Abstract
Purpose
Weight gain after a breast cancer diagnosis is associated with poor cancer outcomes. Limited research describes patterns of weight change by race. The goal of this study was to assess and compare the percent of weight change and change in body mass index (BMI) after chemotherapy in Black and White breast cancer patients.
Methods
Black and White women diagnosed with invasive non-metastatic breast cancer were recruited from two metropolitan areas. Medical records were abstracted to obtain clinical (e.g. cancer stage) and treatment variables (e.g. chemotherapy regimen). Weight change was examined in 98 women who underwent chemotherapy. Differences in baseline characteristics by race were evaluated using the chi-square or Fisher's exact test for categorical variables and t-test for continuous variables. We performed bivariate associations between study variables and relative weight change.
Results
Most (62%) participants maintained their pre-treatment weight; 38% gained more than 5% of their baseline weight by the end chemotherapy. Normal weight women had the highest mean increase (3.57; 1.05, 6.10) compared to those that were overweight/obese. Fifteen percent of women shifted to a higher BMI category; 26% of those that were normal became overweight; 17% of overweight patients became obese. Blacks were more likely than whites to shift to a higher BMI (P=0.06).
Conclusions
Results underscore the need for integrating weight control within cancer treatment plans to prevent weight gain in patients undergoing chemotherapy. Future studies that help to elucidate behaviors and/or biological factors that contribute to weight gain overall and in blacks will be important.
Keywords: Weight, Breast cancer, Chemotherapy, Disparities
Introduction
Weight changes are common in breast cancer patients [1-7]. Extreme weight loss or gain has been shown to adversely affect a woman's prognosis [8-10]. For example, women with metastatic breast cancer that demonstrated a weight change (gain or loss) greater than 5% during chemotherapy treatment were at higher risk of increased death than those who maintained their weight during treatment [9]. Furthermore, Nichols et al. [10] found that each 5 kg weight gain or loss was associated with 12% and 24% increase in mortality risk, respectively. Most commonly however, after chemotherapy, women experience weight gains ranging from 1.2-8.3 kg [11-15]. This weight gain persists beyond therapy, and only about 10% of women return to their pre-cancer weight [16].
There are several adverse consequences of weight gain in breast cancer survivors. First, a growing body of literature supports the assertion that obesity is associated with an increased risk of breast cancer recurrence and increased mortality [8-10]. Another important consideration is that chemotherapy treatment plans are established based on patients' pre-treatment weights. Though not as commonly, the practice of dose reduction in overweight and obese women occurs as some medical oncologists base chemotherapy doses on ideal or adjusted body weight [17,18]. Therefore, weight change, especially gain, could further exacerbate this issue and lead to certain patients receiving lower doses of chemotherapy than planned. Both Black and overweight women are at increased risk of experiencing these dose reductions compared to White or normal weight women [19]. Furthermore, excess weight gain confers higher risk of development of comorbid conditions such as diabetes and heart disease [20,21]. Additionally, each 5 kg weight gain following breast cancer diagnosis was associated with a 19% increase in cardiovascular disease mortality. Indeed, breast cancer survivors are as likely to die from heart disease as they are from their cancer [20-23]. Finally, weight gain in female patients generally leads to substantial changes in their body compositions with increased fat, BMI, and waist-hip ratio [24,25].
One explanation of this relationship between chemotherapy and weight gain is that chemotherapy contributes to weight gain through reduced metabolism as a consequence of treatment-induced ovarian failure and the subsequent rapid onset of menopause [26]. Reductions in physical activity and energy balance are also suggested as potential explanations for weight gain post treatment. Regardless of the specific pathophysiologic mechanisms, weight change, particularly weight gain, warrants attention in order to facilitate improved clinical and psychosocial outcomes. While the precise physiologic mechanism of the basis of weight gain following chemotherapy is unclear, the higher likelihood for numerous adverse outcomes in breast cancer patients who are overweight and obese underscores the seriousness of this problem.
Of all racial and ethnic groups, Black women are the most likely at diagnosis to be overweight or obese [27] and to have comorbid conditions (e.g., hypertension, diabetes) [28,29] that can be exacerbated by weight gain. Some reports suggest that Black women are more likely to gain weight during chemotherapy compared to Whites but most studies have not specified data by race [16]. Additionally, those who gained the most weight during treatment were less likely to return to baseline weight [16]. Rock and colleagues showed that being Black was positively and independently associated with weight gain status. Higher energy intake and lower physical activity levels were also associated with increased risk of weight gain in Blacks [15]. However, there are only a handful of studies that have examined this issue in Black breast cancer patients. Because Black women are most at risk of being overweight or obese at diagnosis, data regarding patterns of weight gain are needed for this group. We are unaware of studies that prospectively examine the changes in weight during the course of treatment or that have examined clinical, psychosocial or process of care factors as predictors of weight change in Black women. Since most available data are from retrospective studies, the goal of this study was to characterize changes in weight during chemotherapy treatment in a sample of Black and White women. A better understanding of patterns and predictors of weight change may inform intervention strategies to reduce risk of adverse breast and other outcomes associated with weight gain and obesity in breast cancer survivors.
Methods
Setting and Population
Institutional Review Board approval was received from all organizations in accordance with the Health Insurance Portability and Accountability Act. Study participants were recruited from the Washington, DC and Detroit, MI metropolitan areas. Eligible breast cancer patients self-identified as Black/African American or White/European American, were over the age of 21, had invasive, non-metastatic disease and who initiated and completed chemotherapy.
Data Collection
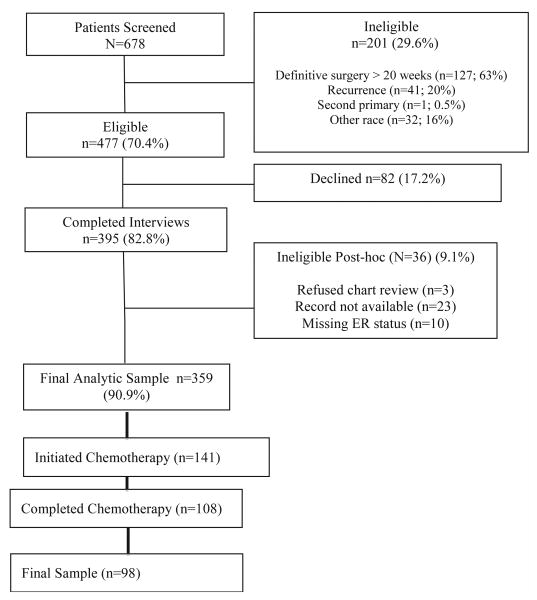
Breast cancer patients were identified from hospital appointment logs and pathology reports by a trained clinical research assistant (CRA) and via fliers and announcements at community organizations. All interviews were completed by phone using a computerized telephone interview (CATI) interview format. Medical records were abstracted to obtain clinical and treatment data. Participants received an incentive of $25 for participation in the study. Information about the screened and eligible women in the study is in Figure 1. The final sample of 359 women met all eligibility criteria for study inclusion. This analysis is limited to women who completed chemotherapy (N=98; 70%).
Figure 1.
Study schema for Total Sample Accrued and Final analytic sample.
Measures
Using weight data abstracted from medical records, the primary outcome measure, “weight change (in pounds)” was defined as: (weight after final cycle of chemotherapy) – (baseline weight). We examined socio-demographic, clinical, and psychosocial factors associated with weight change after chemotherapy for breast cancer. We also examined “relative weight change” defined as weight change relative to baseline weight as an additional outcome variable. However, since results for the two outcome variables were identical, only data on the absolute weight change variable are presented.
Socio-demographic variables were based on self-report and included age, race (Black or White), marital status, education, and insurance status. Clinical factors abstracted from medical records were presence of comorbidities, hormone receptor status (positive vs. negative), nodal status (positive or negative), pathological tumor size (classified as less than 2 cm or above 2 cm), and cancer stage (I, II or III). Treatment information abstracted included surgery type (lumpectomy or mastectomy), chemotherapy regimen (doxorubicin + cyclophosphamide or other), radiation (yes vs. no) or receipt of adjuvant hormonal therapy (yes or no), and type of hormone therapy received (Tamoxifen or Aromatase inhibitors). Psychosocial factors included: self-efficacy, perceived susceptibility of having a recurrence and perceived severity of the diagnosis. Self-efficacy was measured by a 12-item rating scale in which higher scores indicate higher levels [30]. Perceived susceptibility of recurrence (e.g. “I expect to be free of cancer in the future”) (Cronbach's α =.69) and perceived severity of the diagnosis (e.g. “There are many diseases more severe than the kind of cancer I have”) (Cronbach's α = .65) were measured by items taken from the Adherents Determinants Scale [31].
Statistical Analysis
Patient characteristics at baseline including socio-demographic, clinical, treatment, and psychosocial variables were summarized using frequencies for categorical variables and means with standard deviations for continuous variables. Weight change in pounds was analyzed as a continuous variable. We investigated bivariate associations of selected variables with weight change using generalized linear and linear regression models. Generalized linear models were used to calculate the mean weight change associated with categorical variables and linear regression was used to determine weight change associated with one standard deviation change in continuous variables.
Results
Baseline characteristics of breast cancer survivors participating in the study are presented in Table 1. The mean age at baseline was 50 years and 68% of participants were Black. A majority of the participants had a high school diploma or higher education and some type of private insurance. Half of the women were postmenopausal and about 70% had one or more comorbidities. The mean weight at baseline was 176 pounds with a mean BMI of 30 kg/m2. Seventy seven percent of the participants were either overweight or obese at baseline.
Table 1.
Socio-demographic, clinical, and psychosocial characteristics of breast cancer patients pre-chemotherapy.
| Characteristics | Total N (%) |
|---|---|
| Demographics | |
| Mean Age at Diagnosis, Years (SD) | 50.34 (10.68) |
| Race | |
| African-American | 67 (68) |
| White | 31 (32) |
| Marital Status | |
| Married/Living as Married | 43 (44) |
| Currently Single | 55 (56) |
| Education | |
| ≤ High School | 33 (34) |
| High school diploma or higher | 65 (66) |
| Insurance | |
| Private | 68 (70) |
| Public | 30 (30) |
| Clinical | |
| Co-morbid conditions | |
| None | 31 (32) |
| 1 or 2 | 39 (40) |
| 3 or more | 28 (28) |
| Cardiovascular comorbidities | 44 (45) |
| Endocrine or metabolic comorbidities | 23 (23) |
| Post-menopausal | |
| Yes | 44 (50) |
| No | 44 (50) |
| Cancer Stage | |
| Stage I | 22 (23) |
| Stage II | 56 (59) |
| Stage III | 17 (18) |
| HR Status | |
| HR Positive | 61 (63) |
| HR Negative | 36 (37) |
| Surgery Type | |
| Mastectomy | 60 (61) |
| Lumpectomy | 38 (39) |
| Tumor size | |
| <2cm | 38 (39) |
| >=2cm | 59 (61) |
| Nodal status | |
| Positive | 55 (56) |
| Negative | 43 (44) |
| Chemotherapy regimen | |
| AC$ | 45 (46) |
| Other | 53 (54) |
| Hormonal Therapy Initiated | |
| Yes | 53 (87) |
| No | 8 (13) |
| Hormone Treatment | |
| Tamoxifen | 29 (59) |
| Aromatase Inhibitor | 20 (41) |
| Radiation Therapy | |
| Yes | 66 (67) |
| No | 32 (33) |
| Behavioral | |
| Self-efficacy, Mean (SD) | 71.7 (5.5) |
| Adherence determinants scale, Mean (SD) | 7.96 (0.32) |
| Perceived severity subscale | 3.99 (0.10) |
| Perceived susceptibility subscale | 14.33 (2.41) |
| Anthropometric | |
| Weight (in lbs), Mean (SD) | 176.27 (38.82) |
| BMI (kg/m2), Mean (SD) | 29.66 (6.37) |
| Normal | 23 (23) |
| Overweight/Obese | 75 (77) |
Patterns of Weight Change
The mean weight change after chemotherapy among study participants was 4.05 pounds (median: 3.80 pounds). Clinically significant weight gain, defined as ≥ 5% increase in baseline weight, was observed in 30% of the participants after chemotherapy. There was no difference in clinically significant weight gain by race - 30% of Black women and 29% of White women gained 5% or more of baseline weight at follow-up.
Predictors of Weight Change
Associations of weight change with selected socio-demographic and clinical variables are presented in Table 2. Overall, none of the associations between patient characteristics and weight change were statistically significant. However, data suggested increased weight gain in older, married women and those with higher levels of education. Patients with 3 or more co-morbid conditions and/or endocrine/metabolic comorbidities experience more weight gain compared to those without comorbidities, but the differences were not statistically significant. Women with Stage I cancers and those on hormone therapy gained more weight on average compared to those with later stage cancers and women not on hormone therapy, respectively. Within the psychosocial factors, there was a positive association between weight change and maintaining a positive attitude and perceived susceptibility. The interaction between race and perceived susceptibility was significant (see Table 3).
Table 2.
Associations of weight change with selected study variables.
| Mean weight change (lb) | |
|---|---|
| Age Range <=50 |
2.79 |
| >50 | 4.59 |
| Race | |
| African-American | 3.46 |
| White | 4.26 |
| Marital Status | |
| Married/Living as Married | 5.54 |
| Currently Single | 2.28 |
| Education | |
| ≤ HS | 2.89 |
| HS+ | 4.14 |
| Insurance | |
| Private | 4.08 |
| Public | 2.73 |
| Co-morbid conditions | |
| None | 3.14 |
| 1 or 2 | 3.96 |
| 3 or more | 5.20 |
| Cardiovascular comorbidities | |
| None | 4.23 |
| 1 or more | 3.84 |
| Endocrine or metabolic comorbidities | |
| None | 3.56 |
| 1 or more | 5.67 |
| Cancer Stage | |
| Stage I | 6.32 |
| Stage II | 3.69 |
| Stage III | 0.74 |
| HR Status | |
| HR Positive | 3.60 |
| HR Negative | 4.15 |
| Surgery Type | |
| Mastectomy | 3.99 |
| Lumpectomy | 3.54 |
| Tumor size | |
| <2cm | 3.27 |
| >=2cm | 4.17 |
| Nodal status | |
| Positive | 3.67 |
| Negative | 3.76 |
| Chemotherapy regimen | |
| AC† | 2.85 |
| Other | 4.41 |
| Hormone Therapy Initiated‡ | |
| Yes | 4.42 |
| No | 2.75 |
| Radiation Therapy | |
| Yes | 3.85 |
| No | 3.42 |
| BMI (kg/m2) | |
| Normal | 4.96 |
| Overweight | 2.55 |
| Obese | 3.98 |
Among women who are hormone receptor positive
Doxorubicin plus cyclophosphamide
P<0.05,
P<0.01
Table 3.
Associations of weight change with behavioral characteristics.
| β1 | Mean weight change (lb) | |
|---|---|---|
| Self-efficacy | 1.53 | |
| Below median | 3.72 | |
| Above median | 4.66 | |
| Maintaining positive attitude | 1.91a | |
| Below median | 2.68 | |
| Above median | 5.20 | |
| Seek and obtain information | 0.56 | |
| Below median | 1.64 | |
| Above median | 4.65 | |
| Understanding and participating in care | -0.07 | |
| Below median | 3.83 | |
| Above median | 4.18 | |
| Adherence determinants sub-scale, Perceived Susceptibility | 3.00b | |
| Below median | 1.09 | |
| Above median | 5.21a | |
| WHITES2 | ||
| Adherence determinants sub-scale, Perceived Susceptibility | 2.85b | |
| Below median | 0.26 | |
| Above median | 7.33a | |
| AFRICAN-AMERICANS2 | ||
| Adherence determinants sub-scale, Perceived Susceptibility | 2.02 | |
| Below median | 2.97 | |
| Above median | 4.42 |
P<0.05,
P<0.01
Weight change with one SD increase in the score using a linear regression model
Pinteraction of perceived susceptibility with race = 0.14
Discussion
The goal of this prospective study was to examine patterns of weight change in a proportional cohort of Black and White patients undergoing chemotherapy for breast cancer. We found that very few women lost more than 5% of their baseline weight which may suggest effective management of side effects such as nausea. In contrast, a notable proportion (38%) of women had weight gain greater than 5% of their baseline weight. This proportion is in concert with other studies of chemotherapy patients [4,5]. Further, although Black women were more likely to be overweight and/or obese than White patients, Black women did not experience a significantly higher change in relative weight in comparison to Whites. This is likely due to the fact that their weight was significantly higher than Whites at the start. Neither psychosocial nor comorbid conditions were associated with changes in weight. These findings add to the limited information about patterns of weight change in Black breast cancer patients in active treatment. Weight gain in breast cancer patients has been shown to have adverse consequences in terms of cancer prognosis and risk of other comorbid conditions (e.g. heart disease) [8-10]. Our data support the need for intervention efforts to promote weight maintenance in breast cancer patients undergoing chemotherapy.
Of those who experienced changes in weight, it is likely a consequence of reduced energy expenditure. Though in this study we did not collect dietary or physical activity data, routine physical activity has been shown to be the strongest predictor of weight stability in breast cancer patients [15]. Additionally, changes in body composition have been noted in previous studies suggesting gains in fat mass and no change or declines in fat-free mass, which could reduce energy expenditure [1,32-36]. Only a handful of studies have provided empirical data regarding Black patients' experiences with weight gain [37]. Future studies are needed to test these hypotheses within both Black and White patients in active treatment.
Therefore, interventions on breast cancer patients undergoing chemotherapy that promote increased physical activity to maintain fat-free mass, particularly muscle mass, may be warranted. A body of literature also exists which support the health and psychological benefits of physical activity interventions targeted towards breast cancer patients [38-40]. Few studies have examined weight maintenance in breast cancer patients or have tested which approach may be best for women undergoing chemotherapy [41]. Furthermore, while the American Institute for Cancer Research recommends that cancer patients see a dietitian during treatment, it is unclear to what extent these recommendations are being implemented in breast cancer treatment settings. Baldwin et al (2006) found that few patients with gastrointestinal cancer were referred to a dietitian for dietary assessment, and only 40% of these patients with weight loss greater than 10% of initial body weight were referred to a dietitian [42]. This suggests that dietitians may be underutilized in the clinical oncology care setting, and that to a large extent women's opportunities to receive nutrition advice regarding weight loss or weight maintenance may be potentially limited to research studies. We are not aware of any weight loss/maintenance interventions with Black survivors in active treatment. Less than a handful of studies have focused on Black survivors [43]. Similarly, more information is needed regarding level of physical activity in breast cancer patients – particularly Black breast cancer patients.
We found that women who had normal weight were most at risk to gain weight by the end of their treatment. This is important because normal weight women are not generally targeted in interventions and thus may escape the scrutiny of providers who are more likely to counsel or discuss weight maintenance strategies with those presenting excess weight at baseline. All women, irrespective of weight status at time of breast cancer diagnosis, are at risk for weight gain, and there is a need to educate women on the risks that accompany this gain [24].
This study provides novel findings regarding the importance of differentiation among various weight classifications as well as patterns of shifts across BMI categories. Most studies have only examined changes in relative weight. Taken together, our findings suggest that a focus only on overall percent change or gain in weight may mask small groups of women who are more likely to experience weight gain or changes in their BMI post-diagnosis. We were specifically interested in examining race-specific patterns in weight change given that Black women are more likely to be overweight and/or obese at diagnosis. To date, there have been relatively few studies that have focused on weight loss or maintenance in Black breast cancer patients. While empirical data is limited, it appears that Black survivors are less likely to engage in physical activity. Sheppard and colleagues found that 61% of Black breast cancer patients did not engage in rigorous physical activity [44].
Clinical implications of weight gain during treatment include potential increased risk for relapse, development of new breast cancers or development of other serious health conditions (e.g. hypertension). Therefore, obese women may be receiving lower doses of treatment. Griggs et al. demonstrated systematic differences in the administration of chemotherapy given to Black and to overweight/obese women [19].
This study has several strengths such as the inclusion of proportional Black and White breast cancer patients, the reliance on medical records for treatment data, and the prospective nature of the study design. However, some limitations should be noted. First, the size of the sample may have limited our statistical power. Second, we focused on weight change during a relatively short amount of time and were not able to assess whether women continued to gain weight after treatment completion. Current data suggest that weight gain persists over time [4]. Information about energy expenditure was not captured in this study but will be important for future studies of women undergoing chemotherapy. Finally, women in our study were all insured and from urban areas so findings may not be applicable to those with no insurance or who reside in more rural areas. Nevertheless, this study adds to the limited information about weight gain in Black women and also changes in BMI across breast cancer patients in general. Noting patterns in weight gain can help identify whether or not adjustments should be made in chemotherapy regimens.
Weight gain following breast cancer treatment is a serious side effect and concern for many survivors that may ultimately impact survival and quality of life. The American Cancer Society recommends that breast cancer patients control their weight yet weight control is not routinely integrated within cancer treatment plans. None of the women that were obese or overweight improved their weight during treatment. Future interventions to promote weight control during active treatment may have long-term benefits for all patients, particularly those that are overweight. Studies that include Black patients – a subgroup most vulnerable to being overweight or obese are also needed in order to contribute to better outcomes for these survivors.
Acknowledgments
Grant Support: This work was funded in part by grants from the American Cancer Society (Sheppard: PI MRSGT-06-132 CPPB) and NIH-NCI (PI: Sheppard/Adams-Campbell: 1R21CA149996).
References
- 1.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Relationship of obesity and physical activity with C-peptide, leptin, and insulin-like growth factors in breast cancer survivors. Cancer Epidemiol Biomarkers Prev. 2005;14:2881–2888. doi: 10.1158/1055-9965.EPI-05-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demark-Wahnefried W, Winer EP, Rimer BK. Why women gain weight with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1993;11:1418–1429. doi: 10.1200/JCO.1993.11.7.1418. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin PJ, Ennis M, Pritchard KI, Trudeau ME, Koo J, Madarnas Y, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20:42–51. doi: 10.1200/JCO.2002.20.1.42. [DOI] [PubMed] [Google Scholar]
- 4.Trédan O, Bajard A, Meunier A, Roux P, Fiorletta I, Gargi T, et al. Body weight change in women receiving adjuvant chemotherapy for breast cancer: a French prospective study. Clin Nutr. 2010;29:187–191. doi: 10.1016/j.clnu.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Nissen MJ, Shapiro A, Swenson KK. Changes in weight and body composition in women receiving chemotherapy for breast cancer. Clin Breast Cancer. 2011;11:52–60. doi: 10.3816/CBC.2011.n.009. [DOI] [PubMed] [Google Scholar]
- 6.Friedenreich CM, Woolcott CG, McTiernan A, Ballard-Barbash R, Brant RF, Stanczyk FZ, et al. Alberta physical activity and breast cancer prevention trial: sex hormone changes in a year-long exercise intervention among postmenopausal women. J Clin Oncol. 2010;28:1458–1466. doi: 10.1200/JCO.2009.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoskin PJ, Ashley S, Yarnold JR. Weight gain after primary surgery for breast cancer--effect of tamoxifen. Breast Cancer Res Treat. 1992;22:129–132. doi: 10.1007/BF01833342. [DOI] [PubMed] [Google Scholar]
- 8.Chlebowski RT, Weiner JM, Reynolds R, Luce J, Bulcavage L, Bateman JR. Long-term survival following relapse after 5-FU but not CMF adjuvant breast cancer therapy. Breast Cancer Res Treat. 1986;7:23–30. doi: 10.1007/BF01886732. [DOI] [PubMed] [Google Scholar]
- 9.Thivat E, Thérondel S, Lapirot O, Abrial C, Gimbergues P, Gadéa E, et al. Weight change during chemotherapy changes the prognosis in non metastatic breast cancer for the worse. BMC Cancer. 2010;10:648. doi: 10.1186/1471-2407-10-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nichols HB, Trentham-Dietz A, Egan KM, Titus-Ernstoff L, Holmes MD, Bersch AJ, et al. Body mass index before and after breast cancer diagnosis: associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol Biomarkers Prev. 2009;18:1403–1409. doi: 10.1158/1055-9965.EPI-08-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, et al. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2009;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 12.Makari-Judson G, Judson CH, Mertens WC. Longitudinal patterns of weight gain after breast cancer diagnosis: observations beyond the first year. Breast J. 2007;13:258–265. doi: 10.1111/j.1524-4741.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 13.Yaw YH, Shariff ZM, Kandiah M, Mun CY, Yusof RM, Othman Z, et al. Weight changes and lifestyle behaviors in women after breast cancer diagnosis: a cross-sectional study. BMC Public Health. 2011;11:309. doi: 10.1186/1471-2458-11-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodwin PJ, Ennis M, Pritchard KI, Mc Cready D, Koo J, Sidlofsky S. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 15.Rock CL, Flatt SW, Newman V, Caan BJ, Haan MN, Stefanick ML, et al. Factors associated with weight gain in women after diagnosis of breast cancer. Women's Healthy Eating and Living Study Group. J Am Diet Assoc. 1999;99:1212–1221. doi: 10.1016/s0002-8223(99)00298-9. [DOI] [PubMed] [Google Scholar]
- 16.Saquib N, Flatt SW, Natarajan L, Thomson CA, Bardwell WA, Caan B. Weight gain and recovery of pre-cancer weight after breast cancer treatments: evidence from the women's healthy eating and living (WHEL) study. Breast Cancer Res Treat. 2007;105:177–186. doi: 10.1007/s10549-006-9442-2. [DOI] [PubMed] [Google Scholar]
- 17.Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, et al. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012 May 1;30(13):1553–1561. doi: 10.1200/JCO.2011.39.9436. [DOI] [PubMed] [Google Scholar]
- 18.Field KM, Kosmider S, Jefford M, Michael M, Jennens R, Green M, et al. Chemotherapy dosing strategies in the obese, elderly, and thin patient: results of a nationwide survey. J Oncol Pract. 2008;4:108–113. doi: 10.1200/JOP.0832001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81:21–31. doi: 10.1023/A:1025481505537. [DOI] [PubMed] [Google Scholar]
- 20.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle C, Kushi LH, Byers T, Courneya KS, Demark-Wahnefried W, Grant B, et al. Nutrition and physical activity during and after cancer treatment: an American Cancer Society guide for informed choices. CA Cancer J Clin. 2006;56:323–353. doi: 10.3322/canjclin.56.6.323. [DOI] [PubMed] [Google Scholar]
- 22.Petrelli JM, Calle EE, Rodriguez C, Thun MJ. Body mass index, height, and postmenopausal breast cancer mortality in a prospective cohort of US women. Cancer Causes Control. 2002;13:325–332. doi: 10.1023/a:1015288615472. [DOI] [PubMed] [Google Scholar]
- 23.Rayson D, Richel D, Chia S, Jackisch C, van der Vegt S, Suter T. Anthracycline-trastuzumab regimens for HER2/neu-overexpressing breast cancer: current experience and future strategies. Ann Oncol. 2008;19:1530–1539. doi: 10.1093/annonc/mdn292. [DOI] [PubMed] [Google Scholar]
- 24.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118:2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Protani M1, Coory M, Martin JH. Effect of obesity on survival of women with breast cancer: systematic review and meta-analysis. Breast Cancer Res Treat. 2010;123:627–635. doi: 10.1007/s10549-010-0990-0. [DOI] [PubMed] [Google Scholar]
- 26.Gordon AM1, Hurwitz S, Shapiro CL, LeBoff MS. Premature ovarian failure and body composition changes with adjuvant chemotherapy for breast cancer. Menopause. 2011;18:1244–1248. doi: 10.1097/gme.0b013e31821b849b. [DOI] [PubMed] [Google Scholar]
- 27.Flegal KM, Carroll MD, Ogden CL. Effects of trimming weight-for-height data on growth-chart percentiles. Am J Clin Nutr. 2012;96:1051–1055. doi: 10.3945/ajcn.112.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose DP, Haffner SM, Baillargeon J. Adiposity, the metabolic syndrome, and breast cancer in African-American and white American women. Endocr Rev. 2007;28:763–777. doi: 10.1210/er.2006-0019. [DOI] [PubMed] [Google Scholar]
- 29.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002 Jan 16;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 30.Wolf MS, Chang CH, Davis T, Makoul G. Development and validation of the Communication and Attitudinal Self-Efficacy scale for cancer (CASE-cancer) Patient Educ Couns. 2005;57:333–341. doi: 10.1016/j.pec.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 31.DiMatteo M Robin, Hays Ron D, Gritz Ellen R, Bastani Roshan, et al. Patient adherence to cancer control regimens: Scale development and initial validation. Psych Asses. 1993;5:102–112. [Google Scholar]
- 32.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev. 2011;12:282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 33.Ingram C, Brown JK. Patterns of weight and body composition change in premenopausal women with early stage breast cancer: has weight gain been overestimated? Cancer Nurs. 2004;27:483–490. doi: 10.1097/00002820-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. J Am Diet Assoc. 1997;97:519–526. 529. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 35.Kutynec CL, Mc Cargar L, Barr SI, Hislop TG. Energy balance in women with breast cancer during adjuvant treatment. J Am Diet Assoc. 1999;99:1222–1227. doi: 10.1016/s0002-8223(99)00301-6. [DOI] [PubMed] [Google Scholar]
- 36.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, et al. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89:2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 37.Halbert CH, Weathers B, Esteve R, Audrain-McGovern J, Kumanyika S, De Michele A, et al. Experiences with weight change in African-American breast cancer survivors. Breast J. 2008;14:182–187. doi: 10.1111/j.1524-4741.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 38.Duijts SF, Faber MM, Oldenburg HS, van Beurden M, Aaronson NK. Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors--a meta-analysis. Psychooncology. 2011 Feb;20(2):115–126. doi: 10.1002/pon.1728. [DOI] [PubMed] [Google Scholar]
- 39.Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(Suppl 1):S52–73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fong DY, Ho JW, Hui BP, Lee AM, Macfarlane DJ, Leung SS, Cerin E. Physical activity for cancer survivors: meta-analysis of randomised controlled trials. BMJ. 2012;344:e70. doi: 10.1136/bmj.e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Loprinzi CL, Athmann LM, Kardinal CG, O'Fallon JR, See JA, Bruce BK, et al. Randomized trial of dietician counseling to try to prevent weight gain associated with breast cancer adjuvant chemotherapy. Oncology. 1996 May-Jun;53:228–232. doi: 10.1159/000227565. [DOI] [PubMed] [Google Scholar]
- 42.Baldwin C, McGough C, Norman AR, Frost GS, Cunningham DC, Andreyev HJ. Failure of dietetic referral in patients with gastrointestinal cancer and weight loss. Eur J Cancer. 2006;42:2504–2509. doi: 10.1016/j.ejca.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 43.Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Prev Chronic Dis. 2009;6:A22. [PMC free article] [PubMed] [Google Scholar]
- 44.Sheppard VB, Makambi K, Taylor T, Wallington SF, Sween J, Adams-Campbell L. Physical activity reduces breast cancer risk in African American women. Ethn Dis. 2011;21:406–411. [PMC free article] [PubMed] [Google Scholar]