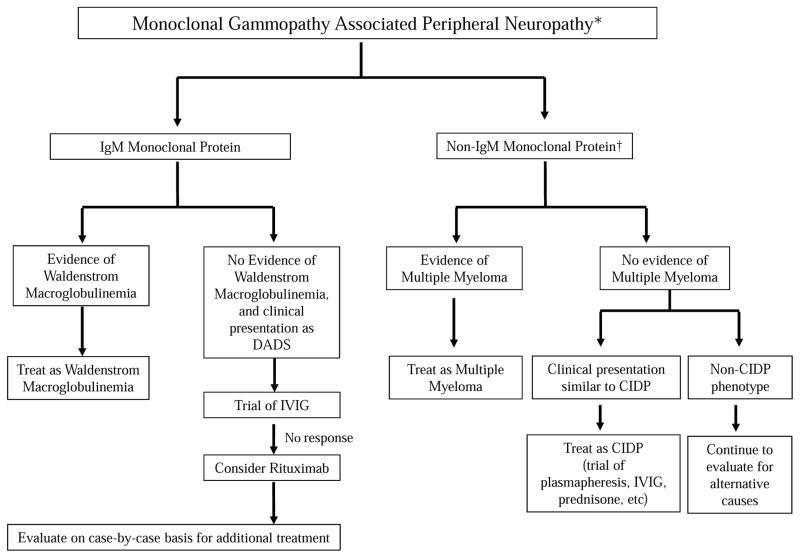
Unfortunately there are very limited data to guide clinical practice in patients with monoclonal gammopathy associated peripheral neuropathy. Further, most treatment results have been disappointing. In many patients, the treatments used can be more risky or cumbersome than the peripheral neuropathy. When considering therapy it is important to recognize that most associations of neuropathy and M proteins are coincidental. If the M protein is not of the IgM type, data on causal association with neuropathy, and data on treatment efficacy are very limited. In order to consider any type of intervention, there must be high probability that the neuropathy is secondary to the monoclonal gammopathy. Other causes of neuropathy and other monoclonal plasma cell disorders that cause neuropathy (POEMS syndrome, AL amyloidosis) must have been considered and excluded. Finally the peripheral neuropathy should be considered to be severe and progressive enough to warrant therapy. An approach to the treatment of monoclonal gammopathy associated peripheral neuropathy is provided in Figure 2. The results of studies evaluating treatments for monoclonal gammopathy associated peripheral neuropathy are discussed below.
Figure 2.
Approach to Management of Monoclonal Gammopathy Associated Peripheral Neuropathy
*High probability of monoclonal gammopathy associated peripheral neuropathy after excluding other causes of peripheral neuropathy, POEMS syndrome, and AL amyloid neuropathy. Neuropathy considered severe and/or progressive enough to warrant intervention
†Unlikely that M protein is causally related to neuropathy; most associations are likely coincidental.
DADS, distal, acquired, demyelinating, symmetric neuropathy; IVIG, intravenous immune globulin; CIDP, chronic inflammatory demyelinating polyneuropathy
Intravenous Immunoglobulin
Various studies have shown limited short-term benefit with treatment with intravenous human immunoglobulin (IVIG) in IgM monoclonal gammopathy associated peripheral neuropathy 11. The mechanism of action is not well understood; it may be related to a neutralization of anti-MAG antibodies or to an antibody response to the anti-MAG producing CD-5 positive B-cells 42.
IVIG was suggested as a treatment option in 1990 by Cook et al. because of its efficacy in treating myasthenia gravis, Kawasaki disease, CIDP, and polymyositis 43. The authors conducted an uncontrolled study of two patients with demyelinating neuropathies; one case was associated with an anti-ganglioside IgM gammopathy, and the other with an anti-MAG IgM gammopathy. The patients observed no benefit from previous treatments including prednisone, chlorambucil, azathioprine, and plasmapheresis. Initial improvement in strength was seen in each patient at least 8 days after treatment with high dose IVIg, and was sustained with continued high dose treatment.
A subsequent study of IVIG for 13 cases of IgM neuropathy and 4 cases of IgG neuropathies showed limited benefit.44 IVIG was administered in a dose of 2g/kg every 4 weeks for a period ranging from 3 months to 3 years. Among 7 patients with IgM M proteins that had no benefit from prior immunosuppressive treatment, 4 showed some improvement in neurological scores, but no change in IgM levels or antibody response. Among 3 of the patients with no prior treatment, 2 showed significant improvement in neurological scores. Among patients considered to have IgG neuropathy, 2 with axonal neuropathy had no benefit, while, 2 patients with chronic demyelinating polyneuropathy had a clinical response to treatment. In a study of 33 patients with anti-MAG IgM associated neuropathies, a short-term improvement was noted in 6 patients (35%), and objective benefit with increased ability and strength for activities of daily living in 4 patients (24%).45 No change in serum IgM levels or in electrophysiology studies was seen with treatment. In this study, plasmapheresis and prednisone had no significant effect. These open label single-arm studies have limitations, but suggest that IVIG may produce some limited benefit in patients with IgM monoclonal gammopathy associated peripheral neuropathy.
In a randomized, double-blind study, 11 patients with IgM demyelinating polyneuropathy received either placebo or IVIG for a 3 month period, and then the opposite treatment for 3 months after a washout period.46 The authors assessed muscle strength as well as sensory and neuromuscular symptoms using the Medical Research Council (MRC) scale. None of the 4 patients treated initially with placebo showed significant improvement (MRC score increase by 10 or more points) in these parameters during either the placebo or the IVIG phases of their treatment. Two of the 7 patients in the initial IVIG group showed improvements in strength and neuromuscular symptoms that affected their activities of daily living. These improvements regressed during the placebo phase. Mariette et al compared IVIG (2g/kg and then 1 g/kg every three weeks) versus recombinant interferon-alpha (IFN-alpha; 3 MU/m2 subcutaneously three times weekly) in 20 patients with polyneuropathy associated with an IgM M protein.47 Only one of 10 patients assigned to IVIG improved compared to 8 of 10 patients treated with IFN-alpha; however, a subsequent study found no benefit with IFN-alpha either.48 More promising results were seen with IVIG in a randomized double blind crossover trial, with 45% of patients (10 of 22 patients) improving during therapy with IVIG compared to 18% (4 of 22) during the placebo treatment period.49 Overall, these studies show a modest potential short-term benefit of IVIG in patients with IgM neuropathies. Data on the role of IVIG in patients with non IgM monoclonal gammopathy is even more limited. A retrospective study of IVIG treatment for 20 patients with polyneuropathy associated with IgG gammopathy suggested an improvement among 8 patients (40%), as evaluated by an increase in MRC strength and sensory scores and Rankin Disability Scale scores 50. Six patients showed a decline after the initial improvement and therefore required additional therapy; the other 2 patients had sustained improvement after the initial course. The patients who responded to IVIG had shorter symptom duration at baseline assessment, more symptoms of numbness, more falling episodes, and more proximal leg weakness before therapy compared to patients who did not respond. The authors found that these clinical features were more consistent with demyelinating neuropathies. Since this is a retrospective study, we cannot determine how convinced the treating providers were about a causal association.
Plasmapheresis
Treatment with plasmapheresis (also known as plasma exchange) has shown conflicting results. The rationale is to remove the offending M protein and thereby reduce ongoing target antibody mediated injury to neuronal fibers.31 Initial case reports supported the potential for application of plasmapheresis for treatment of non IgM (IgG and IgA) neuropathies, with sensory symptoms and strength improving with treatment.51,52 Some of these patients experienced relapses without plasmapheresis that were reversible with continued treatment or increased frequency of exchange. Based on the clinical course it is likely that these were probably patients with CIDP in whom the M protein was an incidental abnormality causally unrelated to the clinical symptoms.
In a double blind trial of 39 patients with neuropathies associated with IgG, IgA or IgM MGUS, patients were randomized into sham plasmapheresis or true plasmapheresis twice per week for three weeks 31. Patients who were assigned to the sham group eventually received plasmapheresis in an open label trial. Although the overall differences in response to sham or true plasmapheresis were not significant, the sham exchange group showed an average decrease in neuropathy disability score by 2 points while the plasma exchange group showed an average decrease by 12 points. While comparing IgG or IgA gammopathies to IgM gammopathy, patients with IgG or IgA related neuropathy showed greater improvement in average weakness scores than those with IgM related neuropathy in both the double blind and open trials. The results of this study must be taken in the context of the fact that we now recognize that some, if not all, of the non IgM related neuropathies may simply represent CIDP with incidental unrelated M proteins, and the fact that plasmapheresis does have benefit in CIDP regardless of an association with monoclonal gammopathy. Nevertheless the study shows the importance of a different approach to therapy based on the type of the M protein (Figure 2).
Subsequent studies have looked at the role of plasmapheresis in IgM related neuropathy. A prospective, randomized, open label clinical trial compared chlorambucil alone versus chlorambucil with plasmapheresis in 44 IgM neuropathy patients 53. Clinical neuropathy disability scores (CNDS) after 12 months were used to assess efficacy. No significant difference was found between the average CNDS decrease in the chlorambucil alone group (2.1 point decrease) and the chlorambucil plus plasma exchange group (1.8 point decrease). In both treatment groups, only the sensory component of the CNDS contributed to the improvement. By the end of the study, there was an improvement in neuropathy among 15 patients, 8 from the chlorambucil alone group and 7 from the combined treatment group, after treatment. The neuropathy remained stable or worsened in the remaining patients. Overall, the study results indicate that plasmapheresis provides no additional benefit to chlorambucil treatment for IgM monoclonal gammopathy associated peripheral neuropathy.
Fludarabine
The purine nucleoside analog fludarabine has long been used for the treatment of WM. Based on these data, fludarabine has been studied in patients with IgM related neuropathy 54. The goal is to eradicate the clone that produces IgM. The study included 4 patients, one of whom was ultimately diagnosed with WM. Intravenous fludarabine (25 mg/m2) was administered for 5 days every 4–6 weeks, for 5–6 months. Three of the four patients treated had previously received treatment with other therapies including steroids, plasmapheresis, and IVIG, with minimal improvement in signs or symptoms. In all of the cases, both subjective and objective improvement occurred. Objective improvements were assessed by in sensory scores, times taken to walk, and disability scores using the Rankin scale. There was also a decrease in IgM concentration by 25% or more in 3 of the four patients. The authors found no difference in response to fludarabine between the patients with or without anti-MAG antibodies. Although this is a small case-series, it suggests that fludarabine (and possibly other methods targeting the underlying neoplastic clone) may be a useful treatment for IgM monoclonal gammopathy associated peripheral neuropathy refractory to other treatment methods. Further study of fludarabine and its clinical effects on IgM neuropathy is warranted.
Rituximab
There have been a number of pilot studies and case reports on the treatment of IgM related neuropathy with the anti-CD20 monoclonal antibody rituximab, which targets the underlying clonal population.11,55 The treatment protocol in most of these studies was weekly infusions of 375 mg/m2 of rituximab over four weeks.55–58 These studies suggested a limited beneficial effect in some patients. Case reports have shown decreased IgM levels in IgM monoclonal gammopathy associated neuropathy and improvement of neuropathy in cases of anti-myelin-associated glycoprotein (MAG)/sulfated glucoronyl paragloboside (SGPG) associated Waldenström macroglobulinemia with rituximab treatment 56,58. There have also been case reports of a neuropathy flare in WM following rituximab therapy.59,60
A prospective study of 10 patients with anti-MAG IgM related neuropathy found that all patients improved with regard to sensory ataxia and muscle strength at 12 months, eight patients improved at 24 months, and 6 patients sustained improvement at 36 months 61. Patients who did not improve showed a slow and progressive increase in ataxia and weakness that led to a decline in gait function. The study on the long-term effect of rituximab also showed significant median reduction in anti-MAG antibody titers by 93% at 12 months, by 80% at 24 months, and close to 60% at 36 months. In a pilot study of 5 patients with anti-GM1 or anti-MAG IgM neuropathy, all five patients showed an increase in strength, both subjectively and objectively, 3 to 6 months after treatment with rituximab 62. An average of 47% reduction in IgM autoantibody titers was seen in 3 patients at 6 months.
A non-randomized comparative study followed strength and antibody levels in 21 patients treated with rituximab and 13 untreated controls over the course of two years in patients with IgM polyneuropathies.63 Patients had either anti-ganglioside (GM1 or Ga1NAc-GD1) or anti-MAG IgM positive neuropathies. Quantitative strength was tested bilaterally by dynamometry, with 12 measurements every 6 months; changes by 12% or more of normal were significant (p<0.05). None of the untreated patients showed significant improvement in strength, while strength improved by at least 12% among 18 of the 21 (86%) treated patients. The mean change in strength among treated patients at one year was 13% and at two years it was 23%. No change in total antibody or levels directed against antigens was found among the untreated patients. Among treated patients total IgM levels decreased to 74% of initial values at one year, and to 55% of baseline values at two years.
Two randomized control trials have been conducted in order to assess the efficacy of treatment of anti-MAG positive IgM neuropathy with rituximab. In one study a total of 26 patients were studied comparing rituximab versus placebo.55 Patients selected had a demyelinating neuropathy, an IgM monoclonal protein, anti-MAG/SGPG antibodies, and diminished function as measured by the Inflammatory Neuropathy Course and Treatment (INCAT) disability score greater than or equal to one. Improved INCAT scores were seen in 4 of the rituximab patients (31%) over 8 months, while no improvement or worsening was seen in placebo patients. Improvement began at 3 months in those who showed improvement, but was sustained at 6 months to a year after treatment. IgM levels decreased by 34% and MAG antibody titers were reduced by 50% at 8 months after treatment in the rituximab group. The results of this study must be interpreted with caution, since one patient enrolled was later found to be ineligible, and was removed from the analysis.
In another double blind, placebo-controlled trial, 54 patients with anti-MAG IgM chronic demyelinating neuropathy were randomized to receive either placebo or rituximab.57 Patients selected had an INCAT sensory score (ISS) above 4, a visual analog pain scale above 4 or an ataxia score greater than 2. The primary outcome of absolute improvement in ISS from baseline at 12 months was not achieved in the study as no significant difference in the change in ISS was seen between rituximab and placebo groups. However, secondary outcomes were achieved as 20% of patients in the rituximab group improved by at least 2 points on the INCAT disability scale, while none improved in the placebo group. Overall the authors concluded that rituximab was not effective for improvement in ISS in anti-MAG IgM demyelinating neuropathy patients, but that dosing regimens and combination therapies with rituximab remain an area for further study.
Further study of rituximab treatment through placebo-controlled trials with larger sample sizes are needed to elucidate the efficacy of rituximab treatment for IgM associated neuropathies.
Approach to therapy
As discussed, there are very limited data to make definitive treatment recommendations. In patients with overt WM or MM, who require therapy for the underlying malignancy, the treatment of the underlying disease will be the primary approach. Symptomatic treatments can be administered as needed. Some care should be made in selection of agents for therapy of the malignancy, so as to avoid drugs with known neurotoxic potential as much as possible.
Our approach to the treatment of monoclonal gammopathy associated peripheral neuropathy is provided in Figure 2. Most treatment results have been disappointing, and studies testing the value of newer treatments used in WM, such as ibrutinib, are needed in patients with IgM monoclonal gammopathy associated peripheral neuropathy. In patients with patients IgM monoclonal gammopathy associated peripheral neuropathy in whom there is severe refractory or progressive neuropathy after treatment with IVIG and rituximab, a decision on further treatment needs to be made with care. In many instances, these patients may need to be managed with symptomatic methods. Further consideration should be given to other causes of neuropathy. If the patient understands the risks, and the neuropathy is severe or disabling, one could consider treatments used in WM on a case-by-case basis (Figure 1). These include rituximab plus bendamustine, ibrutinib, and fludarabine. The patient must be advised that these treatments carry long-term risks, and that they may be more toxic or cumbersome than the peripheral neuropathy.
In patients with non-IgM related peripheral neuropathy presenting with features similar to CIDP, should be treated as CIDP, with treatments such as plasmapheresis, IVIG, prednisone, etc as warranted by the severity of the symptoms. A causal relationship should not be considered in patients with non-IgM M proteins who have peripheral neuropathy with features that do not resemble CIDP. In most of these patients, the relationship between the M protein and neuropathy is likely coincidental and there is greater potential for harm with therapy.
Conclusion
Additional studies are needed investigating new options for therapy. We recognize that many patients have disabling symptoms, and as new treatments emerge for plasma cell malignancies, some of these can be translated to the treatment of severe monoclonal gammopathy associated peripheral neuropathy. We need a better understanding of the pathophysiologic mechanisms, and better biomarkers to assess the value and effectiveness of therapy. Further studies are also needed to determine if there is a true association between non-IgM monoclonal gammopathy and peripheral neuropathy.
Acknowledgments
Supported in part by grants CA186781, CA 107476 and CA 168762 from the National Cancer Institute, Rockville, MD, USA.
HMC, MLM, and SVR conceived of the paper, researched the literature, and wrote the manuscript. All authors reviewed and approved the paper.
Abbreviations
- AL
immunoglobulin light chain
- CIDP
chronic inflammatory demyelinating polyneuropathy
- CNDS
clinical neuropathy disability scores
- DADS
distal, acquired, demyelinating, symmetric neuropathy
- DADS-M
demyelinating, symmetric neuropathy with M protein
- EMG
electromyographic
- Ig
immunoglobulin
- INCAT
Inflammatory Neuropathy Course and Treatment
- ISS
INCAT sensory score
- IVIG
intravenous immune globulin
- POEMS
polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes
- M
monoclonal
- MAG
myelin-associated glycoprotein
- MGUS
monoclonal gammopathy of undetermined significance
- MM
multiple myeloma
- MRC
Medical Research Council
- SGPG
sulfated glucoronyl paragloboside
- WM
Waldenstrom Macroglobulinemia
Footnotes
Disclosure of Conflicts of Interest
The authors declare no conflict of interest.
Authorship Contributions and Disclosure of Conflicts of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA. Immunoglobulin light chain amyloidosis: 2014 update on diagnosis, prognosis, and treatment. Am J Hematol. 2014;89(12):1132–1140. doi: 10.1002/ajh.23828. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Gertz MA, Kyle RA. Prognosis of patients with primary systemic amyloidosis who present with dominant neuropathy. Am J Med. 1998;104(3):232–237. doi: 10.1016/s0002-9343(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 4.Dispenzieri A. POEMS syndrome: update on diagnosis, risk-stratification, and management. Am J Hematol. 2015;90(10):951–962. doi: 10.1002/ajh.24171. [DOI] [PubMed] [Google Scholar]
- 5.Kyle RA, Therneau TM, Rajkumar SV, et al. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006;354(13):1362–1369. doi: 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 6.Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346(8):564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 7.Bida JP, Kyle RA, Therneau TM, et al. Disease associations with monoclonal gammopathy of undetermined significance: a population-based study of 17,398 patients. Mayo Clin Proc. 2009;84(8):685–693. doi: 10.4065/84.8.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen P, Leegaard OF. Peripheral neuropathy and paraproteinemia: an immunohistochemical and serologic study. Clinical Neuropathol. 1985;4(3):99–104. [PubMed] [Google Scholar]
- 9.Isobe T, Osserman EF. Pathologic conditions associated with plasma cell dyscrasias: a study of 806 cases. Ann NY Acad Sci. 1971;190:507–518. doi: 10.1111/j.1749-6632.1971.tb13560.x. [DOI] [PubMed] [Google Scholar]
- 10.Kelly JJ, Jr, Kyle RA, O’Brien PC, Dyck PJ. Prevalence of monoclonal protein in peripheral neuropathy. Neurology. 1981;31(11):1480–1483. doi: 10.1212/wnl.31.11.1480. [DOI] [PubMed] [Google Scholar]
- 11.Ramchandren S, Lewis RA. An update on monoclonal gammopathy and neuropathy. Cur Neurol Neurosci Rep. 2012;12(1):102–110. doi: 10.1007/s11910-011-0237-4. [DOI] [PubMed] [Google Scholar]
- 12.Yeung KB, Thomas PK, King RH, et al. The clinical spectrum of peripheral neuropathies associated with benign monoclonal IgM, IgG and IgA paraproteinaemia. Comparative clinical, immunological and nerve biopsy findings. Journal of neurology. 1991;238(7):383–391. doi: 10.1007/BF00319857. [DOI] [PubMed] [Google Scholar]
- 13.Nobile-Orazio E, Barbieri S, Baldini L, et al. Peripheral neuropathy in monoclonal gammopathy of undetermined significance: prevalence and immunopathogenetic studies. Acta neurol Scand. 1992;85(6):383–390. doi: 10.1111/j.1600-0404.1992.tb06033.x. [DOI] [PubMed] [Google Scholar]
- 14.Zivkovic SA, Lacomis D, Lentzsch S. Paraproteinemic neuropathy. Leukemia & lymphoma. 2009;50(9):1422–1433. doi: 10.1080/10428190903111922. [DOI] [PubMed] [Google Scholar]
- 15.Vital C, Vital A, Deminiere C, Julien J, Lagueny A, Steck AJ. Myelin modifications in 8 cases of peripheral neuropathy with Waldenstrom’s macroglobulinemia and anti-MAG activity. Ultrastructural pathology. 1997;21(6):509–516. doi: 10.3109/01913129709016367. [DOI] [PubMed] [Google Scholar]
- 16.Lach B, Rippstein P, Atack D, Afar DE, Gregor A. Immunoelectron microscopic localization of monoclonal IgM antibodies in gammopathy associated with peripheral demyelinative neuropathy. Acta neuropathol. 1993;85(3):298–307. doi: 10.1007/BF00227726. [DOI] [PubMed] [Google Scholar]
- 17.Bleasel AF, Hawke SH, Pollard JD, McLeod JG. IgG monoclonal paraproteinaemia and peripheral neuropathy. J Neurol Neurosurg Psych. 1993;56(1):52–57. doi: 10.1136/jnnp.56.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stork AC, Jacobs BC, Tio-Gillen AP, et al. Prevalence, specificity and functionality of anti-ganglioside antibodies in neuropathy associated with IgM monoclonal gammopathy. j Neuroimmunol. 2014 doi: 10.1016/j.jneuroim.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Nobile-Orazio E, Marmiroli P, Baldini L, et al. Peripheral neuropathy in macroglobulinemia: incidence and antigen-specificity of M proteins. Neurology. 1987;37(9):1506–1514. doi: 10.1212/wnl.37.9.1506. [DOI] [PubMed] [Google Scholar]
- 20.Dellagi K, Dupouey P, Brouet JC, et al. Waldenstrom’s macroglobulinemia and peripheral neuropathy: a clinical and immunologic study of 25 patients. Blood. 1983;62(2):280–285. [PubMed] [Google Scholar]
- 21.Meucci N, Baldini L, Cappellari A, et al. Anti-myelin-associated glycoprotein antibodies predict the development of neuropathy in asymptomatic patients with IgM monoclonal gammopathy. Ann Neurol. 1999;46(1):119–122. doi: 10.1002/1531-8249(199907)46:1<119::aid-ana18>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 22.Garces-Sanchez M, Dyck PJ, Kyle RA, et al. Antibodies to myelin-associated glycoprotein (anti-Mag) in IgM amyloidosis may influence expression of neuropathy in rare patients. Muscle & nerve. 2008;37(4):490–495. doi: 10.1002/mus.20955. [DOI] [PubMed] [Google Scholar]
- 23.Lozeron P, Adams D. Monoclonal gammopathy and neuropathy. Curr Opin Neurol. 2007;20(5):536–541. doi: 10.1097/WCO.0b013e3282ef79e3. [DOI] [PubMed] [Google Scholar]
- 24.Latov N. Pathogenesis and therapy of neuropathies associated with monoclonal gammopathies. Ann Neurol. 1995;37(Suppl 1):S32–42. doi: 10.1002/ana.410370705. [DOI] [PubMed] [Google Scholar]
- 25.Nobile-Orazio E. Neuropathy and monoclonal gammopathy. Handbook of clinical neurology. 2013;115:443–459. doi: 10.1016/B978-0-444-52902-2.00025-4. [DOI] [PubMed] [Google Scholar]
- 26.Filosto M, Cotelli M, Todeschini A, et al. Clinical spectrum and evolution of monoclonal gammopathy-associated neuropathy: an observational study. The neurologist. 2012;18(6):378–384. doi: 10.1097/NRL.0b013e31826a99e9. [DOI] [PubMed] [Google Scholar]
- 27.Gosselin S, Kyle RA, Dyck PJ. Neuropathy associated with monoclonal gammopathies of undetermined significance. Ann Neurol. 1991;30(1):54–61. doi: 10.1002/ana.410300111. [DOI] [PubMed] [Google Scholar]
- 28.Di Troia A, Carpo M, Meucci N, et al. Clinical features and anti-neural reactivity in neuropathy associated with IgG monoclonal gammopathy of undetermined significance. J Neurol Sci. 1999;164(1):64–71. doi: 10.1016/s0022-510x(99)00049-0. [DOI] [PubMed] [Google Scholar]
- 29.Anonymous. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society--first revision. J Periph Nerve Sys: JPNS. 2010;15(4):295–301. doi: 10.1111/j.1529-8027.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- 30.Vrethem M, Reiser N, Lauermann C, Svanborg E. Polyneuropathy associated with IgM vs IgG monoclonal gammopathy: comparison between clinical and electrophysiological findings. Acta neurol Scand. 2010;122(1):52–57. doi: 10.1111/j.1600-0404.2009.01259.x. [DOI] [PubMed] [Google Scholar]
- 31.Dyck PJ, Low PA, Windebank AJ, et al. Plasma exchange in polyneuropathy associated with monoclonal gammopathy of undetermined significance. N Engl J Med. 1991;325(21):1482–1486. doi: 10.1056/NEJM199111213252105. [DOI] [PubMed] [Google Scholar]
- 32.Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle & nerve. 2001;24(3):311–324. doi: 10.1002/1097-4598(200103)24:3<311::aid-mus1001>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Suarez GA, Kelly JJ., Jr Polyneuropathy associated with monoclonal gammopathy of undetermined significance: further evidence that IgM-MGUS neuropathies are different than IgG-MGUS. Neurology. 1993;43(7):1304–1308. doi: 10.1212/wnl.43.7.1304. [DOI] [PubMed] [Google Scholar]
- 34.Kyle RA, San-Miguel JF, Mateos MV, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Hematol Oncol Clin North Am. 2014;28(5):775–790. doi: 10.1016/j.hoc.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Mauermann ML, Sorenson EJ, Dispenzieri A, Mandrekar J, Suarez GA, Dyck PJ. Uniform demyelination and more severe axonal loss distinguish POEMS syndrome from CIDP. J Neurol Neurosurg Psych. 2012;83(5):480–486. doi: 10.1136/jnnp-2011-301472. [DOI] [PubMed] [Google Scholar]
- 36.Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psych. 2012;83(5):476–479. doi: 10.1136/jnnp-2011-301706. [DOI] [PubMed] [Google Scholar]
- 37.Nobile-Orazio E, Terenghi F, Giannotta C, Gallia F, Nozza A. Serum VEGF levels in POEMS syndrome and in immune-mediated neuropathies. Neurology. 2009;72(11):1024–1026. doi: 10.1212/01.wnl.0000344569.13496.ff. [DOI] [PubMed] [Google Scholar]
- 38.Karam C, Klein CJ, Dispenzieri A, et al. Polyneuropathy improvement following autologous stem cell transplantation for POEMS syndrome. Neurology. 2015;84(19):1981–1987. doi: 10.1212/WNL.0000000000001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vital C, Vital A, Bouillot-Eimer S, Brechenmacher C, Ferrer X, Lagueny A. Amyloid neuropathy: a retrospective study of 35 peripheral nerve biopsies. J Periph Nerve Sys: JPNS. 2004;9(4):232–241. doi: 10.1111/j.1085-9489.2004.09405.x. [DOI] [PubMed] [Google Scholar]
- 40.Niermeijer JM, Fischer K, Eurelings M, Franssen H, Wokke JH, Notermans NC. Prognosis of polyneuropathy due to IgM monoclonal gammopathy: a prospective cohort study. Neurology. 2010;74(5):406–412. doi: 10.1212/WNL.0b013e3181ccc6b9. [DOI] [PubMed] [Google Scholar]
- 41.Nobile-Orazio E, Meucci N, Baldini L, Di Troia A, Scarlato G. Long-term prognosis of neuropathy associated with anti-MAG IgM M-proteins and its relationship to immune therapies. Brain. 2000;123(Pt 4):710–717. doi: 10.1093/brain/123.4.710. [DOI] [PubMed] [Google Scholar]
- 42.Rajkumar SV, Kyle RA, Suarez GA, Dispenzieri A. Hematologic malignancies: multiple myeloma and related plasma cell disorders. Berlin; New York: Springer; 2003. Neuropathy Associated With Plasma Cell Proliferative Disorders; p. x.p. 272. [Google Scholar]
- 43.Cook D, Dalakas M, Galdi A, Biondi D, Porter H. High-dose intravenous immunoglobulin in the treatment of demyelinating neuropathy associated with monoclonal gammopathy. Neurology. 1990;40(2):212–214. doi: 10.1212/wnl.40.2.212. [DOI] [PubMed] [Google Scholar]
- 44.Leger JM, Younes-Chennoufi AB, Chassande B, et al. Human immunoglobulin treatment of multifocal motor neuropathy and polyneuropathy associated with monoclonal gammopathy. J Neurol Neurosurg Psych. 1994;57(Suppl):46–49. doi: 10.1136/jnnp.57.suppl.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellie E, Vital A, Steck A, Boiron JM, Vital C, Julien J. Neuropathy associated with “benign” anti-myelin-associated glycoprotein IgM gammopathy: clinical, immunological, neurophysiological pathological findings and response to treatment in 33 cases. J Neurol. 1996;243(1):34–43. doi: 10.1007/BF00878529. [DOI] [PubMed] [Google Scholar]
- 46.Dalakas MC, Quarles RH, Farrer RG, et al. A controlled study of intravenous immunoglobulin in demyelinating neuropathy with IgM gammopathy. Ann Neurol. 1996;40(5):792–795. doi: 10.1002/ana.410400516. [DOI] [PubMed] [Google Scholar]
- 47.Mariette X, Chastang C, Clavelou P, Louboutin JP, Leger JM, Brouet JC. A randomised clinical trial comparing interferon-alpha and intravenous immunoglobulin in polyneuropathy associated with monoclonal IgM. The IgM-associated Polyneuropathy Study Group. J Neurol Neurosurg Psych. 1997;63(1):28–34. doi: 10.1136/jnnp.63.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariette X, Brouet JC, Chevret S, et al. A randomised double blind trial versus placebo does not confirm the benefit of alpha-interferon in polyneuropathy associated with monoclonal IgM. J Neurol Neurosurg Psych. 2000;69(2):279–280. doi: 10.1136/jnnp.69.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Comi G, Roveri L, Swan A, et al. A randomised controlled trial of intravenous immunoglobulin in IgM paraprotein associated demyelinating neuropathy. J Neurol. 2002;249(10):1370–1377. doi: 10.1007/s00415-002-0808-z. [DOI] [PubMed] [Google Scholar]
- 50.Gorson KC, Ropper AH, Weinberg DH, Weinstein R. Efficacy of intravenous immunoglobulin in patients with IgG monoclonal gammopathy and polyneuropathy. Arch Neurol. 2002;59(5):766–772. doi: 10.1001/archneur.59.5.766. [DOI] [PubMed] [Google Scholar]
- 51.Fineman SM, McKendall RR. Plasma exchange: a treatment for neuropathy associated with IgG-kappa gammopathy. J Neurol. 1990;237(2):85–87. doi: 10.1007/BF00314667. [DOI] [PubMed] [Google Scholar]
- 52.Frayne J, Stark RJ. Peripheral neuropathy with gammopathy responding to plasmapheresis. Clin Exp Neurol. 1985;21:195–200. [PubMed] [Google Scholar]
- 53.Oksenhendler E, Chevret S, Leger JM, Louboutin JP, Bussel A, Brouet JC. Plasma exchange and chlorambucil in polyneuropathy associated with monoclonal IgM gammopathy. IgM-associated Polyneuropathy Study Group. J Neurol Neurosurg Psych. 1995;59(3):243–247. doi: 10.1136/jnnp.59.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson HC, Lunn MP, Schey S, Hughes RA. Successful treatment of IgM paraproteinaemic neuropathy with fludarabine. J Neurol Neurosurg Psych. 1999;66(5):575–580. doi: 10.1136/jnnp.66.5.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dalakas MC, Rakocevic G, Salajegheh M, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein antibody demyelinating neuropathy. Ann Neurol. 2009;65(3):286–293. doi: 10.1002/ana.21577. [DOI] [PubMed] [Google Scholar]
- 56.Kodaira M, Yamamoto K. Rituximab Improves Subclinical Temporal Dispersion of Distal Compound Muscle Action Potential in Anti-MAG/SGPG Neuropathy Associated with Waldenstrom Macroglobulinemia: A Case Report. Case Rep Neurol. 2013;5(1):34–39. doi: 10.1159/000348395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leger JM, Viala K, Nicolas G, et al. Placebo-controlled trial of rituximab in IgM anti-myelin-associated glycoprotein neuropathy. Neurology. 2013;80(24):2217–2225. doi: 10.1212/WNL.0b013e318296e92b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goldfarb AR, Weimer LH, Brannagan TH., 3rd Rituximab treatment of an IgM monoclonal autonomic and sensory neuropathy. Muscle & nerve. 2005;31(4):510–515. doi: 10.1002/mus.20244. [DOI] [PubMed] [Google Scholar]
- 59.Gironi M, Saresella M, Ceresa L, et al. Clinical and immunological worsening in a patient affected with Waldenstrom macroglobulinemia and anti-mag neuropathy after treatment with rituximab. Haematologica. 2006;91(6 Suppl):ECR17. [PubMed] [Google Scholar]
- 60.Noronha V, Fynan TM, Duffy T. Flare in neuropathy following rituximab therapy for Waldenstrom’s macroglobulinemia. J Clin Oncol. 2006;24(1):e3. doi: 10.1200/JCO.2005.04.6474. [DOI] [PubMed] [Google Scholar]
- 61.Benedetti L, Briani C, Franciotta D, et al. Long-term effect of rituximab in anti-mag polyneuropathy. Neurology. 2008;71(21):1742–1744. doi: 10.1212/01.wnl.0000335268.70325.33. [DOI] [PubMed] [Google Scholar]
- 62.Levine TD, Pestronk A. IgM antibody-related polyneuropathies: B-cell depletion chemotherapy using Rituximab. Neurology. 1999;52(8):1701–1704. doi: 10.1212/wnl.52.8.1701. [DOI] [PubMed] [Google Scholar]
- 63.Pestronk A, Florence J, Miller T, Choksi R, Al-Lozi MT, Levine TD. Treatment of IgM antibody associated polyneuropathies using rituximab. J Neurol Neurosurg Psych. 2003;74(4):485–489. doi: 10.1136/jnnp.74.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]