Abstract
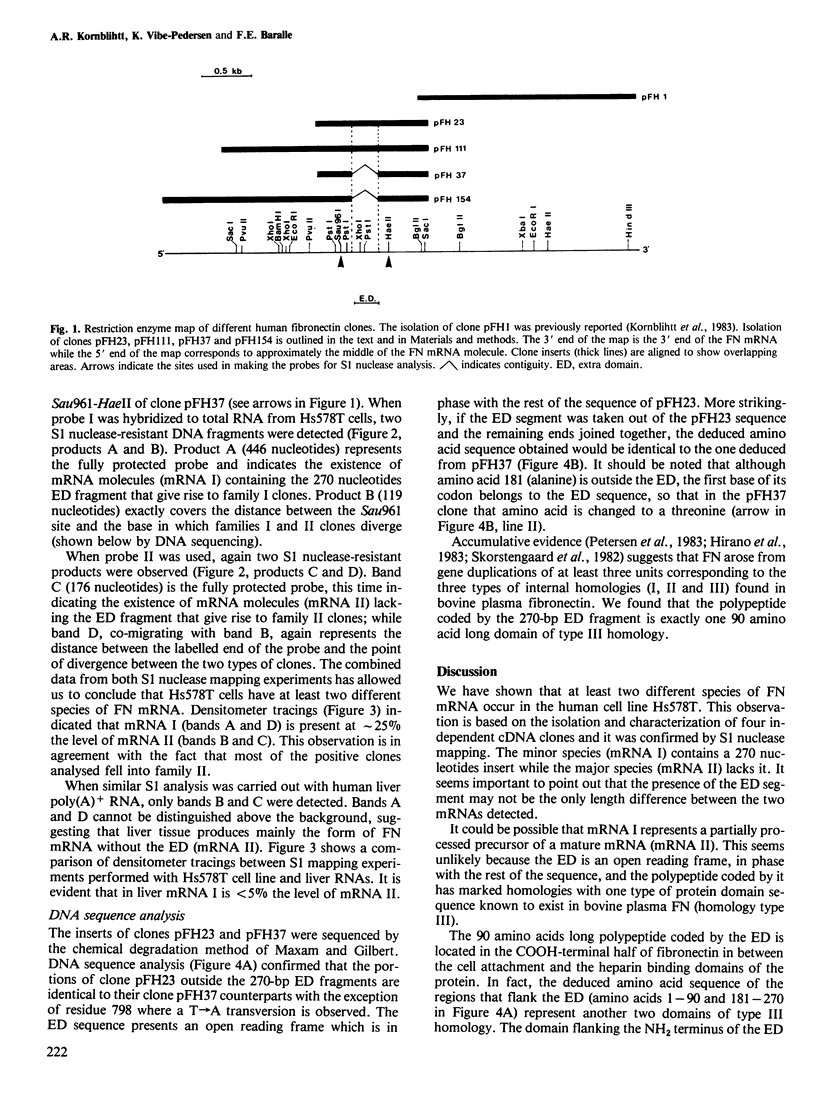
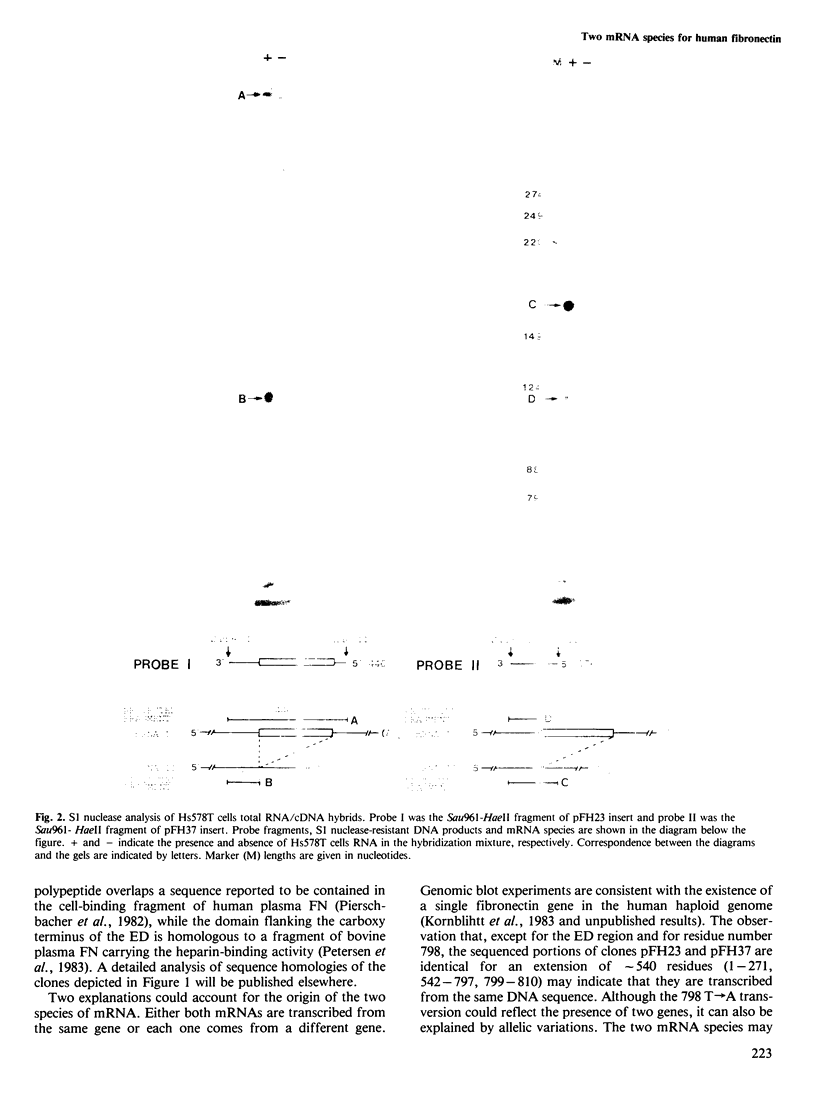
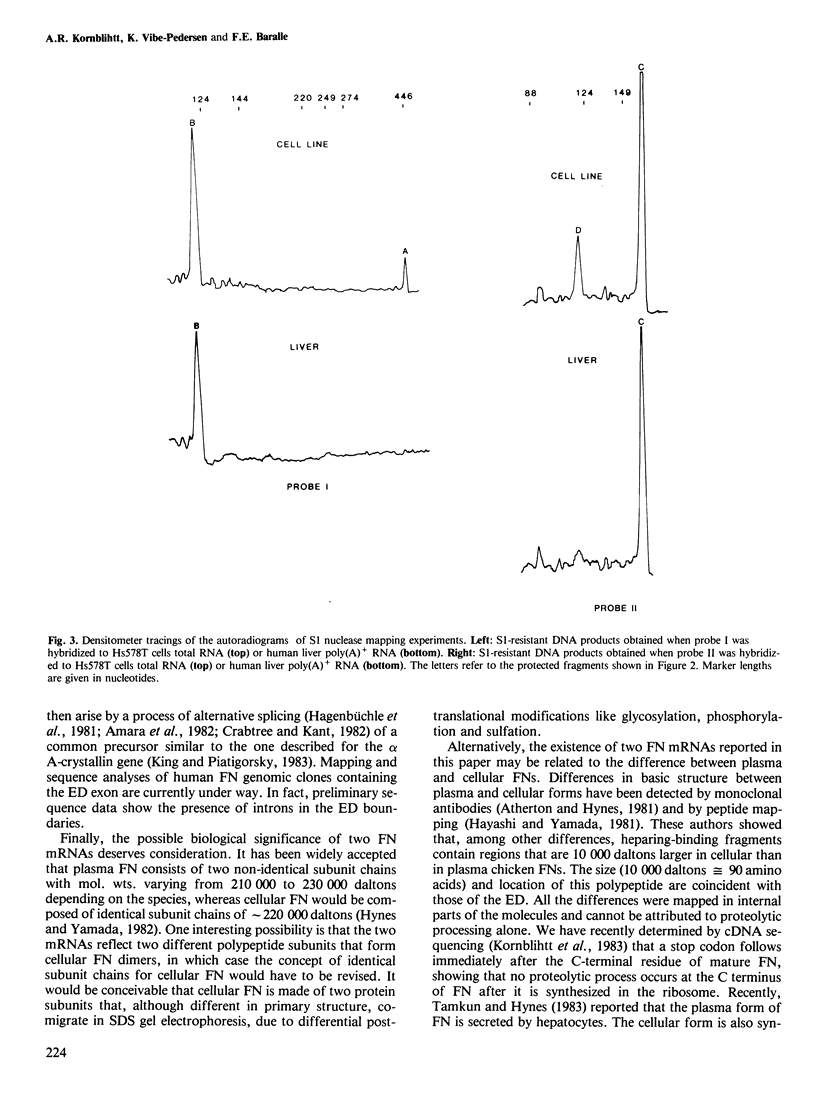
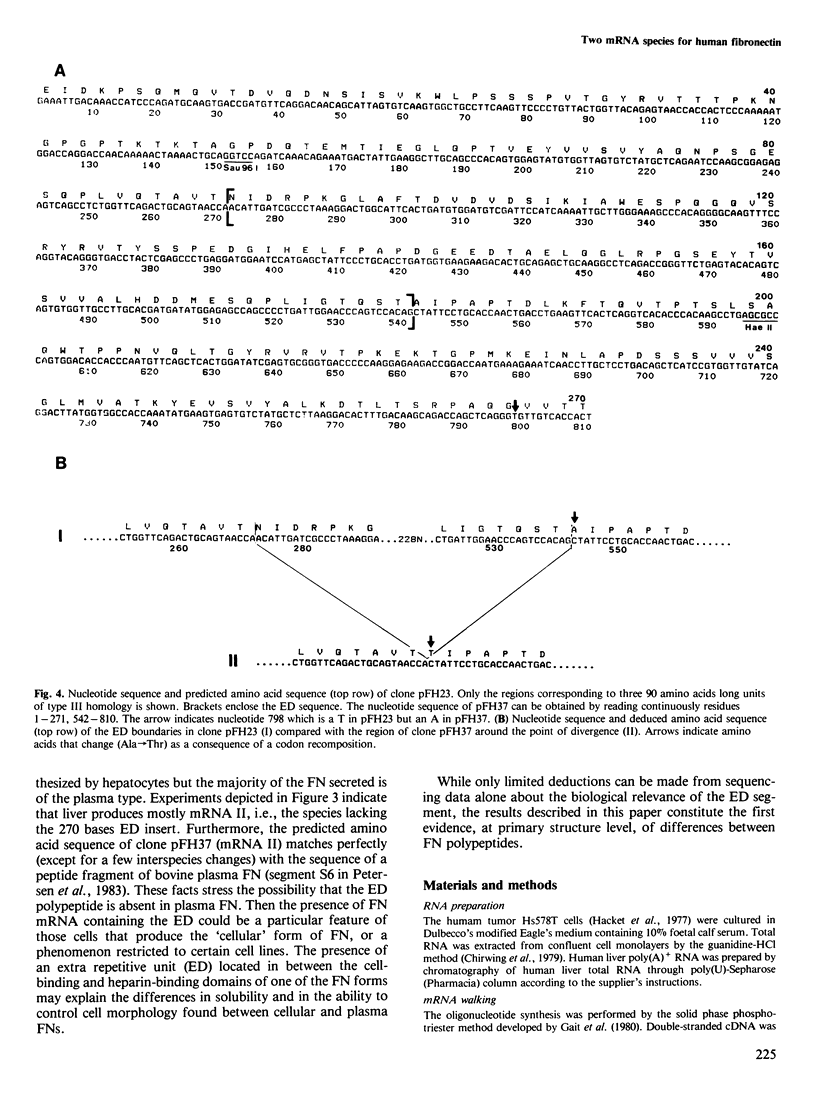
Two different fibronectin (FN) mRNA species were detected in the human cell line Hs578T. One species, mRNA I, contains an additional 270 nucleotide long insert (ED) that encodes exactly one of the internally repeated structural domains of the protein. The 90 amino acid extra domain belongs to the so-called type III homology and it is located in the carboxy-terminal half of FN, in between the cell attachment and the heparin binding sites of the protein. The evidence of two mRNAs is provided by the isolation and characterisation of four independent cDNA clones from a library prepared with a synthetic oligonucleotide primer, and it was confirmed by S1 nuclease analysis of cDNA/mRNA hybrids. This kind of analysis also showed that in the human cell line, mRNA I is present at a lower level than mRNA II (the mRNA species without the ED), whilst in human liver, mRNA I is virtually undetectable. Since liver tissue has recently been reported to be the source of plasma FN, our results indicate that the presence of the ED insert could be a particular feature of cellular FN.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Atherton B. T., Hynes R. O. A difference between plasma and cellular fibronectins located with monoclonal antibodies. Cell. 1981 Jul;25(1):133–141. doi: 10.1016/0092-8674(81)90237-3. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gait M. J., Singh M., Sheppard R. C., Edge M. D., Greene A. R., Heathcliffe G. R., Atkinson T. C., Newton C. R., Markham A. F. Rapid synthesis of oligodeoxyribonucleotides. IV. Improved solid phase synthesis of oligodeoxyribonucleotides through phosphotriester intermediates. Nucleic Acids Res. 1980 Mar 11;8(5):1081–1096. doi: 10.1093/nar/8.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergen J. P., Stern R. H., Wensink P. C. Filter replicas and permanent collections of recombinant DNA plasmids. Nucleic Acids Res. 1979 Dec 20;7(8):2115–2136. doi: 10.1093/nar/7.8.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett A. J., Smith H. S., Springer E. L., Owens R. B., Nelson-Rees W. A., Riggs J. L., Gardner M. B. Two syngeneic cell lines from human breast tissue: the aneuploid mammary epithelial (Hs578T) and the diploid myoepithelial (Hs578Bst) cell lines. J Natl Cancer Inst. 1977 Jun;58(6):1795–1806. doi: 10.1093/jnci/58.6.1795. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Tosi M., Schibler U., Bovey R., Wellauer P. K., Young R. A. Mouse liver and salivary gland alpha-amylase mRNAs differ only in 5' non-translated sequences. Nature. 1981 Feb 19;289(5799):643–646. doi: 10.1038/289643a0. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Yamada K. M. Differences in domain structures between plasma and cellular fibronectins. J Biol Chem. 1981 Nov 10;256(21):11292–11300. [PubMed] [Google Scholar]
- Hirano H., Yamada Y., Sullivan M., de Crombrugghe B., Pastan I., Yamada K. M. Isolation of genomic DNA clones spanning the entire fibronectin gene. Proc Natl Acad Sci U S A. 1983 Jan;80(1):46–50. doi: 10.1073/pnas.80.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. Extensive disulfide bonding at the mammalian cell surface. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2855–2859. doi: 10.1073/pnas.74.7.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O., Yamada K. M. Fibronectins: multifunctional modular glycoproteins. J Cell Biol. 1982 Nov;95(2 Pt 1):369–377. doi: 10.1083/jcb.95.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Isolation and characterization of cDNA clones for human and bovine fibronectins. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3218–3222. doi: 10.1073/pnas.80.11.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Petersen T. E., Thøgersen H. C., Skorstengaard K., Vibe-Pedersen K., Sahl P., Sottrup-Jensen L., Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci U S A. 1983 Jan;80(1):137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E., Sundelin J., Lind P., Peterson P. A. The cell attachment domain of fibronectin. Determination of the primary structure. J Biol Chem. 1982 Aug 25;257(16):9593–9597. [PubMed] [Google Scholar]
- Shoulders C. C., Baralle F. E. Isolation of the human HDL apoprotein A1 gene. Nucleic Acids Res. 1982 Aug 25;10(16):4873–4882. doi: 10.1093/nar/10.16.4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorstengaard K., Thøgersen H. C., Vibe-Pedersen K., Petersen T. E., Magnusson S. Purification of twelve cyanogen bromide fragments from bovine plasma fibronectin and the amino acid sequence of eight of them. Overlap evidence aligning two plasmic fragments, internal homology in gelatin-binding region and phosphorylation site near C terminus. Eur J Biochem. 1982 Nov 15;128(2-3):605–623. doi: 10.1111/j.1432-1033.1982.tb07007.x. [DOI] [PubMed] [Google Scholar]
- Tamkun J. W., Hynes R. O. Plasma fibronectin is synthesized and secreted by hepatocytes. J Biol Chem. 1983 Apr 10;258(7):4641–4647. [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W. Fibroblast cellular and plasma fibronectins are similar but not identical. J Cell Biol. 1979 Feb;80(2):492–498. doi: 10.1083/jcb.80.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]




