Abstract
Background
Medial prefrontal cortex (mPFC) dysfunction is present in heavy alcohol consumers. Dopamine signaling in mPFC is associated with executive functioning and affects drinking behavior; however, direct measurement of extracellular mPFC dopamine during appetitive and consummatory ethanol (EtOH) self-administration behavior has not been reported.
Methods
We used in vivo microdialysis in freely behaving, adult, male, Long Evans rats to determine extracellular dopamine concentration in the mPFC during operant self-administration of an EtOH-plus-sucrose or sucrose solution. The model separated appetitive/seeking from consummatory phases of the operant session. Dopamine was also monitored in an untrained handling control group, and dialysate EtOH was measured in the EtOH-drinking group.
Results
Home cage baseline dopamine was lower in rats that experienced a week of drinking sweetened EtOH compared with sucrose-drinking and handling controls. Transfer into the operant chamber and the initiation of consumption stimulated a relatively higher change in dopamine over baseline in the sweetened EtOH group compared with sucrose and handling controls. However, all groups show a dopamine response during transfer into the operant chamber, and the sucrose group had a relatively higher change in dopamine over baseline during initiation of consumption compared with handling controls. The time courses of dopamine and EtOH in the mPFC differ in the EtOH-consuming rats.
Conclusions
Differences in extracellular mPFC dopamine between EtOH drinkers compared with control groups suggest that mPFC dopamine is involved in the mechanism of operant self-administration of sweetened EtOH and sucrose. Furthermore, the increase in dopamine during consumption is consistent with a role of mPFC dopamine in reward prediction.
Keywords: Ethanol, Operant Self-Administration, Consumption, Ethanol-Seeking, Mesocortical, Appetitive
Medial prefrontal cortex (mPFC) dysfunction, which is frequently noted in heavy alcohol consumers, is associated with increased impulsivity and perseveration, as well as deficits in executive functions (e.g., working memory, decision making, attention, goal-directed behavior; Bechara and Damasio, 2002; Bechara and Van Der Linden, 2005; Chanraud et al., 2007; Goldstein et al., 2004; Sullivan et al., 1993). Similarly, acute ethanol (EtOH) can also disrupt working memory (Ralevski et al., 2012). When exposed to drug-related cues, detoxified alcoholics showed significantly greater mPFC activation compared with controls (Heinz et al., 2004). These findings suggest that EtOH use may be associated with enhanced sensitivity to drug-related cues and decreased behavioral control due to compromised prefrontal cortical function.
While the mechanisms by which EtOH alters mPFC function are unknown, EtOH-induced changes in dopaminergic activity in the mPFC may be involved (Ding et al., 2014; Trantham-Davidson et al., 2014; Tu et al., 2007). Altered dopaminergic signaling has been shown to affect reinstatement of drug-seeking behaviors (Kehagia et al., 2010; McFarland et al., 2004; Sinha, 2013); however, the relationship between mPFC dopamine and EtOH self-administration behavior is still unclear. Recent studies show that EtOH increases extracellular dopamine in the mPFC in naive rats after intravenous administration (Schier et al., 2013), or a single microinjection of EtOH directly into the ventral tegmental area (Ding et al., 2011), although earlier work suggested that EtOH administration had no effect (Bassareo et al., 1996; Engleman et al., 2006; Hegarty and Vogel, 1993). Furthermore, modulation of dopamine receptor signaling in the mPFC has been shown to change EtOH self-administration behaviors (Ding et al., 2014; Hodge et al., 1996; Samson and Chappell, 2003), but due to the experimental designs used, it is unclear whether mPFC dopamine was important for drug-seeking or consummatory behaviors.
Previous work indicated that EtOH-associated stimuli can increase extracellular dopamine in the nucleus accumbens (NAC) during operant sweetened EtOH self-administration (Carrillo and Gonzales, 2011; Doyon et al., 2005; Howard et al., 2009), and therefore, we hypothesized that extracellular dopamine would also increase in the mPFC when an experienced, nondependent, rat is exposed to drinking-associated stimuli. To test this, we used microdialysis to monitor extracellular dopamine within the mPFC during an operant self-administration session, in which rats drank a 10% sucrose plus 10% EtOH solution (10S10E), a 10% sucrose-only solution (10S; to control for sucrose in the EtOH solution), or no solution (handling; to control for experimenter handling and experience within the operant chamber). Additionally, our operant self-administration sessions separated anticipatory/seeking and consummatory behavioral phases. Thus, we monitored mPFC dopamine concentrations affected by contextual cues during transfer into the drinking environment (operant chamber) before exposure to stimuli experienced during the drink period (taste, smell, and consumption).
Materials and Methods
Materials
Drinking solutions (10S: 10% sucrose [w/v] or 10S10E: 10% EtOH [v/v] in 10S) were made from 95% EtOH (AAPER Alcohol and Chemical Co., Shelbyville, KY), ultra-pure sucrose (MP Biomedicals, LLC, Solon, OH), and distilled water. Carprofen (Pfizer, New York, NY) and gentamicin (APP Pharmaceuticals, Schaumburg, IL) were used during surgery.
Animals
Final statistical analyses used 27 male, young adult, Long Evans rats from Charles River Laboratories (Portage, MI or Raleigh, NC; 200 to 225 g upon arrival). An additional 16 male Long Evans rats were used for an independent replication study (see Supporting information). Animals were maintained on a 12-hour light/dark schedule, at 23 ±2°C, with ad libitum food and water (except where noted); rats were weighed each day. All animal procedures complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Texas at Austin.
Surgery
After a week of habituation to the facility and experimenters, rats were anesthetized with isoflurane (5% induction, 2.5% maintenance), and using stereotaxic equipment, a 21-gauge guide cannula was surgically placed (Plastics One, Roanoke, VA) above the left mPFC (mm relative to Bregma and skull surface: +3.0 AP, +0.6 ML, −2.0 DV; Paxinos and Watson, 1986). Three skull screws and dental cement secured the cannula and a tether bolt to the skull. We administered carprofen (5 mg/kg, subcutaneously) to minimize postsurgical malaise, placed a dummy cannula into the cannula to prevent blockage, and monitored weight and health over a 7-day recovery period prior to beginning operant training.
Self-Administration Training and Protocols
Groups
Rats were initially trained to lever-press for access to the 10S solution, and then 2 groups were formed, one that consumed increasing concentrations of EtOH (2 to 10% EtOH [v/v] in 10S; Table 1), and a group that continued to drink 10S. The handling control group was exposed to all the same procedures as the 10S10E and 10S groups (physical handling, water deprivation during lever-press training, time in the operant chamber, tethering, and dialysis), but they were not exposed to drinking solutions or operant training.
Table 1. Eight-Session Protocol Parameters and Consumption.
| Training day | Wait period (minutes) | Lever presses required | Ethanol (EtOH) in 10% sucrose drinking solution(%) | EtOH intake (g/kg) | Sucrose intake (g/kg) |
|---|---|---|---|---|---|
| 1 | 2 | 2 | 0 | n/a | 2.94 0.31 |
| 2 | 5 | 2 | 2 | 0.43 ±0.05 | 2.99 ±0.30 |
| 3 | 8 | 2 | 2 | 0.57 ±0.06 | 2.88 ±0.36 |
| 4 | 13 | 2 | 5 | 1.06 0.10 | 2.78 0.55 |
| 5 | 18 | 4 | 5 | 1.07 ±0.17 | 3.44 ±0.31 |
| 6 | 23 | 4 | 10 | 1.12 ±0.18 | 3.63 ±0.34 |
| 7 (Tethered) | 28 | 4 | 10 | 1.28 ±0.12 | 3.56 ±0.28 |
| 8 (Dialysis) | 28 | 4 | 10 | 1.65 ±0.14 | 3.27 ±0.38 |
Lever-Press Training and Operant Protocol
A week after surgery, animals were habituated to operant chambers (Med Associates, Inc., St. Albans, VT) and then trained to lever-press for a 10S solution (10S10E and 10S groups only). Water deprivation (maximum 22 h/d) was used to expedite lever-press training. Animals typically learned to lever-press within 3 training sessions (1 session per day), after which they regained ad libitum access to water for the remainder of the experiment. Chambers were as previously described by Howard and colleagues (2009). Briefly, chambers had a retractable lever, sipper tube bottle, house light, cue light and lick-ometer circuit. Chambers were contained in sound-attenuating boxes. Operant programs were run and data were collected using Med Associates software.
Once trained to lever-press, animals began an 8-session testing schedule during which a pre-lever-press wait period was lengthened from 0 to 28 minutes, and the response requirement was increased from 2 to 4 (Table 1). Following completion of the response requirement, the sipper tube containing the drinking solution entered the chamber for 21 minutes, during which animals had ad libitum access to the drinking solution. No further responding was required. For the 10S10E group, EtOH started at a 2% (v/v) concentration in 10% (w/v) sucrose and gradually increased to 10% EtOH in 10% sucrose (10S10E) (Table 1). This procedure is modified from the Samson (1986) sucrose fading procedure; however, we did not fade sucrose out of the solution because we wanted to maximize EtOH consumption. Sessions were run once a day, 4 to 6 days per week. Animals received a total of 3 to 4, but never more than 2 sequential days off from training once the 8-session protocol began. The handling control group completed the same procedures, except drinking solutions were not available, and the lever was present, but pressing had no consequences. Solution consumption was measured by the volume of solution before and after the drinking session (to the nearest 0.25 ml, accounting for spillage), and pattern of consumption was monitored using the lickometer.
Following the sixth operant session, a spring was attached to the tether bolt on the animal's head and connected to a swivel suspended above the rat by a counterbalance lever arm. Rats were tethered in their home cages (placed next to their operant chamber) during the seventh operant session to facilitate habituation to the apparatus and environment. The tethering apparatus did not interfere with the rats' abilities to move freely about their home cage or to lever-press in the operant chamber.
Microdialysis
After the seventh operant session, rats were briefly anesthetized with isoflurane to implant the laboratory-constructed microdialysis probe (3.25-mm active area, 13,000 MW cut off, constructed similar to Pettit and Justice, 1991). Probes were perfused with artificial cerebral spinal fluid (ACSF: 149 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2, 0.2 mM ascorbic acid, and 5.4 mM D-glucose), at a 0.2 μl/min flow rate overnight, and then to a 1.0 μ;l/min flow rate at least 2 hours prior to dialysis sampling. For the 10S10E group, 2 samples before the lever extended into the chamber and all samples after were evaluated for EtOH concentration (described below). The dialysis samples were immediately frozen on dry ice and stored at −80°C until dopamine analysis.
Experimental Timeline
Microdialysis samples were manually collected every 7 minutes before and during the eighth operant session (Fig. 1). Four baseline samples were taken in the home cage. During the last minute of the fourth baseline sample, the rat was transferred into the operant chamber. The operant program began with turning on the house light and sound-attenuating fan, and the sample collection vial was changed to the first wait period sample. Four wait period samples were taken. The time it took the rat to meet the response requirement of 4 lever presses was collapsed into the last wait period sample. The wait/lever-press sample was changed to the first drink sample as the drinking bottle entered the chamber. Three samples were taken during the drink period, after which the bottle retracted and the house light turned off. Then, 3 samples were taken during the postdrink period. The rat was then returned to its home cage, and the ACSF was changed to calcium-free ACSF. Approximately 1.5 hours later, 2 additional calcium-free samples were taken.
Fig. 1.
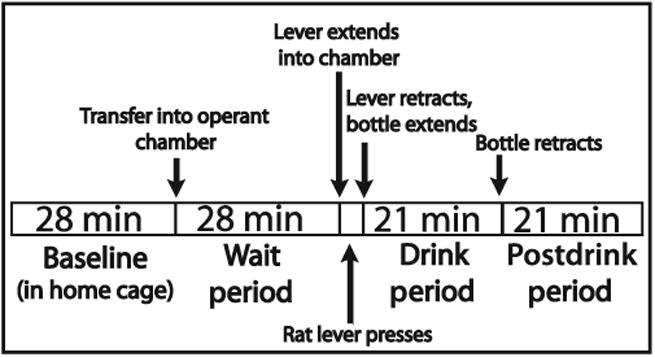
Time course of the operant self-administration session. On the eighth day of operant testing, consecutive 7-minute dialysis samples were taken during all behavioral phases except for the final wait period sample which was variable due to the bar-press period. Figure adapted from Schier and colleagues (2013).
Dopamine Analysis
We evaluated the dopamine concentration in all samples using reverse-phase high performance liquid chromatography with electrochemical detection. All samples were run with accompanying external standards (0.03125 to 1.0 nM dopamine). Samples and standards were run using a 8125 manual injector (Rheodyne, Cotati, CA), a Luna 50 x 1.0 mm (C18, 3 micron particle size; Phenomenex, Torrance, CA) or Haisil 100 50 x 0.5 mm column (C18, 3 micron particle size; Higgins Analytical Inc., Mountain View, CA), and a 2mm glassy carbon working electrode (SenCell or VT03 with ISAAC reference electrode; Antec Leyden, Zoeterwoude, the Netherlands) at potential +345 or +395 mV. Mobile phase aqueous solution consisted of approximately 2.1 mM octanesulfonic acid, 0.04 to 0.3 mM decanesulfonic acid (adjusted to optimize chromatography), 0.34 mM ethylenedianimetetraacetic acid, 71 mM sodium phosphate monobasic dihydrate, and 60 mM potassium chloride, adjusted to 5.60 pH with 1 M sodium hydroxide. Prior to use, 150 ml/l of methanol was added and the mobile phase solution was sparged with helium. Mobile phase flow rates ranged from 0.1 to 0.12 ml/min for 50 x 1.0 mm columns, and 0.025 to 0.032 ml/min for 50 x 0.5 mm columns. Four to 5.5 μl of dialysate was mixed with 1 to 3 μl of ascorbate oxidase (EC 1.10.3.3; 102.3 U/mg) prior to injecting 5 μl of the mixture into the system. The amount of ascorbate oxidase was adjusted to optimize dopamine detection. EZChrome Elite software (Agilent Technologies, Wilmington, DE) was used for chromatogram acquisition and peak integration. The dopamine signal was required to be at least 3 times greater than the background noise. See Supporting information for details regarding the dopamine analyses for the independent replication study.
Ethanol Analysis
Samples were analyzed for EtOH concentration on the day they were collected (Schier et al., 2012). Briefly, 1 μl aliquots of dialysate or external standards (0.3125 to 20 mM EtOH) were sealed in 2-ml glass vials, heated in an autosampler tray (50 to 65°C), and analyzed for EtOH content by a gas chromatograph with flame ionization detection.
Histological Analysis
Within 3 days of dialysis, animals were overdosed with sodium pentobarbital (150 mg/kg, intraperitoneal) and perfused through the heart with saline and 10% formalin in saline prior to brain extraction. Brains were fixed with 10% formalin in saline, coronally sectioned (100 μm thick), and stained with cresyl violet for verification of the microdialysis probe placement (Paxinos et al., 1998).
Exclusion Criteria
For inclusion of rats in data analysis, dopamine concentrations in home cage baseline samples were required to have a relative standard deviation < 0.25. We also required a 40% decrease in dopamine concentration in calcium-free ACSF samples compared with basal ACSF samples to verify that dopamine release was exocytotic. Rats were required to acquire the lever-press behavior within 6 training sessions, complete the lever-press requirement on the day of dialysis, and the 10S10E group was required to consume at least 0.8 g/kg on the day of dialysis. Finally, rats were excluded if technical errors resulted in loss of critical dopamine samples (before and after transfer into the operant chamber, or initiation of drinking).
Statistical Analysis
Raw dopamine concentrations (nM) were analyzed using repeated measures analysis of variance (ANOVA) with group (10S10E, 10S, and handling) as between-subjects factor, and time (14 time points: 4 home cage baseline, 4 wait period, 6 drink and postdrink) as within-subjects repeated factor. Post hoc analyses (using pooled error and Bonferroni corrections) separated the experiment into 3 phases: home cage baseline, wait period, and drink/ postdrink. Specifically, when significant interactions between main effects were observed for the overall experiment, simple effects analyses were performed to determine the source of the significant interaction. As significant group differences occurred in raw dopamine concentrations during the home cage baseline (see Results; Fig. 2), we also analyzed dopamine expressed as a percentage of home cage baseline levels (% BL). Behavioral data were analyzed using independent-samples t-tests; except licks separated into 7-minute bins were analyzed with repeated measures ANOVA with group (10S10E and 10S) as between-subjects factor, and bin as within-sub-jects factor. Data were analyzed using SPSS software (IBM, Chicago, IL). Significance was assigned if p < 0.05; ns = not significant.
Fig. 2.
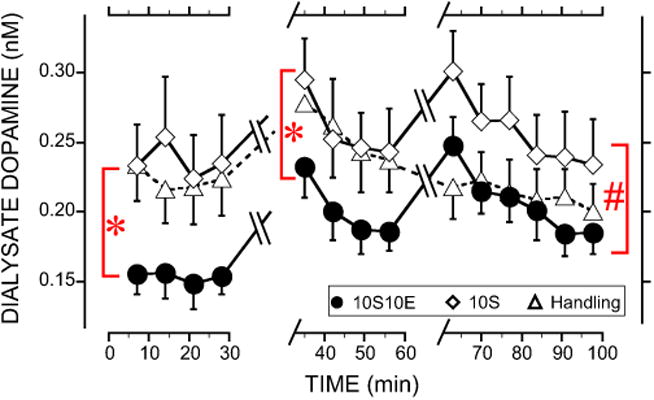
Dialysate dopamine concentrations in medial prefrontal cortex (mPFC) during home cage baseline, wait, drink and postdrink periods for the sucrose and handling controls along with rats trained to drink 10S10E. For clarity, only group comparisons are shown on the figure as follows. *Significant difference between the 10S10E group compared with either the 10S or handling groups during the baseline and wait periods. #Drink and postdrink period dopamine concentrations were significantly different in 10S compared with either the 10S10E or handling groups. Not indicated in the figure are significant increases in dopamine at the first sample during the wait period compared with the remaining samples. Similarly, there was a significant increase in dopamine during the first drink sample compared with the remaining drink and postdrink samples. Overall significant main effects of time and a group-by-time interaction occurred. Data represented as mean ±SEM for most points, but selected error bars are omitted for clarity (n =9 for each group). 10S10E = 10% sucrose + 10% ethanol, 10S = 10% sucrose.
Results
Consumption During Operant Self-Administration Sessions
EtOH and sucrose consumption data for the 8 operant sessions are represented in Table 1. In the group consuming sweetened EtOH, rats increased their EtOH intake over the 8 sessions and consumed at least 1 g/kg during the 4 sessions prior to microdialysis.
Overall Analysis of Raw Dopamine Concentrations and Home Cage Baseline
In general, dopamine concentrations in both the 10S10E and 10S groups peaked once during the first wait period time point, and again during the first drink period time point. In contrast, dopamine in the handling group only peaked during the first wait period time point and remained at baseline levels during the drink and postdrink periods. Comparison of overall raw dopamine concentrations across the entire experiment (Fig. 2) resulted in significant main effects of time, F(13, 306) = 11.9, p < 0.001, and a group-by-time interaction, F(26, 306) = 3.0, p < 0.001. Post hoc analyses of the significant group-by-time interaction separated the experiment into the 3 phases: home cage baseline, wait period, and drink/postdrink.
Significant group differences occurred in raw dopamine concentrations during the home cage baseline (Fig. 2), F(2, 33) = 12.1, p < 0.001. Following up on the main effect of group, an ANOVA revealed that the 10S10E group exhibited significantly lower baseline dopamine concentrations compared with both the 10S, F(1, 33) = 20.9, p < 0.05, and handling groups, F(1, 32) = 14.8, p < 0.05, while no difference occurred between 10S and handling groups. A separate experiment utilizing the same experimental protocol also found that 10S10E animals show significantly lower baseline dopamine concentrations in the PFC compared with 10S controls, t(14) = 1.76, p < 0.05 (Fig. S1).
Raw Dopamine Concentrations and Lever-Press Behavior During the Wait Period
After collecting the home cage baseline samples, the rats were transferred into the operant chamber and the program was started. During the wait period (Fig. 2), dopamine concentrations significantly differed between groups, F(2, 33) = 6.3, p < 0.01, and changed over time, F(3, 306) = 12.5, p < 0.001, but there was not a group-by-time interaction (ns). Post hoc analyses on the main effect of group during the wait period showed that dopamine in the 10S10E group significantly differed from both the 10S, F(1, 33) = 10.1, p < 0.05, and handling groups, F(1, 33) = 8.6, p < 0.05. Dopamine concentrations were similar between 10S and handling groups during the wait period. Analysis of the change in dopamine during the wait period collapsed across all groups showed that dopamine concentrations peaked during the first wait period time point and then decreased back toward baseline levels (first sample differed from samples 2 to 4, p < 0.05).
At the end of the wait period, the lever was presented to all groups. 10S10E and 10S groups were required to press the lever 4 times for access to the drinking solution. The 10S10E group began lever-pressing significantly sooner than the 10S group after lever presentation, t(16) =−2.3, p <0.05, yet both groups showed similar lever-press rates, t(16) = 1.1, ns, and similar time to complete the lever-press requirement, t (16) =−0.1, ns (Table 2). The time taken to complete the lever presses was accounted for in the final wait period dialysis sample, making that sampling period variable (7 minutes 3 seconds to 10 minutes 4 seconds).
Table 2. Behavioral Parameters During Microdialysis Session.
| Parameter | 10S10E group | 10S group |
|---|---|---|
| Time to complete presses (seconds) | 24 ± 16 | 25 ± 3.2 |
| Latency to press (seconds) | 7.0 ± 3.8* | 18.3 ± 3.2 |
| Latency to drink (seconds) | 6.1 ± 2.2 | 2.8 ± 0.2 |
| Length of first bout (minutes) | 4.9 ± 0.3* | 8.4 ±1.1 |
| Licks in first bout | 1,239 ± 80* | 2,478 ±310 |
| First bout lick rate (licks/min) | 259 ± 21 | 305 ± 20 |
| Solution consumed (ml) | 8.8 ± 0.7* | 13.8 ± 0.9 |
| Total licks | 1,503 ± 100* | 2,704 ± 265 |
Significant difference (p < 0.05) between ethanol-plus-sucrose solution-consuming (10S10E) and sucrose solution-consuming (10S) groups. Data represented as mean ±SEM.
Drinking Behavior and Raw Dopamine Concentrations During the Drink and Postdrink Periods
Once the response requirement was completed, the lever retracted and the bottle entered the chamber, at which time the dialysis sample was changed and the drink period began. The 10S10E and 10S groups showed similar latency to drink, rate of licking during the first drinking bout, and total number of drinking bouts (Table 2), bout defined as a minimum of 25 licks without a 2-minute break; t(16) = 1.5, −1.6, and −0.7, respectively, ns. The 10S10E group drank significantly less solution, had significantly fewer licks overall and during the first bout, and had a significantly shorter first bout compared with the 10S group (Table 2), t(16) =−4.7, −4.2, −3.9, and −3.1, respectively, p <0.01. When licks were binned per 7 minutes, the 10S10E group had significantly fewer licks during the first 2 bins compared with the 10S group, but both groups had similar licks during the third bin (Fig. 3), group-by-time interaction F(2, 32)=12.4, p < 0.001; bin 1: F(1, 32)=45.4, p < 0.05; bin 2: F (1, 32) = 13.0, p < 0.01; bin 3: F(1, 32) = 0.1, ns. On dialysis day, the 10S10E group consumed 1.7 ±0.1 g/kg EtOH during the drink period (Table 1).
Fig. 3.
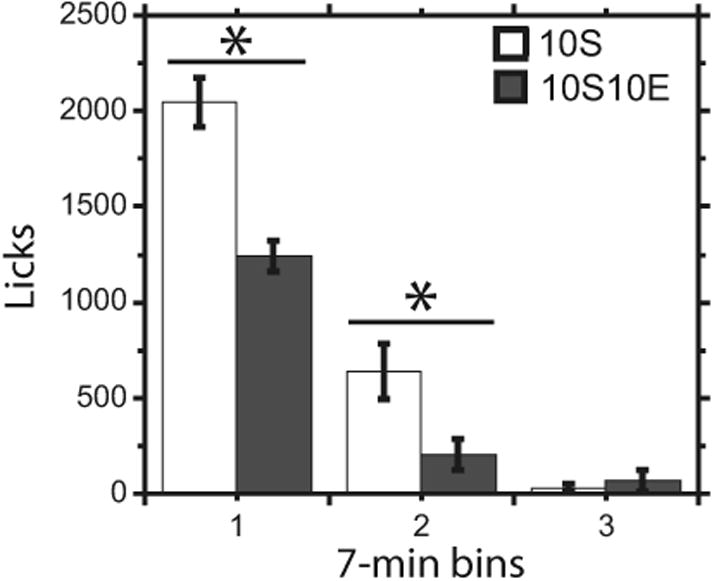
Licks in 7-minute bins. *Significant difference in licks between 10S10E (n =9; 10% sucrose + 10% ethanol) and 10S (n =9; 10% sucrose) groups. Data represented as mean ±SEM. 10S10E = 10% sucrose + 10% ethanol, 10S = 10% sucrose.
During the drink and postdrink periods (Fig. 2), dopamine concentrations significantly differed between groups, F(2, 33) = 6.9, p < 0.01, and changed over time, F(5, 306) = 9.4, p < 0.001. Following up on the main effect of group, a post hoc ANOVA revealed that dopamine in the 10S10E group significantly differed from the 10S group, F(1, 33) = 11.4, p < 0.05, but not the handling group (ns). Dopamine concentrations were also significantly different between 10S and handling groups, F(1, 33) = 9.2, p < 0.05. Because there was not a significant group-by-time interaction in the overall 1ANOVA conducted on the drink and postdrink periods, the groups were collapsed to analyze the change in dopamine over time during the experimental periods. These analyses revealed that dopamine concentrations peaked during the first drink period time point, and then decreased back toward baseline levels (first drink sample differed from drink sample 3 and postdrink samples 1 to 3, p < 0.05).
Dopamine Concentration as a Percentage of Home Cage Baseline Levels
Due to significant group differences in raw dopamine concentrations during the home cage baseline (results above; Fig. 2), we also analyzed dopamine expressed as % BL (Fig. 4). In general, analysis of raw and % BL dopamine resulted in similar conclusions; therefore, with % BL analysis, we mainly describe differences compared to that obtained with raw dopamine concentrations. For example, analysis of all 10 time points within the operant chamber (wait period, drink, and postdrink) revealed a significant main effect of group, F(2, 24) = 7.7, p < 0.01, in addition to significant time and group-by-time effects seen with the raw concentration analysis. Simple effects analyses between groups showed that each group significantly differs from one another: 10S10E versus 10S, F(1, 63) = 44.7, p < 0.001; 10S10E versus handling, F(1, 63) = 85.0, p < 0.001; and 10S versus handling, F(1, 63) = 6.4, p < 0.05.
Fig. 4.
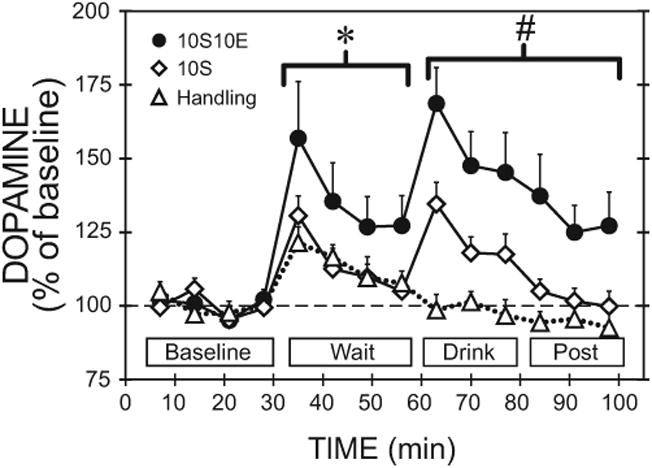
Medial prefrontal cortex dopamine relative to home cage baseline during home cage baseline, wait, drink and postdrink periods. The data shown in Fig. 2 were transformed to percent of home cage baseline. For clarity, only group comparisons are shown on the figure as follows. *Significant difference between the 10S10E group compared with either the 10S or handling groups during the wait period. #Drink and postdrink period dopamine responses above baseline were significantly different in each group compared with the other 2 groups. Overall, significant main effects of time, group, and a group-by-time interaction occurred. Data represented as mean ±SEM for most points, but selected error bars are omitted for clarity (n =9 for each group). 10S10E = 10% sucrose + 10% ethanol, 10S = 10% sucrose.
Following up on these simple effects analyses, we identified where significant group differences occurred within each experimental phase. During the wait period, the % BL analysis revealed similar results compared to raw dopamine concentrations. During the drink and post drink periods, the % BL analysis also revealed similar results compared to raw dopamine concentrations, with a significant main effect of group and significant interaction of group by time. However, post hoc analyses following up on the main effect of group also indicated significant differences between 10S10E and handling groups, F(1, 30) = 81.2, p <0.05, which was not observed in the analyses on the raw basal dopamine concentrations. Subsequent simple effects analysis of the group-by-time interaction showed that dopamine concentrations in the 10S10E group differed from the 10S group during the first drink time point, F(1, 30) = 7.7, p < 0.05, and differed from the handling group during all 3 drink and the first post-drink time points (p < 0.05). Dopamine concentrations in the 10S group only differed from the handling group during the first drink time point, F(1, 30) = 8.5, p < 0.05.
Dialysate Ethanol Concentrations During and After the Drink Period
At the initiation of drinking, dialysate EtOH was lowest and then increased throughout the drink and postdrink period to peak at 5.2 ±0.8 mM EtOH (Fig. 5). Dialysate EtOH concentrations are not corrected with an in vivo extraction fraction, and are therefore lower than true tissue EtOH concentrations.
Fig. 5.
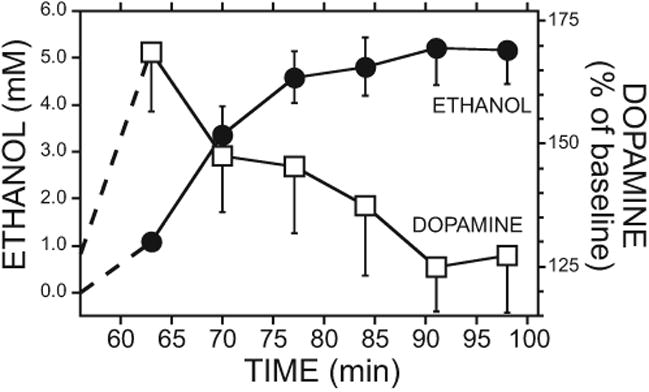
Dopamine and ethanol (EtOH) concentrations in medial prefrontal cortex (mPFC) during the drink and postdrink periods in the 10S10E group. Left y-axis shows the dialysate EtOH concentrations (circles). Right y-axis shows the percent change in mPFC dopamine concentration during the drink and postdrink periods relative to home cage baseline (squares, same data shown in Fig. 3). The bottle retracted from the chamber after the third sample. Data represented as mean ±SEM (n =9). 10S10E = 10% sucrose + 10% ethanol.
Histology, Body Weight, and Calcium-Dependent Dopamine Concentration
Histologies showed that the percent of probe active area within the infralimbic and prelimbic regions was not significantly different between groups (Fig. 6), F(2, 24) = 2.1, ns. Probe active area was required to be at least 50% in the infralimbic and prelimbic regions of the mPFC. 10S10E, 10S, and handling groups were 76 ±3, 66 ±5, and 65 ±5% within these regions, respectively. Body weights on the day of dialysis were not significantly different between groups, F(2, 24)=2.7, ns; range 337 to 477 g. Calcium-dependent dopamine release was confirmed by a minimum 40% dialysate dopamine concentration decrease when calcium-free ACSF was perfused through the probe. 10S10E, 10S, and handling groups showed an average of 67 ±3, 57 ±4, and 61 ±5% decrease in dopamine in calcium-free ACSF samples compared with concentrations at the conclusion of the operant session, respectively.
Fig. 6.
Microdialysis probe placements within the medial prefrontal cortex. Coronal slices 2.7, 3.2, and 3.7 mm from bregma showing microdialysis probe placements for all experimental groups. Lines represent 3.25 mm active dialysis area. 10S10E = 10% sucrose + 10% ethanol, 10S = 10% sucrose. Histology figure adapted from Paxinos and colleagues (1998).
Discussion
This is the first report of changes in mPFC extracellular dopamine during operant self-administration of sweetened EtOH. Dopamine concentrations during the home cage baseline were lower in the mPFC of rats that had experienced about a week of drinking sweetened EtOH versus sucrose-drinking and handling controls. Upon transfer into the operant chamber, dopamine increased to a greater degree relative to baseline in the mPFC of the sweetened EtOH group compared with sucrose and handling controls; however, all groups showed a dopamine response during the transfer. At the start of the drink period, extracellular dopamine increased in the PFC in both the 10S10E and 10S groups, and the magnitude of this effect relative to baseline was greater in the 10S10E group. Overall, we used a behaviorally relevant operant model and show differences in mPFC dopamine concentrations that are unique to rats drinking sweetened EtOH.
Compared with animals in the control groups, rats that had self-administered a sweetened EtOH solution for about a week demonstrated significantly lower basal dopamine concentrations in the mPFC. The reliability of this effect is demonstrated by our own independent replication (Supporting information). No baseline mPFC dopamine differences occurred between sucrose-experienced and handling control groups, which highlight the specificity of EtOH experience on mPFC baseline dopamine levels. We speculate that this limited voluntary EtOH-drinking experience may be sufficient to induce synaptic adaptations that alter the regulation of basal extracellular dopamine concentrations in the mPFC, and raises the possibility that lower mPFC dopamine concentration contributes to EtOH-related seeking or drinking behaviors. In the present studies, rats consumed on average 1 to 1.3 g/kg in the 4 sessions prior to the microdialysis session. At these doses, it is unlikely that basal dopamine levels were influenced by the aversive, lingering effects of alcohol intoxication during the microdialysis sessions. Previous work has demonstrated that with high doses of acute EtOH (3 or 4 g/kg), rats show conditioned place aversion 10 hours post-EtOH administration, but this behavior is not observed with lower doses (2 g/kg; Morse et al., 2000). Furthermore, we did not observe a relationship between EtOH intake on the day prior to microdialysis and basal mPFC dopamine concentrations (data not shown). This supports our argument that the observed reduction in basal mPFC dopamine concentrations in the EtOH-experienced animals is related to repeated self-administration of intoxicating doses of EtOH and not to the lingering effects of the dose consumed the day prior to microdialysis.
Consistent with the suggestion of EtOH-specific effects on basal PFC dopamine, Engleman and colleagues (2006) reported lower basal dopamine concentrations in mPFC of naive alcohol-preferring “P” rats compared with the outbred Wistar strain. However, not all alcohol-preferring strains of rats demonstrate reduced basal dopamine activity in the mPFC relative to controls. For example, Leggio and colleagues (2003) demonstrated higher basal dopamine content in the mPFC of Sardinian alcohol-preferring (sP) rats compared with Wistar control rats. Furthermore, we did not observe baseline dopamine differences in the NAC between EtOH-experienced rats and controls using similar procedures (Doyon et al., 2005; Howard et al., 2009). This clear contrast between mPFC and NAC suggests that low tonic basal dopamine concentrations selectively in the mPFC might be important for operant self-administration of EtOH.
Differences between EtOH and sucrose-drinking groups and handling controls were noted in the dopamine response during transfer from the home cage into the operant chamber. Rats in all 3 groups exhibited peak increases in mPFC dopamine when transferred into the operant chamber, and the largest increase in dopamine relative to baseline occurred in the 10S10E group. In contrast, we did not observe a difference between sucrose and handling groups. Across all groups, we attribute some of the dopamine increase to the physical handling of the rat and environment change. Physical handling increases extracellular dopamine in the mPFC, as does transfer into a novel environment (Feenstra and Botterblom, 1996; Feenstra et al., 1998, 2000). While the operant chamber environment was not novel, it was an environment change. In both EtOH and sucrose groups, transfer into the operant chamber exposed the rats to reward-associated contextual cues that could have contributed to the observed stimulation of mPFC dopamine. The present data, along with previous studies of the NAC (Doyon et al., 2005; Howard et al., 2009), suggest that both regions respond to EtOH-associated contextual stimuli with significant increases in extracellular dopamine relative to baseline compared with sucrose controls. Therefore, dopamine may be acting in the mPFC and NAC to stimulate EtOH-seeking behavior in response to EtOH-associated stimuli.
Our data clearly show that dopamine activity is enhanced in the mPFC during operant self-administration of sweetened EtOH or sucrose alone. As both solutions are rewarding and can act as reinforcers, the current work provides novel data to support the reward prediction role of mPFC dopamine, as has been found for the NAC (Carrillo and Gonzales, 2011; Day et al., 2007; Doyon et al., 2003, 2005, 2006; Howard et al., 2009; Stuber et al., 2008). The original reward prediction theory arose, in part, from experimental findings of single unit recording of presumed dopamine cell activity in the midbrain (Schultz et al., 1997). However, single unit recording in the midbrain does not allow conclusions about where dopamine release occurs in response to cues that predict reward. Recent work measuring changes in dopamine release in the NAC has added strong support for the role of dopamine as a reward prediction signal (Brown et al., 2011; Day et al., 2007; Hart et al., 2015; Stuber et al., 2008; Wassum et al., 2012). The current work using microdialysis of dopamine in the mPFC is also consistent with the idea that dopamine release may also act as a reward prediction signal similar to that in the NAC, at least in a qualitative manner. This was possible through the use of microdialysis followed by chromatographic separation of dopamine from other biogenic amines found in the mPFC. The time course of the dopamine signals in our microdialysis work is much longer (7 minutes) than observed with single unit recording or fast-scan cyclic voltammetry methods in which the reward signals are observed within 10 seconds.
We present our dopamine microdialysis data in 2 forms: raw dialysate concentration and percent of home cage baseline. In general, the results and interpretations are similar for both analyses across the different phases of the experiment. However, there are minor, but critical differences that should be noted. Specifically, the net increase in concentration of dopamine is similar in the EtOH and sucrose groups during the initiation of drinking when analyzed as raw concentration data, but the EtOH group shows a significantly greater increase as a percent of baseline. This is due to lower baseline concentrations in the EtOH group. The biological significance of either way of looking at this dopamine response is not clear, and both could be important. It has been suggested that dopamine signaling in the mPFC has an optimal level and increases or decreases from this level contribute to cognitive deficits (Cools and D'Esposito, 2011; Floresco and Magyar, 2006). However, without assessing the functional consequences of reduced basal dopamine concentrations in the mPFC of our animals, we cannot make assumptions about the implications of our findings on cognition.
In conclusion, we present novel data using a behaviorally relevant model that within the mPFC, the amount of extracellular dopamine is significantly different in rats that drink sweetened EtOH, compared to sucrose and handling controls. Rats trained to drink sweetened EtOH not only demonstrated reduced basal mPFC dopamine concentrations, but also exhibited a different dopamine response in anticipation of the drinking event and once consumption was initiated. Compared to previous reports on the NAC, our results also highlight important regional differences in the dopamine response to EtOH or EtOH-associated cues that are specific to the mPFC. Thus, our current data provides a critical foundation for future studies that may help identify mechanisms behind compromised prefrontal cortical executive function that are frequently observed in heavy alcohol consumers.
Supplementary Material
Fig. S1. Basal dopamine concentrations in the mPFC of rats trained to consume 10S or 10S10E
Fig. S2. Microdialysis probe placements within the mPFC
Table S1. Ethanol and sucrose consumption per session.
Acknowledgments
Authors thank So Yoon Lee, Hannah Bang, and Wonbin Song for technical assistance and Dr. Regina Mangieri for assistance with statistical analysis. Support provided by NIH/NIAAA (R37 AA11852 and T32 AA007471 to RAG), the Bruce–Jones Fellowship (CJS), and Undergraduate Research Fellowship (Geoff Dilly) at the University of Texas at Austin. GAD contributed to data acquisition. CJS contributed to the experimental design, data acquisition, analysis, interpretation, and writing. AAV performed the replication study and contributed to the interpretation and writing. JMD contributed to analysis, interpretation, and writing. RAG contributed to the experimental design, analysis, interpretation, and writing.
Footnotes
Supporting information: Additional Supporting Information may be found online in the supporting information tab for this article:
Conflict of interest: The authors have no conflict of interests.
References
- Bassareo V, Tanda G, Petromilli P, Giua C, Di Chiara G. Non-psychostimulant drugs of abuse and anxiogenic drugs activate with differential selectivity dopamine transmission in the nucleus accumbens and in the medial prefrontal cortex of the rat. Psychopharmacology. 1996;124:293–299. doi: 10.1007/BF02247433. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Van Der Linden M. Decision-making and impulse control after frontal lobe injuries. Curr Opin Neurol. 2005;18:734–739. doi: 10.1097/01.wco.0000194141.56429.3c. [DOI] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo J, Gonzales RA. A single exposure to voluntary ethanol self-administration produces adaptations in ethanol consumption and accumbal dopamine signaling. Alcohol. 2011;45:559–566. doi: 10.1016/j.alcohol.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Ding ZM, Ingraham CM, Rodd ZA, McBride WJ. The reinforcing effects of ethanol within the posterior ventral tegmental area depend on dopamine neurotransmission to forebrain corticolimbic systems. Addict Biol. 2014;20:458–468. doi: 10.1111/adb.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Oster SM, Hall SR, Engleman EA, Hauser SR, McBride WJ, Rodd ZA. The stimulating effects of ethanol on ventral tegmental area dopamine neurons projecting to the ventral pallidum and medial prefrontal cortex in female Wistar rats: regional difference and involvement of serotonin-3 receptors. Psychopharmacology. 2011;216:245–255. doi: 10.1007/s00213-011-2208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, Anders SK, Ramachandra VS, Czachowski CL, Gonzales RA. Effect of operant self-administration of 10% ethanol plus 10% sucrose on dopamine and ethanol concentrations in the nucleus accumbens. J Neurochem. 2005;93:1469–1481. doi: 10.1111/j.1471-4159.2005.03137.x. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Howard EC, Shippenberg TS, Gonzales RA. Kappa-Opioid receptor modulation of accumbal dopamine concentration during operant ethanol self-administration. Neuropharmacology. 2006;51:487–496. doi: 10.1016/j.neuropharm.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon WM, York JL, Diaz LM, Samson HH, Czachowski CL, Gonzales RA. Dopamine activity in the nucleus accumbens during consummatory phases of oral ethanol self-administration. Alcohol Clin Exp Res. 2003;27:1573–1582. doi: 10.1097/01.ALC.0000089959.66222.B8. [DOI] [PubMed] [Google Scholar]
- Engleman EA, Ingraham CM, McBride WJ, Lumeng L, Murphy JM. Extracellular dopamine levels are lower in the medial prefrontal cortex of alcohol-preferring rats compared to Wistar rats. Alcohol. 2006;38:5–12. doi: 10.1016/j.alcohol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH. Rapid sampling of extracellular dopamine in the rat prefrontal cortex during food consumption, handling and exposure to novelty. Brain Res. 1996;742:17–24. doi: 10.1016/s0006-8993(96)00945-6. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, Mastenbroek S. Dopamine and noradrenaline efflux in the prefrontal cortex in the light and dark period: effects of novelty and handling and comparison to the nucleus accumbens. Neuroscience. 2000;100:741–748. doi: 10.1016/s0306-4522(00)00319-5. [DOI] [PubMed] [Google Scholar]
- Feenstra MG, Botterblom MH, van Uum JF. Local activation of metabotropic glutamate receptors inhibits the handling-induced increased release of dopamine in the nucleus accumbens but not that of dopamine or noradrenaline in the prefrontal cortex: comparison with inhibition of ionotropic receptors. J Neurochem. 1998;70:1104–1113. doi: 10.1046/j.1471-4159.1998.70031104.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology. 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Leskovjan AC, Hoff AL, Hitzemann R, Bashan F, Khalsa SS, Wang GJ, Fowler JS, Volkow ND. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42:1447–1458. doi: 10.1016/j.neuropsychologia.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Hart AS, Clark JJ, Phillips PE. Dynamic shaping of dopamine signals during probabilistic Pavlovian conditioning. Neurobiol Learn Mem. 2015;117:84–92. doi: 10.1016/j.nlm.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty AA, Vogel WH. Modulation of the stress response by ethanol in the rat frontal cortex. Pharmacol Biochem Behav. 1993;45:327–334. doi: 10.1016/0091-3057(93)90247-q. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D (2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Gonzales RA. The dopamine response in the nucleus accumbens core-shell border differs from that in the core and shell during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:1355–1365. doi: 10.1111/j.1530-0277.2009.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Leggio B, Masi F, Grappi S, Nanni G, Gambarana C, Colombo G, DeMontis MG. Sardinian alcohol-preferring and non-preferring rats show different reactivity to aversive stimuli and a similar response to a natural reward. Brain Res. 2003;973:275–284. doi: 10.1016/s0006-8993(03)02533-2. [DOI] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse AC, Schulteis G, Holloway FA, Koob GF. Conditioned place aversion to the “hangover” phase of acute ethanol administration in the rat. Alcohol. 2000;22:19–24. doi: 10.1016/s0741-8329(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Kus L, Ashwell K, Watson C. Chemoarchitectonic Atlas of the Rat Forebrain. Academic Press; San Diego, CA: 1998. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Orlando, FL: 1986. [Google Scholar]
- Pettit HO, Justice JB., Jr Effect of dose on cocaine self-administration behavior and dopamine levels in the nucleus accumbens. Brain Res. 1991;539:94–102. doi: 10.1016/0006-8993(91)90690-w. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Perry EB, Jr, D'Souza DC, Bufis V, Elander J, Limoncelli D, Vendetti M, Dean E, Cooper TB, McKee S, Petrakis I. Preliminary findings on the interactive effects of IV ethanol and IV nicotine on human behavior and cognition: a laboratory study. Nicotine Tob Res. 2012;14:596–606. doi: 10.1093/ntr/ntr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A. Dopaminergic involvement in medial pre-frontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: a dual-site microinjection study in the rat. Physiol Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- Schier CJ, Dilly GA, Gonzales RA. Intravenous ethanol increases extracellular dopamine in the medial prefrontal cortex of the Long-Evans rat. Alcohol Clin Exp Res. 2013;37:740–747. doi: 10.1111/acer.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schier CJ, Mangieri RA, Dilly GA, Gonzales RA. Microdialysis of ethanol during operant ethanol self-administration and ethanol determination by gas chromatography. J Vis Exp. 2012;67:ii, 4142. doi: 10.3791/4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sinha R. The clinical neurobiology of drug craving. Curr Opin Neurobiol. 2013;23:649–654. doi: 10.1016/j.conb.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A. Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons. Science. 2008;321:1690–1692. doi: 10.1126/science.1160873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, Burnett EJ, Gass JT, Lopez MF, Mulholland PJ, Centanni SW, Floresco SB, Chandler LJ. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial pre-frontal cortex. J Neurosci. 2014;34:3706–3718. doi: 10.1523/JNEUROSCI.0623-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, Woodward JJ. Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci. 2007;27:4765–4775. doi: 10.1523/JNEUROSCI.5378-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT. Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol Psychiatry. 2012;71:846–854. doi: 10.1016/j.biopsych.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Basal dopamine concentrations in the mPFC of rats trained to consume 10S or 10S10E
Fig. S2. Microdialysis probe placements within the mPFC
Table S1. Ethanol and sucrose consumption per session.


