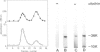Abstract
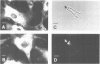
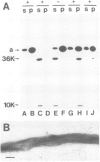
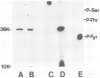
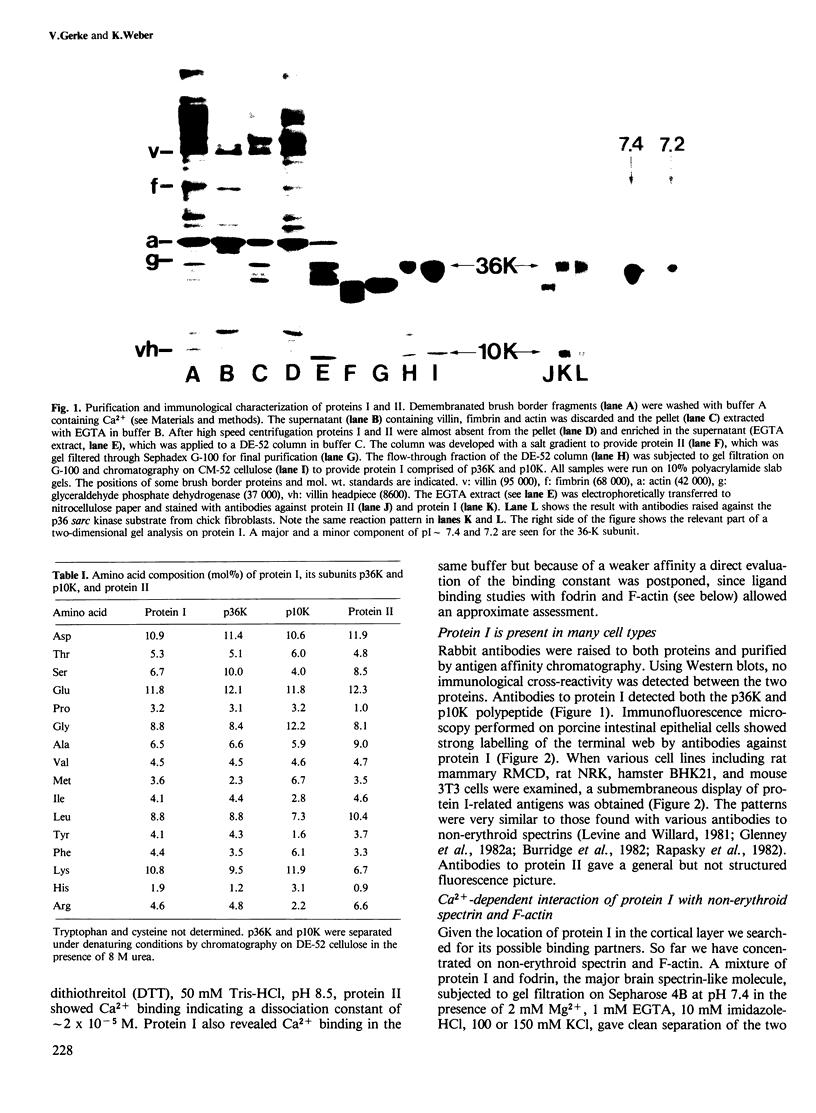
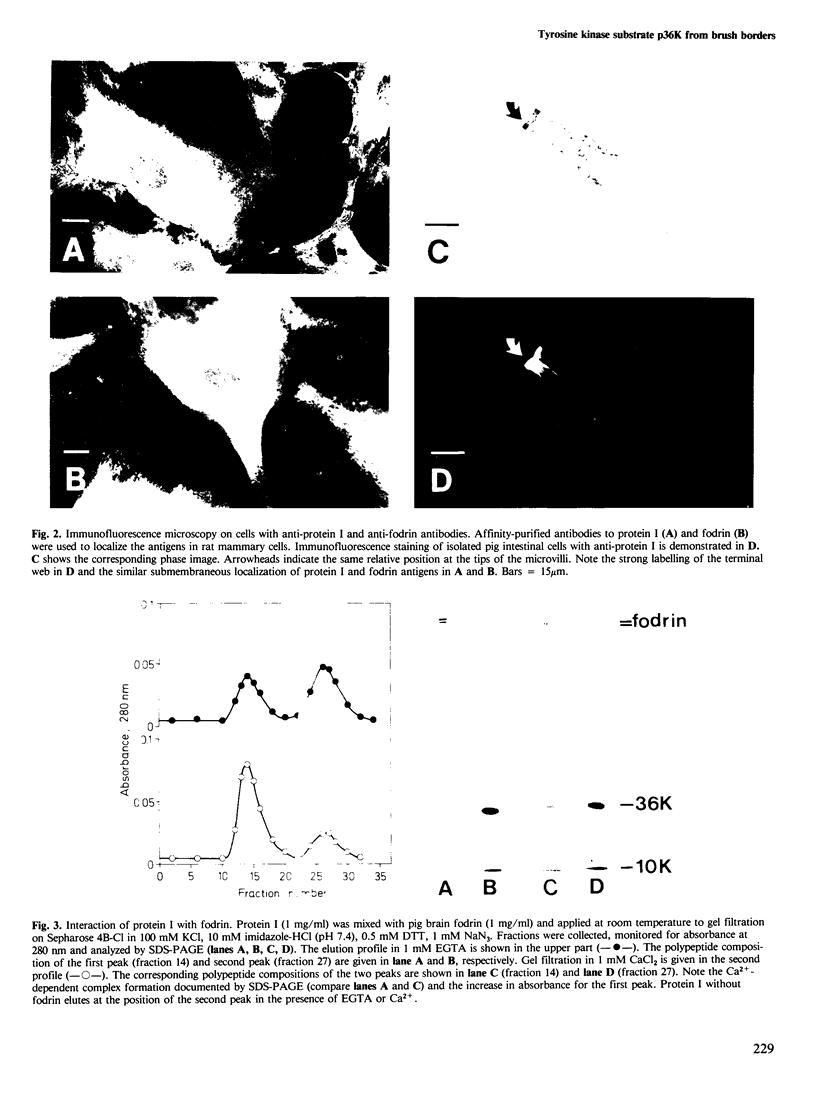
Membrane vesicles derived from the apical side of procine intestinal epithelial cells retain, after demembranation in the presence of calcium, two major proteins (I, II) which are released by the addition of calcium chelators. We have purified and characterized these two calcium-binding proteins. Protein I has a mol. wt. of 85 000 and contains two copies of a 36-K subunit and an additional 10-K subunit. It binds in a calcium-dependent manner to F-actin as well as to non-erythroid spectrin. Immunofluorescence microscopy reveals protein I-related antigens in the terminal web of the intestinal cell and in a submembraneous cortical layer in various tissue culture cells. Biochemical and immunological results document that the 36-K subunit of protein I is identical with the cellular p36K recognized as a major substrate for tyrosine phosphorylation by the sarc gene kinase in Rous sarcoma virus-transformed cells. The biochemical properties of protein I agree with its location seen in immunofluorescence microscopy and cell fractionation and suggest that the actin-spectrin network in the cortical layer may be affected by virus transformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amini S., Kaji A. Association of pp36, a phosphorylated form of the presumed target protein for the src protein of Rous sarcoma virus, with the membrane of chicken cells transformed by Rous sarcoma virus. Proc Natl Acad Sci U S A. 1983 Feb;80(4):960–964. doi: 10.1073/pnas.80.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Fimbrin is a cytoskeletal protein that crosslinks F-actin in vitro. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6849–6853. doi: 10.1073/pnas.78.11.6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A., Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980 Jul;20(3):839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burridge K., Feramisco J. R. Non-muscle alpha actinins are calcium-sensitive actin-binding proteins. Nature. 1981 Dec 10;294(5841):565–567. doi: 10.1038/294565a0. [DOI] [PubMed] [Google Scholar]
- Burridge K., Kelly T., Mangeat P. Nonerythrocyte spectrins: actin-membrane attachment proteins occurring in many cell types. J Cell Biol. 1982 Nov;95(2 Pt 1):478–486. doi: 10.1083/jcb.95.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. S., Chen L. B. Detection of phosphotyrosine-containing 34,000-dalton protein in the framework of cells transformed with Rous sarcoma virus. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2388–2392. doi: 10.1073/pnas.78.4.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Discrete primary locations of a tyrosine-protein kinase and of three proteins that contain phosphotyrosine in virally transformed chick fibroblasts. J Cell Biol. 1982 Aug;94(2):287–296. doi: 10.1083/jcb.94.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudrier E., Reggio H., Louvard D. Characterization of an integral membrane glycoprotein associated with the microfilaments of pig intestinal microvilli. EMBO J. 1983;2(3):469–475. doi: 10.1002/j.1460-2075.1983.tb01446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S., Ralston R., Alitalo K., Bishop J. M. Subcellular location of an abundant substrate (p36) for tyrosine-specific protein kinases. Mol Cell Biol. 1983 Mar;3(3):340–350. doi: 10.1128/mcb.3.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson E., Shealy D. J., Erikson R. L. Evidence that viral transforming gene products and epidermal growth factor stimulate phosphorylation of the same cellular protein with similar specificity. J Biol Chem. 1981 Nov 25;256(22):11381–11384. [PubMed] [Google Scholar]
- Erikson R. I., Collett M. S., Erikson E., Purchio A. F., Brugge J. S. Protein phosphorylation mediated by partially purified avian sarcoma virus transforming-gene product. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):907–917. doi: 10.1101/sqb.1980.044.01.098. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Isolation and characterization of mammalian villin and fimbrin, the two bundling proteins of the intestinal microvilli. Eur J Cell Biol. 1983 Sep;31(2):249–255. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Geisler N., Kaulfus P., Weber K. Demonstration of at least two different actin-binding sites in villin, a calcium-regulated modulator of F-actin organization. J Biol Chem. 1981 Aug 10;256(15):8156–8161. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. F-actin-binding and cross-linking properties of porcine brain fodrin, a spectrin-related molecule. J Biol Chem. 1982 Aug 25;257(16):9781–9787. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. The spectrin-related molecule, TW-260/240, cross-links the actin bundles of the microvillus rootlets in the brush borders of intestinal epithelial cells. J Cell Biol. 1983 May;96(5):1491–1496. doi: 10.1083/jcb.96.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Kaulfus P., Matsudaira P., Weber K. F-actin binding and bundling properties of fimbrin, a major cytoskeletal protein of microvillus core filaments. J Biol Chem. 1981 Sep 10;256(17):9283–9288. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Weber K. Calmodulin-binding proteins of the microfilaments present in isolated brush borders and microvilli of intestinal epithelial cells. J Biol Chem. 1980 Nov 25;255(22):10551–10554. [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. Comparison of the 34,000-Da pp60src substrate and a 38,000-Da phosphoprotein identified by monoclonal antibodies. J Biol Chem. 1983 Jul 10;258(13):8497–8502. [PubMed] [Google Scholar]
- Greenberg M. E., Edelman G. M. The 34 kd pp60src substrate is located at the inner face of the plasma membrane. Cell. 1983 Jul;33(3):767–779. doi: 10.1016/0092-8674(83)90019-3. [DOI] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Cheney R. E., Willard M. Location of a protein of the fodrin-spectrin-TW260/240 family in the mouse intestinal brush border. Cell. 1983 Mar;32(3):953–965. doi: 10.1016/0092-8674(83)90080-6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Leonard K., Jockusch B. M. Structural aspects of vinculin-actin interactions. J Mol Biol. 1982 Jun 25;158(2):231–249. doi: 10.1016/0022-2836(82)90431-4. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Hanafusa H. Viral and cellular src genes contribute to the structure of recovered avian sarcoma virus transforming protein. Cell. 1981 Apr;24(1):155–164. doi: 10.1016/0092-8674(81)90511-0. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. doi: 10.1016/0092-8674(82)90454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Paasivuo R., Ralston R., Alitalo K. The p36 substrate of tyrosine-specific protein kinases co-localizes with non-erythrocyte alpha-spectrin antigen, p230, in surface lamina of cultured fibroblasts. EMBO J. 1983;2(10):1701–1705. doi: 10.1002/j.1460-2075.1983.tb01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J., Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981 Sep;90(3):631–642. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. T., Burgess D. R. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979 Dec;83(3):667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S., Graves T. A., Wharton K. A., Falco N., Howe C. L. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J Cell Biol. 1980 Dec;87(3 Pt 1):809–822. doi: 10.1083/jcb.87.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cooper J. A., Hunter T. Immunofluorescent localization of a 39,000-dalton substrate of tyrosine protein kinases to the cytoplasmic surface of the plasma membrane. J Cell Biol. 1983 Jun;96(6):1601–1609. doi: 10.1083/jcb.96.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rübsamen H., Saltenberger K., Friis R. R., Eigenbrodt E. Cytosolic malic dehydrogenase activity is associated with a putative substrate for the transforming gene product of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1982 Jan;79(2):228–232. doi: 10.1073/pnas.79.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz J., Preiser H., Maestracci D., Ghosh B. K., Cerda J. J., Crane R. K. Purification of the human intestinal brush border membrane. Biochim Biophys Acta. 1973 Sep 27;323(1):98–112. doi: 10.1016/0005-2736(73)90434-3. [DOI] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Weber K., Glenney J. R., Jr Microfilament-membrane interaction: the brush border of intestinal epithelial cells as a model. Philos Trans R Soc Lond B Biol Sci. 1982 Nov 4;299(1095):207–214. doi: 10.1098/rstb.1982.0127. [DOI] [PubMed] [Google Scholar]
- Weber K., Wehland J., Herzog W. Griseofulvin interacts with microtubules both in vivo and in vitro. J Mol Biol. 1976 Apr 25;102(4):817–829. doi: 10.1016/0022-2836(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Yeaton R. W., Lipari M. T., Fox C. F. Calcium-mediated degradation of epidermal growth factor receptor in dislodged A431 cells and membrane preparations. J Biol Chem. 1983 Aug 10;258(15):9254–9261. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Purification and structural properties of gelsolin, a Ca2+-activated regulatory protein of macrophages. J Biol Chem. 1980 Oct 10;255(19):9490–9493. [PubMed] [Google Scholar]