Abstract
Aggressive systemic mastocytosis is a rare hematologic neoplastic disease that presents with a poor prognosis and low survival rate. It typically manifests with symptoms associated to mast cell release of bioactive substances, causing anaphylaxis, flushing, autonomic and hemodynamic instability, gastric distress and headache. Moreover, more than 95% of cases are related to a mutation in codon 816 of the KIT gene, located on human chromosome 4q12 which codes for a type III receptor tyrosine kinase. We present a 78 year-old Hispanic man diagnosed with the aggressive subtype of systemic mastocytosis, who had an atypical manifestation and a KIT negative variant. The diagnosis was confirmed based on pathologic and serologic findings which included mast cell infiltration of the spleen and bone marrow, malignant ascites and an unusually elevated serum tryptase.
Keywords: systemic mastocytosis, KIT mutation, tryptase, myeloproliferative disorders
Introduction
Systemic mastocytosis (SM) is one of the seven myeloproliferative disorders (MPD) classified by WHO 2008 with the other six been Chronic Myelogenous Leukemia (CML), Chronic Neutrophilic Leukemia (CNL), Polycythemia Vera (PV), Primary Myelofibrosis (PM), Essential Thrombocythemia (ET) and Chronic Eosinophilic Leukemia (CEL) [1]. SM is a rare, somatic disorder, characterized by an abnormal expansion and accumulation of neoplastic mast cells (MCs) in various organ systems [2]. Depending of the extent of organ involvement, SM can be divided into cutaneous mastocytosis (CM), systemic mastocytosis (SM), and localized MC tumors. CM has skin limited involvement and occurs most commonly in the pediatric population. Localized MC tumors consist of MC conglomerates that form either an MC sarcoma or an extracutaneous mastocytoma, depending on the involved tissues. SM is characterized by mast cell infiltration more than one organ system, which may include spleen, GI tract and bone marrow (2).
SM is further classified into five subtypes: Indolent SM (ISM), Smoldering SM (SSM), SM with an associated hematologic non-MC-lineage disease (SM-AHNMD), Aggressive SM (ASM), and Mast cell leukemia (MCL, these last three conferring poor prognosis. Symptoms of SM are due to mass effect by the neoplastic mast cells, and the release of bioactive substances acting at both local and distal sites. One of these substances is tryptase which may cause anaphylaxis, flushing, palpitations, vascular collapse, gastric distress, lower abdominal crampy pain, and recurrent headache [3]. Other features of aggressive SM may include pancytopenia, lymphadenopathy, hypoalbuminemia, malabsorption, or large osteolyses (possibly as a result of mast cell–mediated fibrotic changes in these organs) as well as hepatosplenomegaly and ascites (due to periportal fibrosis associated with mass cell infiltration of liver parenchyma. The advanced variants of SM have poor prognosis, with an overall survival of less than 12 months (2).
Case Presentation
A 78 year old Hispanic male with arterial hypertension, controlled diabetes mellitus type II, chronic atrial fibrillation, coronary artery disease, colonic diverticulosis in warfarin, atenolol, lisinopril was consulted to the Hematology-Oncology section in August 2011 due to the incidental discovery of normocytic anemia with thrombo-cytopenia (4.5 WBC, Hgb 11.1, 94.9 MCV, 84,000 platelets-40 segs, 38 lymphs, 20 monocytes). The patient denied use of alcohol or smoking and had pertinent family history of two siblings with colon cancer. Patient had no splenomegaly, lymphadenopathy or ascites. Anemia workup revealed normal levels of B12, folate, iron, ferritin levels. A bone marrow biopsy revealed 80% cellularity with dysmegakaryopoiesis and slight monocytosis, suggestive of myelodysplastic syndrome(MDS). Cytogenetic results were normal. As patient had mild anemia and no symptoms with a low risk score, he continued in observation with no requirement of transfusions or active treatment. In 2013 the patient had a follow up colonoscopy with diverticuli and a sessile benign rectal polyp. However in follow up by July 2015, the patient developed early satiety and 13 lb weight loss since 3 months prior to evaluation. On physical exam he had epigastric pain upon palpation, for which was consulted for an upper endoscopy. The endoscopy revealed mucosal erythema in gastric area and a clean base ulcer; biopsy showed active chronic gastritis with Helicobacter pylori requiring treatment with amoxicillin and clarithromycin for 2 weeks.
Follow up laboratories disclosed WBC 4.1 (29 segs, 38 lymphs, 23 mono, 8 eos); Hgb of 9.3, Htc of 30.1 and Plt count in 75,000. Blood chemistry was significant for alkaline phosphatase in 315 (ref 30-115), but BUN, Cr and electrolytes were within normal ranges. Urinalysis, hepatitis B and C panel, thyroid function tests, HIV were non-contributory.
In the setting of constitutional symptoms and weight loss with worsening anemia, an adbominopelvic CT scan was performed. Abdominal CT showed diffuse lymphadenopathy, absent in previous imaging studies from 2010. Skeletal bone revealed heterogenous densities suggesting diffuse sclerotic metastatic disease vs myeloproliferative disorder. Additional findings included hepatocellular disease (hepatic nodular contour but no discrete enhancing lesions), pelvic ascites, and a left-sided pleural effusion with rounded atelectasis. A PET/CT scan disclosed a “possible mild hypermetabolic borderline size spleen (SUV 3.2) and skeletal bone marrow”. There was no increased capitation in abdominal lymphadenopathy (largest 2.4 cm). Due to above findings, a spleen biopsy was performed by Interventional Radiology. Pathology revealed fibrosis with increased eosinophilic, monocytoid cell infiltrate, and hyalinized granulomas as well as spindled cell areas positive for mast cell tryptase, CD117 and CD68, but negative for CD2, S100, AE1/3, CD34, CD163, CD21 and CD31. Tissue was negative for blasts, Hodgkin cells, CD20, CD3, CD34, CD15 and CD30 markers. AFB and GMS stains were also negative. The pathology was consulted to the Joint Pathology Center which agreed that the morphology of spindle cell areas together with the phenotype were diagnostic of mast cell disease (Figures 1 and 2). A bone marrow aspirate and biopsy also disclosed cluster of spindle cells, positive for CD117 and tryptase confirming the diagnosis of systemic mastocytosis. Cytogenetics were normal, and there were no mutations in JAK-2 or c-KIT (D816V codon) by FISH. Serum tryptase levels were 165 ng/ml (NV 2-10).
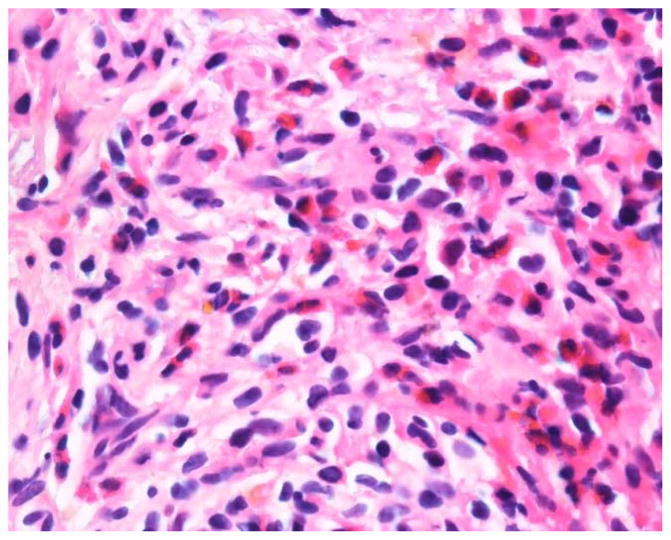
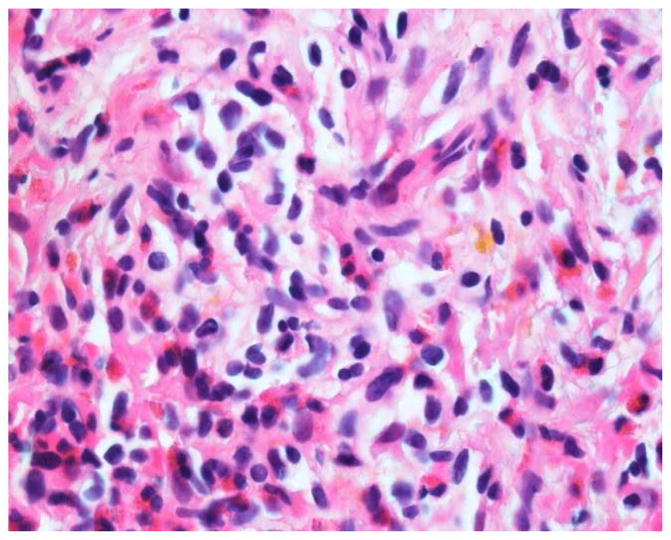
Figures 1 and 2.
H&E 1000x. Needle core biopsy of the spleen shows eosinophilic infiltrate as well as atypical mast cells with clear cytoplasm and somewhat spindle cell morphology. These cells labeled positive with CD117 and tryptase (mast cell markers).
Patient was started on Imatinib 400mg PO, as indicated for the treatment of adult patients with aggressive systemic mastocytosis without the D816V c-KIT mutation or with unknown c-KIT mutational status (4). Alternatives such as interferon or 2-CDA were considered in the patient but he was considered initially too fragile to tolerate those alternatives. Patient evolved with progressive tension ascites and worsening anemia (6.3 gm/dl) requiring PRBC transfusions. The patient had poor tolerance to imatinib, even at lower dose of 100 mg due to worsening diarrhea and tension ascites, requiring an intraperitoneal catheter for chronic drainage.
During the following months, the patient was hospitalized on multiple occasions due to massive ascites and symptomatic anemia. Low dose Prednisone at 5 mg improved the platelet count to over 150,000. Due to poor clinical response to imatinib, patient had a trial of low dose interferon alpha 1 million units s/c three times a week, but his aggressive mastocytosis progressed. The patient died in June 2016 in Hospice Care.
Discussion
Uncommitted and MC-committed progenitors express KIT a Type III receptor tyrosine kinase encoded by a 21-exon containing gene located on human chromosome 4q12 [4]. The ligand of the KIT receptor, stem cell factor (SCF), initiates the development of MCs from their uncommitted and MC-committed precursor cells as well as the proliferation, maturation, survival and proinflammatory mediator release from MCs [5,6]. In mastocytosis, more than 95% of cases undergo SCF-independent differentiation and accumulation of MCs due to a KIT receptor tyrosine kinase gain-of-function mutation (D816V) primarily an aspartic acid to valine substitution, which results in uncontrolled proliferation, enhanced survival and cell autonomous growth of MCs(2). This mutation is often detectable independent of the category of SM, including most aggressive subtypes of SM. Our patient however, tested negative for such mutation further emphasizing the uniqueness of this case. Additional somatic mutations are found in patients with SM-AHNMD, ASM, and MCL. These include mutations in TET2, SRSF2, ASXL1, CBL, RUNX1, and RAS [7–9].
The diagnosis of SM is based on pathologic and laboratory findings. Several criteria have to be met in order to confirm the diagnosis. Criteria are divided into Minor and Major criteria. When one major and one minor or three minor criteria are detected, a diagnosis can be established. Minor criteria are: (1) MCs in BM infiltrates, infiltrates in another extracutaneous organ, or in a BM smear showing spindle-shaped morphology (2) KIT mutation at codon 816 in extracutaneous organs (3) BM MCs express CD2 and/or CD25 by flow or IHC, (4) Serum total tryptase >20 ng/mL. Major criteria are: Multifocal dense infiltrates of MCs in BM sections or other extracutaneous organ(s) (>15 MCs in aggregate) [1].
This patient complies with the major criteria, as multifocal dense infiltrates of mast cells were identified both in bone marrow and spleen. He also complies with the minor criteria of a total serum tryptase >20 ng/mL. Since other flushing disorders were excluded, including carcinoid tumor and pheochromocytoma, this patient was diagnosed with SM.
The aim of initial management is to reduce the burden of systemic mediators of inflammation. H1 antihistamine is usually prescribed for flushing and pruritus in ASM patients. This patient, however, denied such symptoms. H2 antihistamine or proton pump inhibitor for gastric acid hypersecretion and oral cromolyn sodium for diarrhea and abdominal pain; aspirin for severe flushing to block biosynthesis of PGD2. Systemic glucocorticoids appear to alleviate the malabsorption. The patient had improvement in diarrhea with cromolyn sodium.
In terms of management of ASM itself, IFN-A (as long-term subcutaneous therapy) or 2CdA (3–6 cycles) are first-line therapy for patients who can tolerate it. If rapidly progressing ASM more intensive therapy is required. Polychemotherapy with fludarabine or 2CdA, often in combination with cytosine arabinoside is also an option for these patients. In older patients and those who cannot tolerate intensive therapy, conventional cytoreductive agents (2CdA) or palliative drugs [hydroxyurea (HU)] may be prescribed. In cases in which intensive therapy cannot be offered, palliative therapy with HU is an option (2). A recent open label study, demonstrated efficacy of Midostaurin, an oral small molecule agent that inhibits multiple kinases, in patients with advanced systemic mastocytosis, with a response rate of 60% (10). It is an emerging therapeutic option that inhibits non mutant and mutant KITD816V.
Despite treatment with two lines of therapy, such as imatinib and interferon, the patient continued with progressive ascites and worsening functional status.
Conclusion
Mastocytosis is a rare hematologic disease which requires clinical suspicion and pathologic expertise. As demonstrates our patient, treatment of advanced SM is a challenge, and most patients relapse or have resistant disease. There is hope that novel agents, especially tyrosine kinase inhibitors that can target the kit mutations can alter the dim prognosis of these patients (10). For patients that have drug resistance and are young and fit, stem cell transplant is an alternative treatment option (11). Therapy of SM has to be adjusted to the individual patient and the SM category with main aim in indolent disease to control mediator secretion while in aggressive disease to suppress the malignant clone expansion.
References
- 1.Horny HP, Metcalfe DD, Bennett JM, Bain BJ, Akin C, Escribano L, Valent P, Swerdlow SH, Campo E, Harris NL, et al., editors. WHO classification of Tomours of Haematopoietic and Lymphoid Tissues. 4. IARC; Lyon: 2008. Mastocytosis; pp. 54–63. [Google Scholar]
- 2.Valent P. Diagnosis and management of mastocytosis: an emerging challenge in applied hematology. American Society of Hematology. 2015:98–105. doi: 10.1182/asheducation-2015.1.98. [DOI] [PubMed] [Google Scholar]
- 3.Kasper Dennis. Harrison’s Principles of Internal Medicine 19/E. 19. Vol. 1. McGraw-Hill Education / Medical; VitalBook file; p. 20150417. [Google Scholar]
- 4.Arock M, Sotlar K, Cem A, et al. KIT Mutation Analysis in Mast Cell Neoplasms: Recommendations of the European Competence Network on Mastocytosis. Leukemia. 2015 Jun;29(6):1223–1232. doi: 10.1038/leu.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–956. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497–516. doi: 10.1586/ehm.10.42. [DOI] [PubMed] [Google Scholar]
- 7.Tefferi A, Levine Rl, Lim KH, et al. Frequent TET2 mutations in systemic mastocytosis: clinical KITD816V and F1L1P1-PDGFRA correlates. Leukemia. 2009;23:900–904. doi: 10.1038/leu.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson TM, Marie I, Simakova O, et al. Clonal analysis of NRAS activating mutations in KIT-D816V systemic mastocytosis. Haematologica. 2011;96:459–463. doi: 10.3324/haematol.2010.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scwabb J, Schnittger S, Sotlar K, et al. Comprehensive mutational profiling in advanced systemic mastocytosis. Blood. 2013;122:2460–2466. doi: 10.1182/blood-2013-04-496448. [DOI] [PubMed] [Google Scholar]
- 10.Gotlib J, Klum-Nelemans H, George TI, Akin C, et al. Efficacy and safety of Midostaurin in advanced systemic mastocytosis. New Engl J Med. 2016;374:2530. doi: 10.1056/NEJMoa1513098. [DOI] [PubMed] [Google Scholar]
- 11.Ustun C, Gotlib J, Popat U, et al. Consensus opinion on allogeneic hematopoietic cell transplantation in advanced systemic mastocytosis. Biol Blood Marrow Transplant. 2016;22(18):1348–56. doi: 10.1016/j.bbmt.2016.04.018. [DOI] [PubMed] [Google Scholar]