Abstract
Astrocytes are morphologically complex cells that perform a wide variety of critical functions in the brain. As a structurally and functionally integrated component of the synapse, astrocytes secrete proteins, lipids, and small molecules that bind neuronal receptors to promote synaptogenesis and regulate synaptic connectivity. Additionally, astrocytes are key players in circuit formation, instructing the formation of synapses between distinct classes of neurons. This review highlights recent publications on the topic of astrocyte-mediated synaptogenesis, with a focus on the molecular mechanisms through which astrocytes orchestrate the formation of synaptic circuits.
Introduction/Overview
Synapses are the building blocks of neuronal networks in the brain. A synapse is traditionally defined as a specialized cell-cell adhesion between the presynaptic axon of one neuron and the postsynaptic dendrite of another neuron. During synaptogenesis, the presynaptic terminal accumulates vesicles, and the postsynaptic side recruits neurotransmitter receptors. Synaptic cell adhesion molecules facilitate this process by mechanically stabilizing synaptic contacts, organizing presynaptic release machinery and postsynaptic scaffolding proteins, and employing downstream signaling molecules that interact with the cytoskeleton[1]. In the mammalian brain, synapse formation is an intricately regulated developmental process that occurs simultaneously in numerous brain regions and between many different types of neurons. A single neuron receives thousands of synaptic inputs. Neuronal activity, sensory experience, extrinsic cues, and intrinsic signaling pathways all help to shape and define synaptic connections, thus establishing the complex circuitry of the brain.
Numerous studies over the past 20 years have revealed that astrocytes of the gray matter (aka protoplasmic astrocytes) are integral components of synapses that dynamically participate in the control of synapse formation and function[2]. Astrocytes are morphologically complex cells with extensively branched processes terminating in fine structures, called perisynaptic astrocytic processes (PAPs), that structurally and functionally interact with synapses[3]. A single astrocyte can ensheathe over 100,000 synapses in the mouse, and over one million synapses in the human brain[4]. Through their intimate association with synapses, astrocytes regulate key aspects of synapse formation, maturation, and function. In this review, we will focus on recent findings on the topic of astrocyte-induced synaptogenesis, since the previous Current Opinion article[5]. Collectively, these new studies provide a framework for how astrocytes, through a number of distinct molecular pathways, promote formation of synaptic circuits.
Astrocyte-secreted factors induce synaptogenesis and specify circuit formation
The synaptogenic role of astrocytes was initially discovered using a purified retinal ganglion cell (RGC) culture system. RGC neurons grown in the absence of astroglia form very few synapses. However, synapse formation is greatly enhanced upon addition of astrocyte-conditioned media (ACM)[6]. This seminal finding, and the cell culture system that was used to reveal this phenomenon, led to the identification of a number of astrocyte-secreted factors, including proteins, lipids, and small molecules that control different aspects of excitatory synapse formation (Box 1). Furthermore, several recent studies have uncovered distinct molecular mechanisms employed by astrocyte-secreted factors to induce synapse formation. Interestingly, some of these studies highlight a role for astrocytes in directing the formation of specific types of synaptic connections to build different circuits. Below we will highlight some of these molecular mechanisms.
Box 1.
Astrocyte-expressed molecules that regulate synapse formation
| Molecule | Neuronal Receptor1 |
Synapse type | Finding | References |
|---|---|---|---|---|
| Thrombospondin | α2δ-1 | Excitatory | Induces formation of silent structural synapses | [7,8,9] |
| Hevin | NL1B, NRX1α | Excitatory thalamocortical | Bridges NL1B and NRX1α to stabilize thalamocortical excitatory synapses | [13,14,15] |
| SPARC | Unknown | Excitatory | Antagonizes Hevin-induced synaptogenesis | [13] |
| TGF-β1 | TGFBRs? (not tested) | Excitatory and Inhibitory | Induces excitatory and inhibitory synapse formation | [22,23] |
| D-Serine | NMDAR | Excitatory | Promotes synapse formation in adult born neurons | [25] |
| Glypican 4 and 6 | Unknown | Excitatory | Induces excitatory synapse formation; increases synaptic levels of GluA1 AMPARs | [26] |
| Sema3A | Npn1/Plexin A | Excitatory and inhibitory motor neuron inputs | Positional cue required for proper establishment of motor neuron and sensory neuron circuit formation | [36] |
| γ-Protocadherin | γ-protocadherin | Excitatory and Inhibitory | Promotes excitatory and inhibitory synaptogenesis via direct contact with neurons | [29] |
| EphrinA3 | EphA4 | Excitatory | Promotes normal dendritic spine morphology via direct contact with neurons | [30,31] |
| Cholesterol/ApoE | Unknown | Excitatory | Promotes excitatory synapse formation | [28] |
| BDNF | TrkB | Excitatory | Promotes excitatory synapse formation | [27] |
Here we list the neuronal receptor(s) required for the described synaptogenic function. While receptors for some of these factors are known, whether they participate in astrocyte-induced synaptogenesis is not known.
Control of excitatory synapse formation by thrombospondins
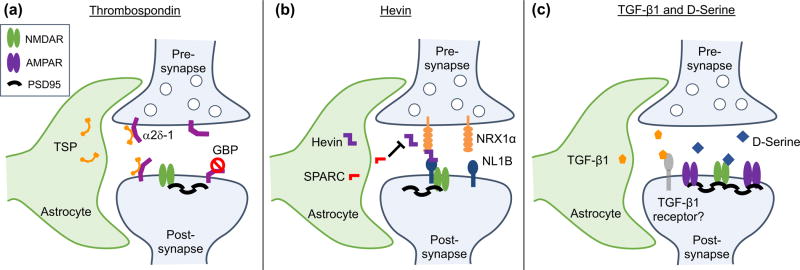
The thrombospondins (TSP), a family of 5 extracellular matrix proteins, were among the first astrocyte-secreted synaptogenic factors to be identified[7,8]. Addition of purified TSPs to cultured RGCs induces the formation of structurally mature, but functionally silent synapses. These silent structural synapses contain NMDA receptors, but lack AMPA receptors[7]. In the developing mouse brain, immature protoplasmic gray-matter astrocytes express TSP1 and TSP2 for the first two postnatal weeks. In agreement for an important role in triggering excitatory synapse formation in vivo, TSP1/2 knockout (KO) mice, display fewer excitatory synapses in the cortex[7]. TSPs induce synaptogenesis by binding to their neuronal receptor, the calcium channel subunit α2δ-1 (Cacna2d-1)[8]. The synaptogenic activity of TSP is mediated through its EGF-like domains, which bind to the von Willebrand Factor A (VWF-A) domain of α2δ-1[8]. This interaction is thought to cause a conformational change in α2δ-1, allowing for the recruitment and assembly of a putative synaptogenic signaling complex[9](Figure 1A). Interestingly, α2δ-1 is also a receptor for Gabapentin, a drug used to treat epilepsy and neuropathic pain. Gabapentin strongly inhibits excitatory synapse formation in vitro and in vivo by preventing TSP from binding α2δ-1, thus blocking TSP-induced synaptogenesis[8]. This finding highlights not only the important role of astrocyte-secreted TSP in synapse formation, but also suggests that astrocyte dysfunction may contribute to neuropathologies such as epilepsy and neuropathic pain[10–12].
Figure 1.
Astrocyte-secreted factors induce excitatory synapse formation. (a) Astrocytes secrete thrombospondins (TSP) which bind to neuronal α2δ-1 to induce the formation of silent, structural synapses. The anti-epileptic drug Gabapentin (GBP) binds to α2δ-1, preventing TSP-induced synaptogenesis. (b) Astrocyte-secreted Hevin/SPARCL1 promotes synapse formation through its interactions with presynaptic NRX1α and postsynaptic NL1B, two proteins that do not normally interact. Astrocyte-secreted SPARC antagonizes Hevin-induced synapse formation through an unknown mechanism. (c) Astrocyte-secreted TGF-β1 promotes the formation of excitatory synapses through a mechanisms that requires NMDA receptor activity, along with the NMDA receptor agonist D-serine.
Circuit specific control of excitatory synapse maturation and plasticity by astrocytic Hevin/SPARCL1
Besides TSPs, two members of the secreted protein acidic, enriched in cysteine (SPARC) family proteins, Hevin (also known as SPARC-like 1/SPARCL1) and SPARC were identified as astrocyte-secreted factors that control synapse formation between cultured RGCs. Similar to TSPs, treatment of RGCs with purified Hevin is sufficient to induce formation of ultrastructurally mature, but postsynaptically silent synapses. Conversely, SPARC is not synaptogenic, and antagonizes Hevin-induced synaptogenesis[13]. In the developing mouse brain, Hevin and SPARC are expressed at high levels in astrocytes during the second and third weeks, coinciding with periods of heightened synapse stabilization and synaptic refinement. In adulthood, Hevin expression remains high, while SPARC expression is significantly reduced[13]. Analyses of developing visual cortices of Hevin-null mice revealed that Hevin is required for proper thalamocortical synaptic connectivity[14]. Hevin KO mice have significantly fewer thalamocortical synapses, while the number of intracortical synapses is significantly increased. Furthermore, ultrastructural analysis revealed that Hevin KO spines and dendrites are structurally immature[14].
Recently, significant progress was made in identifying the molecular mechanism of Hevin-induced synapse formation. Hevin regulates formation of thalamocortical glutamatergic synapses by bridging presynaptic neurexin-1alpha (NRX1α) with postsynaptic neuroligin-1B (NL1B)[15], two neuronal cell adhesion molecules that do not interact with each other (Figure 1B). The region of Hevin between amino acids 351–440 interacts with the extracellular domains of NRX1α and NL1B, and is required for Hevin’s synaptogenic activity. Hevin recruits NL1 and NL1-associated proteins, including PSD95 and NMDA receptor subunits, to synapses. Neurons express several isoforms of presynaptic neurexins and postsynaptic neuroligins. The interaction code of these different isoforms is thought to instruct the formation of a diverse array of synaptic connections[16]. By bridging the connection between two incompatible isoforms, Hevin acts as a synaptic linker protein, modifying the neurexin/neuroligin isoform code to induce thalamocortical synapse formation. Interestingly, mutations in Hevin, neurexins, and neuroligins are strongly associated with Autism Spectrum Disorder, suggesting that these molecules play critical roles in proper brain development[17,18].
Hevin and SPARC have similar domain structures, with an N-terminal acidic domain and C-terminal follistatin-like and SPARC-like domains. They share 60% homology in their C-terminal domains, but differ substantially in their N-terminal domains. The mechanism through which SPARC antagonizes Hevin-induced synaptogenesis is unknown. Hevin and SPARC do not interact with one another[13], but given their C-terminal homology, SPARC may antagonize Hevin function by binding and sequestering common interacting partners. Interestingly, SPARC expression is negatively regulated by enhanced excitatory synaptic activity in astrocyte-neuron co-cultures in vitro. In addition, SPARC negatively regulates AMPAR recruitment to synapses, potentially via interactions with integrins[19]. Furthermore, SPARC was shown to inhibit maturation of cholinergic presynaptic terminals[20] and induce a cell autonomous program of synapse elimination via collapse of presynaptic terminals[21]. The molecular mechanisms through which SPARC exerts these inhibitory actions in synapse formation remains to be elucidated.
TGF-beta promotes formation of excitatory and inhibitory synapse formation
Transforming growth factor beta-1 (TGF-β1) is a secreted protein belonging to the TGFβ superfamily of cytokines, which participate in a plethora of developmental signaling pathways. While studies of astrocyte-secreted synaptogenic factors have mainly identified factors that promote excitatory synapse formation, TGF-β1 application promotes formation of both excitatory and inhibitory synapses. In cultured mouse cortical neurons, addition of TGF-β1 significantly enhances the formation of structural and functional excitatory and inhibitory synapses[22,23]. In vivo, TGF-β1 overexpressing mice display increased levels of AMPA and NMDA receptor subunits in the hippocampus[24]. TGF-β1-induced excitatory synaptogenesis requires NMDA receptor activity and the NMDA co-activator D-serine (Figure 1C). D-serine treatment is sufficient to mimic TGF-β1-induced synaptogenesis, and requires the function of serine racemase, the enzyme which converts L-serine to D-serine[22]. Interestingly, D-serine administration also induces synapse formation in adult-born neurons in vivo[25].
TGF-β1 requires NMDA receptor activity to induce inhibitory synapse formation, but employs distinct signaling mechanisms from excitatory synaptogenesis. TGF-β1 increases phosphorylation of CAMKII, a major downstream signaling component of NMDA receptors, at Thr286, a key site for inhibitory synapse regulation. Accordingly, blocking CAMKII activity prevents TGF-β1-induced inhibitory synapse formation, but has no effect on formation of excitatory synapses. TGF-β1 also increases neuroligin 2 (NL2) expression and gephyrin/NL2 clustering[23], two important components of inhibitory postsynapses. Injection of TGF-β1 into mice increases inhibitory synapse formation in vivo. Collectively, these studies demonstrate that TGF-β1 is capable of inducing both excitatory and inhibitory synapse formation, yet whether TGF-β1 is required for synapse formation in vivo under physiological conditions remains to be determined.
In addition to the factors we highlighted above, several other astrocyte-secreted synaptogenic factors have been identified, including glypican (Gpc) family members Gpc4 and Gpc6[26], Brain-Derived Neurotrophic Factor (BDNF)[27], and cholesterol with apolipoprotein-E[28]. Furthermore, astrocyte-neuron contact may also play roles in synaptogenesis. Along these lines homotypic interactions between gammaprotocadherins and heterotypic interactions between Ephrin A3/EphA were shown to be important for synapse formation [29–31] (See Box 1 for more details).
Astrocyte heterogeneity underlies neural circuit formation
Thus far, we have discussed astrocytes as a homogenous population. However, increasing evidence demonstrates that astrocytes comprise a heterogeneous population of cells with distinct molecular properties and functions[32–34]. Astrocyte heterogeneity occurs not only between brain regions, but may also occur within the same region. While our understanding of astrocyte heterogeneity is in its infancy, a number of recent studies describe how different types of astrocytes differentially instruct synapse formation and neural circuit formation.
Developmentally-encoded astrocyte domains regulate circuit formation in the spinal cord
During development of the mouse spinal cord, astrocytes are allocated to specific domains based on their site of origin in the ventricular zone. Depletion of astrocytes from one domain causes neuronal and synaptic defects that cannot be rescued by astrocytes from neighboring domains[35]. This phenomenon is accomplished via different positional cues expressed by regionally-distinct astrocytes. Gene expression analysis of mouse spinal cord astrocytes identified 38 genes that are differentially expressed between dorsal and ventral astrocytes[36]. Interestingly, many of these genes encode proteins with previously defined roles in positional guidance during brain development.
Semaphorin 3a (Sema3a) expression is highly enriched in ventral spinal cord astrocytes. Sema3a is an extracellular matrix protein that signals through a PlexinA/neuropilin 1 receptor (Nrp1) complex[37]. In the spinal cord, α-Motor neurons (α-MNs) and TrkA+ sensory neurons express high levels of Nrp1. A combination of in vivo and in vitro experiments revealed that astrocyte-secreted Sema3a is required for α-MN survival, circuit integration, and function. Knockdown of Sema3A in astrocytes decreases excitatory inputs and increases inhibitory inputs onto α-MNs[36]. Together, these findings identify a role for astrocytes in positional regulation of circuit formation. Furthermore, they suggest that proper astrocyte development and regional allocation is a prerequisite for establishing and maintaining the complex circuitry of the central nervous system.
In addition to the spinal cord, a recent study examined astrocyte heterogeneity in different brain regions, including the cortex, thalamus, cerebellum, olfactory bulb, and brain stem[38]. This study used a cell sorting approach to identify five different populations of astrocytes in the brain based on their expression of different cell surface antigens. These different populations of astrocytes (named population A, B, C, D, and E) have unique gene signatures and different functional properties. For example, population C astrocytes, which account nearly 50% of all astrocytes in the cortex, cerebellum, and brainstem, are strongly enriched for genes associated with synaptogenesis and synaptic function. Accordingly, population C astrocytes appear more synaptogenic than other populations of astrocytes when cultured with neurons in vitro[38]. Additional studies are needed to determine how these different populations of astrocytes control the formation of specific neural circuits in vivo.
Altered synaptogenic potential in different types of reactive astrocytes
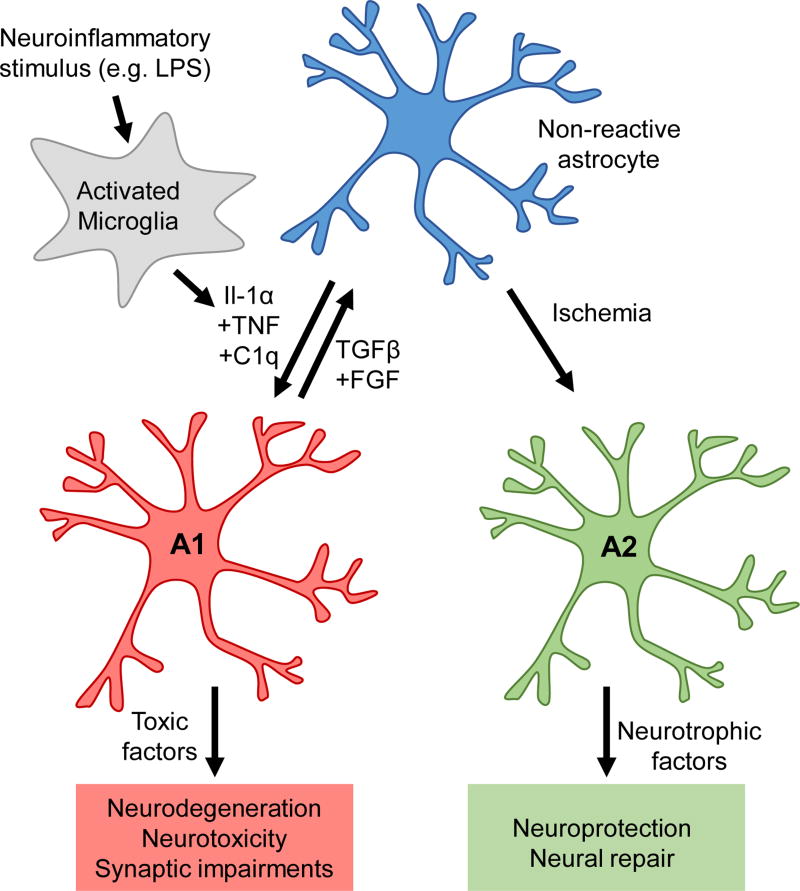
Astrocyte heterogeneity is present not only under normal physiological conditions, but also amongst reactive astrocytes[39]. Following injury or disease, astrocytes enter a reactive state characterized by changes in astrocyte morphology and gene expression. Numerous studies over the past three decades have found that reactive astrocytes are neurotoxic in some instances[40,41], yet neuroprotective in others[42–44], suggesting that different types of reactive astrocytes may serve different functions. Indeed, a recent study found that different types of stimuli induce distinct types of reactive astrocytes. For example, systemic injection of lipopolysaccharide (LPS) induces harmful A1 reactive astrocytes in mice, whereas ischemic insult induces neuroprotective A2 reactive astrocytes[45] (Figure 2).
Figure 2.
Heterogeneity amongst reactive astrocytes. Following insult or injury, astrocytes enter a reactive state, characterized by changes in astrocyte morphology and gene expression. Depending on the stimulus, astrocytes can become neurotoxic A1 type reactive astrocytes, or neuroprotective A2 type reactive astrocytes. Neuroinflammatory stimuli, such as LPS, yield A1 reactive astrocytes, by activating microglia to secrete the inflammatory cytokines Il-1α, TNF, and C1q. A1 reactive astrocytes promote neurodegeneration and neurotoxicity, and are not synaptogenic. Interestingly, application of TGFβ and FGF can revert type A1 reactive astrocytes to a non-reactive state in vitro. Ischemia induces the formation of A2 reactive astrocytes, through an unknown mechanism. These astrocytes have a different gene signature from A1 reactive astrocytes, and secrete neurotrophic factors to promote neuroprotection and neural repair.
How does astrocyte reactivity impact synaptogenesis? In the mouse cortex, activated microglia secrete several cytokines to induce A1 reactive astrocytes, including interleukin 1α (Il1-α), tumor necrosis factor (TNF), and complement component 1, subcomponent q (C1q)[46]. Adding these three microglia-secreted cytokines to cultures of purified primary cortical astrocytes is sufficient to produce A1 reactive astrocytes. Conditioned media from A1 reactive astrocyte cultures (A1 ACM) fails to induce synapse formation in cultured RGC neurons[46]. Interestingly, many astrocyte-secreted synaptogenic factors are expressed by A1 reactive astrocytes. While Hevin and Gpc6 mRNA levels are substantially decreased, and SPARC levels remain unchanged, levels of Gpc4 are increased nearly two-fold, and levels of TSP1 and TSP2 increase 7–8 fold[46]. That A1 astrocytes fail to induce synaptogenesis despite expressing such high levels of TSP1, TSP2, and Gpc4, implies that A1 reactive astrocytes secrete a toxic signal that adversely affects synapse formation.
In addition to having distinct responses to different types of stimuli, different groups of astrocytes may have distinct reactions to the same stimuli[45]. In cortical astrocytes, LPS induces an A1 reactive phenotype via microglia, but has no direct effect on astrocytes[46]. Conversely, a recent study found that hippocampal astrocytes in juvenile mice respond to LPS treatment via a TLR4/MyD88-dependent signaling pathway to activate Erk1/2 signaling and enhance excitatory synapse development[47]. These LPS-treated mice display an increased susceptibility to early-life seizures, which can be rescued by blocking Erk1/2 activation, suggesting a role for reactive gliosis in the pathogenesis of neurological disorders such as epilepsy. In addition to epilepsy, reactive astrocytes are observed in many other neurological disorders, including Huntington’s Disease, Alzheimer’s Disease, Parkinson’s Disease, and Multiple Sclerosis[46], where they may adversely affect synapse and neuronal health. For a more in-depth discussion of astrocyte-synapse interactions in brain disorders, we refer to recent review articles[48,49].
Conclusions and Future Directions
Here we have summarized a number of recent, high-impact studies detailing the molecular mechanisms of astrocyte-mediated synaptogenesis. Together, these studies demonstrate the critical role that astrocytes play in promoting the formation of synaptic circuits. However, there are still a number of important outstanding questions:
What mechanisms control astrocytic expression and secretion of synaptogenic factors?
How do astrocyte-synapse adhesions form, and what is their role in synaptogenesis?
Do astrocytes communicate with each other to coordinate synaptogenesis?
What astrocyte-intrinsic signaling mechanisms are required for astrocyte-induced synaptogenesis?
How does astrocyte-neuron cross-talk shape synapse formation during development
Going forward, new tools for studying astrocytes will be essential for answering these questions. Recently developed tools and resources, including RNA sequencing databases of mouse[50] and human[51] astrocytes, new astrocyte-specific Cre mouse lines[52], and methods for studying human astrocytes[53–55] are paving the way towards furthering our understanding of astrocyte biology. Given the implications for astrocyte dysfunction in human brain disorders, answering these questions will be of fundamental importance not only for deepening our understanding of the mechanisms guiding astrocyte-induced synaptogenesis, but also for developing therapeutic strategies to combat neurological disease.
Highlights.
Astrocytes secrete synaptogenic factors to induce synapse formation.
Astrocytes control formation of specific synapses to build different circuits.
Astrocyte heterogeneity underlies the formation of specific neural circuits.
A1 reactive astrocytes are neurotoxic and do not promote synapse formation.
Acknowledgments
KTB is supported by The Hartwell Foundation Postdoctoral Fellowship and The Foerster- Bernstein Postdoctoral Fellowship. Research in the Eroglu lab is supported by NIH/NIDA DA031833 and NIH/NINDS NS096352-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bukalo O, Dityatev A. Synaptic cell adhesion molecules. Adv Exp Med Biol. 2012;970:97–128. doi: 10.1007/978-3-7091-0932-8_5. [DOI] [PubMed] [Google Scholar]
- 2.Chung WS, Allen NJ, Eroglu C. Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015;7:a020370. doi: 10.1101/cshperspect.a020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardinelli Y, Randall J, Janett E, Nikonenko I, Konig S, Jones EV, Flores CE, Murai KK, Bochet CG, Holtmaat A, et al. Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr Biol. 2014;24:1679–1688. doi: 10.1016/j.cub.2014.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen NJ. Role of glia in developmental synapse formation. Curr Opin Neurobiol. 2013;23:1027–1033. doi: 10.1016/j.conb.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 7.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Risher WC, Eroglu C. Thrombospondins as key regulators of synaptogenesis in the central nervous system. Matrix Biol. 2012;31:170–177. doi: 10.1016/j.matbio.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park J, Yu YP, Zhou CY, Li KW, Wang D, Chang E, Kim DS, Vo B, Zhang X, Gong N, et al. Central Mechanisms Mediating Thrombospondin-4-induced Pain States. J Biol Chem. 2016;291:13335–13348. doi: 10.1074/jbc.M116.723478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DS, Li KW, Boroujerdi A, Peter Yu Y, Zhou CY, Deng P, Park J, Zhang X, Lee J, Corpe M, et al. Thrombospondin-4 contributes to spinal sensitization and neuropathic pain states. J Neurosci. 2012;32:8977–8987. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, Inada H, Roh SE, Kim SJ, Lee G, et al. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 2016;126:1983–1997. doi: 10.1172/JCI82859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc Natl Acad Sci U S A. 2011;108:E440–449. doi: 10.1073/pnas.1104977108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risher WC, Patel S, Kim IH, Uezu A, Bhagat S, Wilton DK, Pilaz LJ, Singh Alvarado J, Calhan OY, Silver DL, et al. Astrocytes refine cortical connectivity at dendritic spines. Elife. 2014;3 doi: 10.7554/eLife.04047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Singh SK, Stogsdill JA, Pulimood NS, Dingsdale H, Kim YH, Pilaz LJ, Kim IH, Manhaes AC, Rodrigues WS, Jr, Pamukcu A, et al. Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1alpha and NL1 via Hevin. Cell. 2016;164:183–196. doi: 10.1016/j.cell.2015.11.034. This paper identifies the molecular mechanism of Hevin-induced synapse formation. Astrocyte-secreted Hevin bridges presynaptic Nrxn1α and postsynaptic NL1B to control the formation of thalamocortical synapses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Iossifov I, O'Roak BJ, Sanders SJ, Ronemus M, Krumm N, Levy D, Stessman HA, Witherspoon KT, Vives L, Patterson KE, et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature. 2014;515:209–215. doi: 10.1038/nature13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones EV, Bernardinelli Y, Tse YC, Chierzi S, Wong TP, Murai KK. Astrocytes control glutamate receptor levels at developing synapses through SPARC-beta-integrin interactions. J Neurosci. 2011;31:4154–4165. doi: 10.1523/JNEUROSCI.4757-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albrecht D, Lopez-Murcia FJ, Perez-Gonzalez AP, Lichtner G, Solsona C, Llobet A. SPARC prevents maturation of cholinergic presynaptic terminals. Mol Cell Neurosci. 2012;49:364–374. doi: 10.1016/j.mcn.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Murcia FJ, Terni B, Llobet A. SPARC triggers a cell-autonomous program of synapse elimination. Proc Natl Acad Sci U S A. 2015;112:13366–13371. doi: 10.1073/pnas.1512202112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diniz LP, Almeida JC, Tortelli V, Vargas Lopes C, Setti-Perdigao P, Stipursky J, Kahn SA, Romao LF, de Miranda J, Alves-Leon SV, et al. Astrocyte-induced synaptogenesis is mediated by transforming growth factor beta signaling through modulation of D-serine levels in cerebral cortex neurons. J Biol Chem. 2012;287:41432–41445. doi: 10.1074/jbc.M112.380824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diniz LP, Tortelli V, Garcia MN, Araujo AP, Melo HM, Silva GS, Felice FG, Alves-Leon SV, Souza JM, Romao LF, et al. Astrocyte transforming growth factor beta 1 promotes inhibitory synapse formation via CaM kinase II signaling. Glia. 2014;62:1917–1931. doi: 10.1002/glia.22713. [DOI] [PubMed] [Google Scholar]
- 24.Bae JJ, Xiang YY, Martinez-Canabal A, Frankland PW, Yang BB, Lu WY. Increased transforming growth factor-beta1 modulates glutamate receptor expression in the hippocampus. Int J Physiol Pathophysiol Pharmacol. 2011;3:9–20. [PMC free article] [PubMed] [Google Scholar]
- 25.Sultan S, Li L, Moss J, Petrelli F, Casse F, Gebara E, Lopatar J, Pfrieger FW, Bezzi P, Bischofberger J, et al. Synaptic Integration of Adult-Born Hippocampal Neurons Is Locally Controlled by Astrocytes. Neuron. 2015;88:957–972. doi: 10.1016/j.neuron.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 26.Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, Barres BA. Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012;486:410–414. doi: 10.1038/nature11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Casati ME, Murtie JC, Rio C, Stankovic K, Liberman MC, Corfas G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc Natl Acad Sci U S A. 2010;107:17005–17010. doi: 10.1073/pnas.1008938107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 29.Garrett AM, Weiner JA. Control of CNS synapse development by {gamma}-protocadherin-mediated astrocyte-neuron contact. J Neurosci. 2009;29:11723–11731. doi: 10.1523/JNEUROSCI.2818-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida H, Okabe S. Direct astrocytic contacts regulate local maturation of dendritic spines. J Neurosci. 2007;27:331–340. doi: 10.1523/JNEUROSCI.4466-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci U S A. 2009;106:12524–12529. doi: 10.1073/pnas.0903328106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khakh BS, Sofroniew MV. Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci. 2015;18:942–952. doi: 10.1038/nn.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haim LB, Rowitch DH. Functional diversity of astrocytes in neural circuit regulation. Nat Rev Neurosci. 2017;18:31–41. doi: 10.1038/nrn.2016.159. [DOI] [PubMed] [Google Scholar]
- 34.Buosi AS, Matias I, Araujo AP, Batista C, Gomes FC. Heterogeneity in Synaptogenic Profile of Astrocytes from Different Brain Regions. Mol Neurobiol. 2017 doi: 10.1007/s12035-016-0343-z. [DOI] [PubMed] [Google Scholar]
- 35.Tsai HH, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SP, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012;337:358–362. doi: 10.1126/science.1222381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Molofsky AV, Kelley KW, Tsai HH, Redmond SA, Chang SM, Madireddy L, Chan JR, Baranzini SE, Ullian EM, Rowitch DH. Astrocyte-encoded positional cues maintain sensorimotor circuit integrity. Nature. 2014;509:189–194. doi: 10.1038/nature13161. Here the authors describe a molecular mechanism underlying the positional regulation of circuit formation by ventral spinal cord astrocytes. These astrocytes secrete the positional cue Sema3A to controlα-MN survival, circuit integration, and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- *38.John Lin CC, Yu K, Hatcher A, Huang TW, Lee HK, Carlson J, Weston MC, Chen F, Zhang Y, Zhu W, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017 doi: 10.1038/nn.4493. This study uses flow sorting combined with surface antigen expression to identify five different populations of astrocytes in the brain. These populations have distinct gene signatures and different functional properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson MA, Ao Y, Sofroniew MV. Heterogeneity of reactive astrocytes. Neurosci Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer's disease. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Anderson MA, Burda JE, Ren Y, Ao Y, O'Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 2016;532:195–200. doi: 10.1038/nature17623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner DS, Liu C, Eroglu C, Kuo CT. Protective astrogenesis from the SVZ niche after injury is controlled by Notch modulator Thbs4. Nature. 2013;497:369–373. doi: 10.1038/nature12069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391–6410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **46.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017 doi: 10.1038/nature21029. Here the authors provide a molecular and functional profile of neurotoxic A1 reactive astrocytes. Microglia secrete Il1-α, TNF, and C1q to produce A1 reactive astrocytes, which are deficient in their ability to promote synapse formation and neuronal survival. A1 reactive astrocytes are found in numerous human neurodegenerative diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen Y, Qin H, Chen J, Mou L, He Y, Yan Y, Zhou H, Lv Y, Chen Z, Wang J, et al. Postnatal activation of TLR4 in astrocytes promotes excitatory synaptogenesis in hippocampal neurons. J Cell Biol. 2016;215:719–734. doi: 10.1083/jcb.201605046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blanco-Suarez E, Caldwell AL, Allen NJ. Role of astrocyte-synapse interactions in CNS disorders. J Physiol. 2016 doi: 10.1113/JP270988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung WS, Welsh CA, Barres BA, Stevens B. Do glia drive synaptic and cognitive impairment in disease? Nat Neurosci. 2015;18:1539–1545. doi: 10.1038/nn.4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, et al. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, et al. Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron. 2016;89:37–53. doi: 10.1016/j.neuron.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Srinivasan R, Lu TY, Chai H, Xu J, Huang BS, Golshani P, Coppola G, Khakh BS. New Transgenic Mouse Lines for Selectively Targeting Astrocytes and Studying Calcium Signals in Astrocyte Processes In Situ and In Vivo. Neuron. 2016 doi: 10.1016/j.neuron.2016.11.030. This paper describes the generation of an Aldh1L1-Cre/ERT2 transgenic mouse line. This mouse line will potentially be a valuable tool for the study of astrocytes in vivo, as it enables the efficient and targeted conditional deletion of genes in a large population of astrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Krencik R, Hokanson KC, Narayan AR, Dvornik J, Rooney GE, Rauen KA, Weiss LA, Rowitch DH, Ullian EM. Dysregulation of astrocyte extracellular signaling in Costello syndrome. Sci Transl Med. 2015;7:286ra266. doi: 10.1126/scitranslmed.aaa5645. This paper details a novel tool for studying astrocyte contribution to human diseases. The authors examined the role of HRAS singaling in Costello Syndrome iPSC-derived human astroctytes. This will be a useful tool for studying astrocytes in a variety of human diseases and developmental processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldman SA, Nedergaard M, Windrem MS. Modeling cognition and disease using human glial chimeric mice. Glia. 2015;63:1483–1493. doi: 10.1002/glia.22862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–353. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]