Abstract
The oral toxicity of phorate oxon (PHO), with emphasis on gender- and age-related effects, was characterized in the Sprague-Dawley rat. The oral LD50 (95% fiducial limits) for PHO in corn oil was 0.88 (0.79, 1.04) mg/kg in males and 0.55 (0.46, 0.63) mg/kg in females with a probit slope of 15. Females had higher baseline blood cholinesterase titers, but males were significantly more tolerant. Younger rats generally had lower absolute cholinesterase blood titers. However as PHO challenges increased, baseline-normalized cholinesterase inhibition was independent of age and gender. Butyrylcholinesterase (BChE) and especially acetylcholinesterase (AChE) in brains of younger females were affected more than that in either males or older females. In summary, while female rats, especially older females, had higher titers relative to males, female rats were more susceptible in terms of absolute cholinesterase inhibition and 24-hr lethality data, but the differences were not observed when titers were normalized to baseline levels.
Keywords: Phorate oxon, Pesticide, Toxicity, Rat, Oral
INTRODUCTION
Phorate (Thimet® 20G, AMVAC, Los Angeles, CA, USA) is an extremely toxic broad use insecticide and ascaricide, commonly applied as a granular agricultural product for long-term release and systemic uptake by plants to control specific pests, such as sucking insects on peanut and nematodes in soybean plants. Phorate is rapidly metabolized by plants, insects, and mammals into several organophosphorus (OP) anti-cholinesterase (anti-ChE) intermediates that are even more toxic than the parent compound, specifically phorate-sulfoxide, phorate-sulfone, phorate oxon (oxygen analog metabolite; also known as phoratoxon (PHO); O,O-diethyl S-(eththiomethyl) phosphorothioate; CAS RN 2600-69-3; Fig. 1), phorate oxon-sulfoxide, and phorate oxon-sulfone (pubchem.ncbi.nlm.nih.gov). These metabolites prevent ChE-mediated hydrolysis and inactivation of the neurotransmitter acetylcholine (ACh). Consequently, the mechanism of phorate toxicosis is similar to other OP compounds, i.e., nondiscriminatory inhibition of acetylcholinesterase (AChE), butyrylcholinesterase (BChE), carboxylesterase (CaE), etc. leading to an over-accumulation of the neurotransmitter acetylcholine (ACh) at neural synapses. Cholinolergic crisis can occur if the resultant increase in brain tissues and peripheral nerves acetylcholine levels are not rapidly controlled (De Bleecker et al., 1994; Rusyniak and Nañagas, 2004; Newmark, 2004). Clinical manifestation of toxicity includes miosis, increased and uncontrolled secretions, lacrimation, urination, defecation, fasciculations, seizures, convulsions, respiratory distress, and even death (Vale and Lotti, 2015).
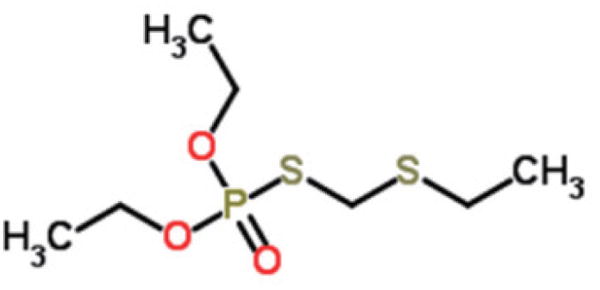
Fig. 1.
Phorate oxon (Phoratoxon).
Adverse environmental consequences associated with the agricultural use of phorate have been reported (Baburaj, 2013; Puschner et al., 2013; Lisker et al., 2011; Holme et al., 2016). Additionally, phorate also presents a challenge to public health. For example, deliberate and/or accidental human exposure to phorate and its metabolites is most probable through inhalation of ambient air in areas recently treated with the pesticide (Han, 2011; Mission, 2006), ingestion of contaminated food (Khatiwada et al., 2012; Zhou and Zhao, 2015) or liquids (Zhang et al., 2012; Salas et al., 2003), and/or dermal contact through occupational exposure (Kashyap et al., 1984; Young et al., 1979). Owing to its high level of toxicity and multiple avenues of exposure to this compound, phorate (and its metabolites) represents a very real poisoning threat. As such, it is critical that gaps in understanding its overall toxicity be addressed.
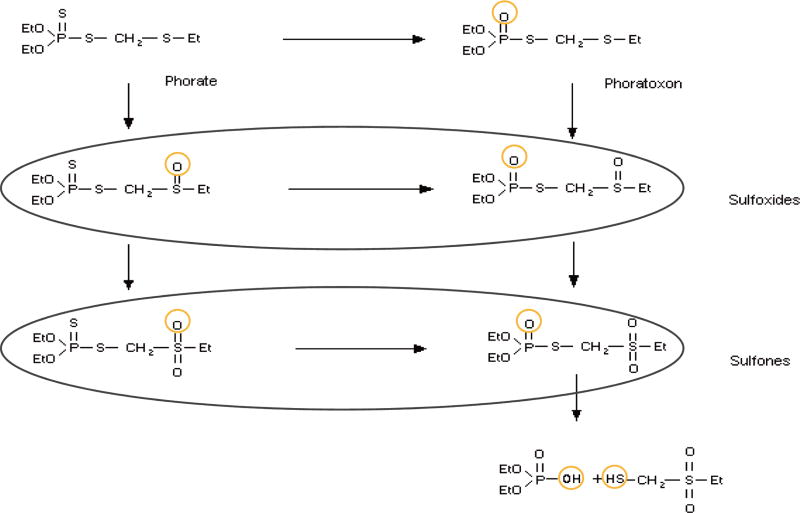
While animal toxicity data of the parent compound phorate has been reported (pmep.cce.cornell.edu; pesticideinfo.org), relatively little is known about its more toxic metabolites. Since OP oxons are typically more toxic than the parent pesticides (Sultatos et al., 1985; Natoff, 1967) and PHO is easily generated via a simple O-substitution in the phosphorodithioate group (Fig. 2, inchem.org) of phorate, it was identified as the first metabolite to be characterized. Previous work involving topical challenges of bioactivated PHO on guinea pigs demonstrated a median lethal dose (LD50) of 98 mg/kg, and by the subcutaneous route, 2.1 mg/kg (unpublished data). From the doselethality probit curves characterized in those works, the subcutaneous and topical LD85 challenge levels were then used to assess the therapeutic efficacy of several oxime AChE reactivators administered in conjunction with the U.S. FDA-recommended pre-hospital level of atropine (Snider et al., 2015). Although some of the tested oximes demonstrated efficacy against PHO in the subcutaneously exposed study (therapies given ~1 min after PHO administration), none was therapeutically effective when exposure occurred dermally (treated at onset of clinical evidence of toxicosis).
Fig. 2.
Metabolic pathways of phorate.
Unfortunately, very little else is known about PHO toxicity by other, more human-relevant routes of exposure. Consequently, the purpose of the current study was to 1) evaluate the oral/ingestion toxicity of PHO in the Sprague-Dawley rat and 2) investigate and characterize any potential differences in susceptibility due to gender and age.
MATERIALS AND METHODS
PHO was synthesized in-house. Under argon, an 11.9-mL volume of triethylamine was slowly added to a suspension of 10.7 g of diethyl phosphite and 2.7 g of solid sulfur in a two-necked round-bottom flask equipped with a reflux condenser and a rubber septum. After full conversion of the phosphite, as monitored by 31P NMR spectroscopy, the suspension was diluted with diethyl ether to 100 mL and then washed with 100 mL aqueous 1M HCl, dried over magnesium sulfate, concentrated under reduced pressure, and finally dried under high vacuum. The resulting suspension was filtered over a small cotton plug to yield the diethyl S-hydrogen phosphorothiolate. The yield was 10.48 g (80%) of product as a yellow oil. A 51-g volume of O,O-diethylthiolphosphoric acid was added to 35 g. of aqueous 33% formaldehyde solution and subsequently 20 g of ethyl sulfide with stirring at 30°C. The mixture was allowed to stand for 2 hr, after which the oily layer separated. The oily layer was extracted with chloroform, washed with 5% aqueous sodium carbonate and dried over sodium sulfate. The solvent was removed, and the residue distilled to give 10.7 g (15% yield). Purity was determined by 31P NMR, (99.6%), 1H NMR (97.6%), and GC/MS (99.4%), and 97.6% purity was used in all dose calculations. PHO was used as a 0.125 mg/mL solution in Mazola® corn oil (CAS RN 8001-30-7; ACH Food Companies, Cordova, TN, USA).
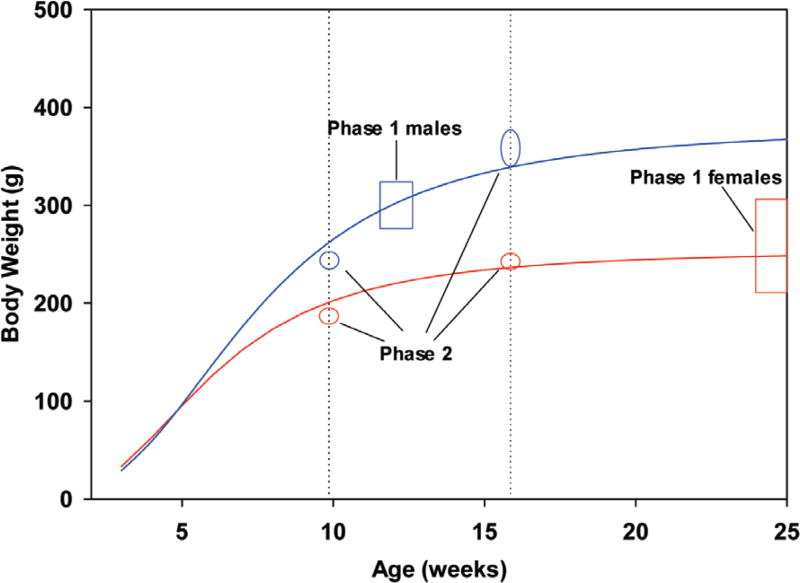
Male and female Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN, USA). Rats were identified with ear tags and same-gender pair housed before challenge in polycarbonate cages labeled with cage cards. During the 7-day quarantine period, the rats were weighed and randomized by body weight into test days. For the first aim of the study (Phase 1), each of four test days comprised of five treatment groups of either two rats receiving PHO/corn oil or one vehicle control rat receiving corn oil. Among the 72 rats used in this aim, body weight means (minimum - maximum) on the day prior to challenge were 297 (270 – 315) g for males (approximately 12 weeks old) and 266 (212 – 306) g for females (approximately 25 weeks old). Among the 24 male rats used in the second aim of the study (Phase 2), body weight means (minimum - maximum) on the day prior to challenge were 242 (233 – 233) g for males at 9.9 weeks old and 360 (339 – 375) g for males at 15.9 weeks old. These groups were roughly equidistant from the Phase 1 males in body weight. Among the 24 female rats used in Phase 2, body weight means (minimum - maximum) on the day prior to challenge were 185 (177 – 196) g at 9.9 weeks old and 245 (231 – 260) g for females at 15.9 weeks old, and both of these were less than Phase 1 females in body weight. Ordered animals were expected to be 9 and 15 weeks of age but arrived closer to 10 and 16 weeks (Fig. 3; Phase 1 and 2 ranges are depicted with rectangles and ovals, respectively).
Fig. 3.
Typical growth curves for Sprague-Dawley male and female rats and selection of weight ranges.
On the morning of each challenge day, a ~0.7-mL baseline blood sample was collected from the vena cava of each animal, placed in K3 EDTA tubes, chilled, processed, and stored at ≤ −70°C. Each rat was administered a 0.1 to 5.0 mL volume of a 0.125 mg/mL solution of PHO in corn oil using a 1-mL or 5-mL syringe with attached feeding needle (18G × 7.6-cm, 2-mm ball). The target PHO dose administered varied across test days in order to obtain relevant and valuable dose/lethality data for assessing PHO toxicity. In Phase 1, the objective was to obtain a median lethal dose (LD50) with 95% fiducial limits and slope using probit analysis for each gender. Then in Phase 2, age and gender effects were investigated by repeating Phase 1 in rats varied both by age and gender.
Clinical observations of survivors were recorded at 0.25, 0.5, 1, 2, 4 and 24 hr post-challenge. After the final observation, each surviving animal was euthanized with an IM injection of euthanasia solution. A 2-mL volume of blood was collected via the intracardial route from euthanized animals, placed into K3 EDTA tubes, and designated as the “terminal” sample. Brains from animals surviving to 24 hr were collected and bisected sagittally. One brain half was placed into a cassette and plunged into liquid nitrogen and the other half was placed into 10% neutral buffered formalin. No additional tissues were collected.
From the chilled blood samples, two 80-µL aliquots of whole blood were removed, diluted, and treated with Hemoglobind™ (Biotech Support Group LLC, Monmouth Junction, NJ, USA) to remove hemoglobin for later assay for cholinesterase activity (McGarry et al., 2013). Half (~1 mL) of the remaining blood sample was centrifuged to obtain plasma for PHO concentration analysis, and the remaining whole blood was processed for PHO concentration analysis via LC/MS/MS (detection limit: 0.5 ng/mL). Processed samples were stored at ≤ −70°C. Cholinesterase activity was assessed using a spectrophotometric assay conducted in a manner similar to Ellman (Ellman et al., 1961). AChE and BChE activity levels were calculated using 1 × 10−3 M acetylthiocholine (ATC) or 3 × 10−3 M butyrylthiocholine (BTC) as the substrate respectively. DTNB [5,5-dithio-bis-(2-nitrobenzoic acid); Ellman’s Reagent; Sigma Aldrich: D8130, St. Louis, MO, USA] at 5 × 10−4 M was used as the indicator. The extinction coefficient used for calculation purposes was 13,600 (M−1 cm−1).
Frozen brain samples were thawed, homogenized, and assayed for ATC and BTC hydrolysis activities, protein, and PHO concentration via LC/MS/MS (detection limit: 0.5 ng/g). Enzyme activities were expressed as international units per gram of protein.
Statistical Analysis
Clinical signs were tabulated by gender and (for Phase 2) age. Probit dose-response models were fitted to lethality data using the method of maximum likelihood (Finney, 1971). Either Fieller’s method or the delta method was used to compute a 95 percent fiducial limit for each LD50 (Oehlert, 1992). STATA® 11.0 (StataCorp, College Station, TX, USA) was used to analyze the survival data. Blood and brain ATC and BTC hydrolysis activities and brain PHO concentrations were tabulated by gender and (for Phase 2) age, and the blood ChE data were normalized by pre-challenge control values to form the terminal relative activity parameters RAATC,t and RABTC,t.
RESULTS AND DISCUSSION
Phase 1
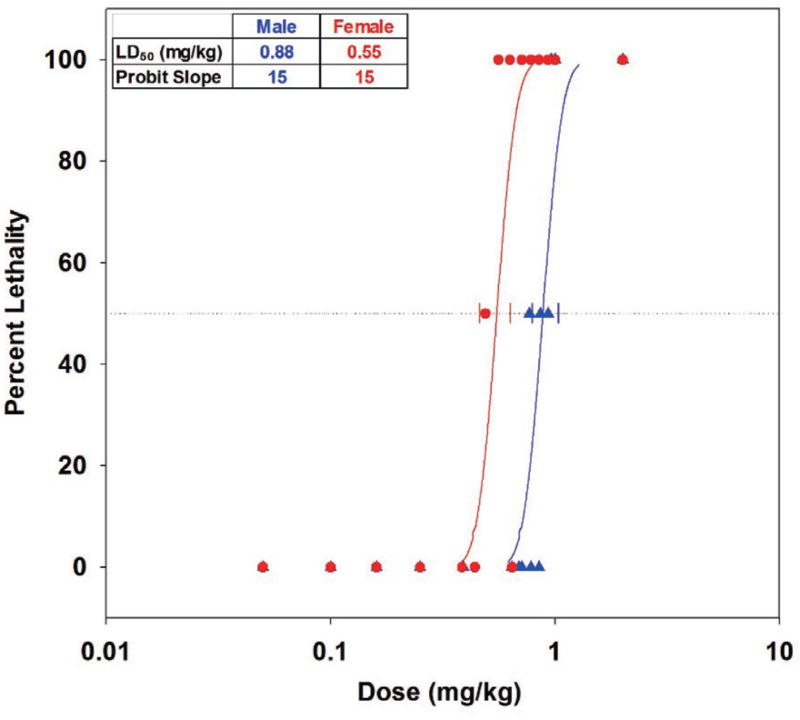
Male rats challenged below 0.77 mg/kg were asymptomatic, while those challenged between 0.77 and 2.00 mg/kg presented ataxia/lethargy (28%), fasciculations (22%), tremors (39%), dyspnea (9%), and death (25%). Female rats challenged below 0.10 mg/kg were asymptomatic, while those challenged between 0.10 and 2.00 mg/kg presented ataxia/lethargy (47%), fasciculations (47%), tremors (58%), dyspnea (33%), and death (47%). Time to death, which if observed, usually occurred within 4 hr of challenge and significantly (p < 0.05) decreased versus log(dose) in both genders. Probit analysis on 24-hr lethality rates (Fig. 4) indicated that males were significantly (p < 0.0001) more tolerant then females to oral challenges of PHO. The 24-hr LD50 (with 95% fiducial limits) among males was 0.55 (46, 63) mg/kg and among females was 0.88 (0.79, 1.04) mg/kg. A test between genders for parallel slopes passed, and the common probit slope was 15 (p = 0.0008). The oral LD01, an estimate of the maximally tolerated dose, was 0.61 mg/kg in males and 0.38 mg/kg in females.
Fig. 4.
Phase 1 probit analysis, phorate oxon/corn oil in Sprague-Dawley rats by oral gavage LD50 with 95% fiducial limits.
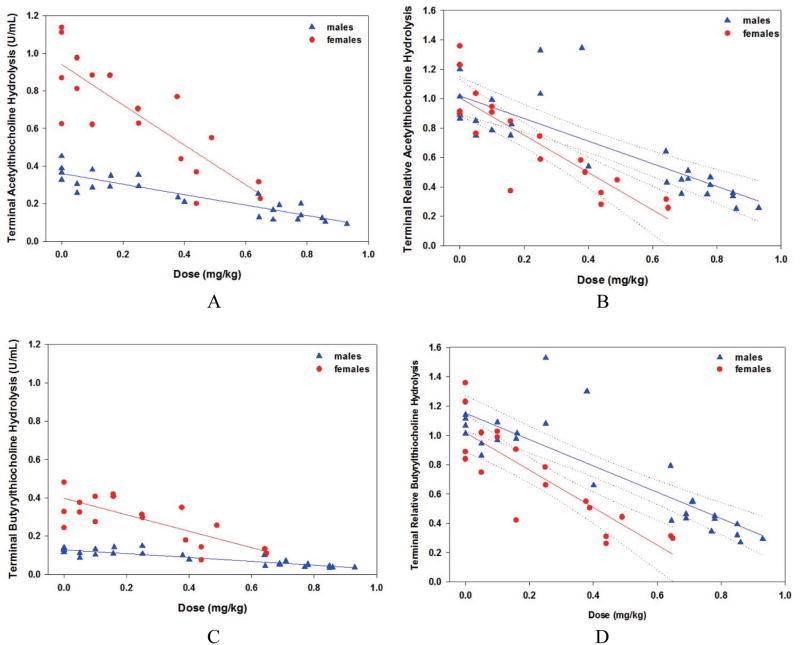
Phase 1, terminal blood ATC hydrolysis data are presented in Fig. 5, Insets A (absolute) and B (baseline-normalized, RAATC,t). Similar plots are presented for BTC hydrolysis data in Fig. 5, Insets C and D. Baseline BTC hydrolysis rates were a fraction of ATC hydrolysis rates, approximately 42% in males and 32% in females. In Fig. 5, Inset A, two-sided t tests indicated that male baseline values (mean ± standard deviation) for ATC hydrolysis rates at 0.36 ± 0.06 U/mL were significantly (p < 0.0001) less than that of females at 1.03 ± 0.37 U/mL. Likewise in Fig. 5, Inset C, male BTC hydrolysis rates at 0.11 ± 0.01 U/mL were significantly (p < 0.0001) less than that of females at 0.44 ± 0.16 U/mL. Twenty-six of the 72 rats challenged died before a 24-hr, terminal blood sample could be collected. In Fig. 5, Inset A linear regression of absolute terminal ATC hydrolysis was significantly correlated with PHO challenge dose for both males (slope = −0.28, p < 0.0001) and females (slope = −1.07, p < 0.0001). This suggested that PHO cholinergic effects increased linearly with dose, and that females were more strongly affected. In Fig. 5, Inset B, baseline-normalized ATC values indicated that (1) individuals from both genders were unaffected by the corn oil vehicle as expected (mean RAATC,t ≈ 1.0), and (2) females (slope = −1.27, p < 0.0001) were more strongly affected from equivalent challenges of PHO relative to males (slope = −0.77, p < 0.0001). In addition, the divergence of the regression 95% fiducial limits indicated that the gender effect was significant above 0.3 mg/kg. In Fig. 5, Inset C, absolute terminal BTC hydrolysis rate was significantly correlated with PHO challenge dose for both males (slope = −0.10, p < 0.0001) and females (slope = −0.43, p = 0.0002). This reinforced that PHO cholinergic effects increased linearly with dose, and that females were more strongly affected. In Fig. 5, Inset D, baseline-normalized BTC values indicated that (1) individuals from both genders were unaffected by the corn oil vehicle as expected (mean RABTC,t ≈ 1.1), and (2) females (slope = −1.28, p < 0.0001) were more strongly affected from equivalent challenges of PHO relative to males (slope = −0.90, p = 0.0001). In addition, divergence of the regression 95% fiducial limits indicated that the gender effect was significant above 0.2 mg/kg.
Fig. 5.
Phase 1 terminal blood, males and females: Inset A, acetylthiocholine hydrolysis, absolute values; Inset B, acetylthiocholine hydrolysis, baseline-normalized values; Inset C, butyrylthiocholine hydrolysis, absolute values; Inset D, butyrylthiocholine hydrolysis, baseline-normalized values.
Phase 1, brain cholinesterase data are presented for both genders in Fig. 6, Insets A (absolute ATC hydrolysis) and B (absolute BTC hydrolysis). Naïve levels of brain BTC hydrolysis were approximately 60% of brain ATC hydrolysis. Two-sided t tests indicated that among vehicle controls, male rat ATC hydrolysis (mean ± standard deviation) at 13.7 ± 3.5 U/g was not significantly (p = 0.4760) different from that of female rats at 12.3 ± 1.5 U/g, and male rat BTC hydrolysis at 7.7 ± 1.7 U/g was not significantly (p = 0.5434) different from that of female rats at 7.1 ± 0.8 U/g. Similar to the blood results, linear regression of brain ATC hydrolysis was significantly correlated with PHO challenge dose for both males (slope = −5.2, p = 0.0015) and females (slope = −9.6, p < 0.0001). Divergence of the regression 95% fiducial limits indicated that the gender effect was significant above 0.4 mg/kg. Again, linear regression of brain BTC hydrolysis was significantly correlated with PHO challenge dose for both males (slope = −4.4, p < 0.0001) and females (slope = −7.1, p < 0.0001). Divergence of the regression 95% fiducial limits indicated that the gender effect was significant above 0.3 mg/kg.
Fig. 6.
Phase 1 brain, males and females, absolute values: Inset A, acetylthiocholine hydrolysis; Inset B, butyrylthiocholine hydrolysis.
With few exceptions, no free PHO, PHO sulfoxide, or PHO sulfone was detected in the blood or brains of the animals. Two brains from male rats challenged at above-average levels contained small but measured phorate oxon sulfoxide. The PHO was likely bound to cholinesterase and other potential biological targets, or metabolized and excreted, precluding detection of the free PHO.
In view of Phase 1 data indicating that older (by a mean of approximately 13 weeks) yet smaller (by a mean of 31 g) female rats were more sensitive to orally administered PHO than younger yet heavier males, Phase 2 was designed to determine whether the effects were truly gender-related or age/weight-related. Similar effects had been observed for chlorpyrifos (Moser et al., 1998) and explained as likely due to chlorpyrifos sequestration by increased liver carboxylesterase in adult male rat liver (Sterri et al., 1985).
Phase 2
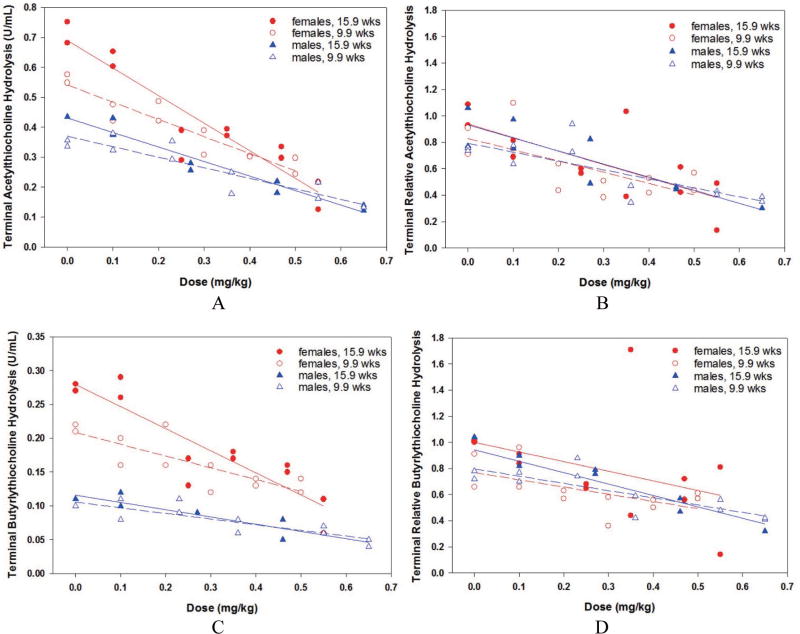
Phase 2 rats were generally asymptomatic at challenges less than 0.25 mg/kg, and at higher challenges sometimes exhibited the same cholinergic signs as in Phase 1. The 15.9-week old males presented somewhat more frequent cholinergic signs (dyspnea, fasciculation, tremors, tremors, ataxia/lethargy, and death) at the upper levels relative to the younger males. Since the challenge doses in this phase were intended to be non-lethal, the scarcity of clinical signs was not surprising. Terminal blood hydrolysis data are presented in Fig. 7, Insets A (absolute ATC), B (baseline-normalized, RAATC,t), C (absolute BTC), and D (baseline-normalized, RABTC,t). Only two male rats challenged at 0.75 mg/kg died before a 24-hr, terminal blood sample could be collected. Two-sided t tests indicated that male baseline values (mean ± standard deviation calculated across both younger and older rats) for ATC hydrolysis rates at 0.45 ± 0.08 U/mL were significantly (p < 0.0001) less than that of females at 0.69 ± 0.19 U/mL, and male BTC hydrolysis rates at 0.12 ± 0.01 U/mL were significantly (p < 0.0001) less that of females at 0.26 ± 0.07 U/mL. Thus, as presented in Phase 1, naïve female rats had higher ChE levels relative to males. The effects of age were very small and not statistically significant (p > 0.05) in either gender for either enzyme. In Fig. 7, Inset A, linear regression of absolute terminal ATC hydrolysis was significantly correlated with PHO challenge dose for all groups: 9.9-week males (slope = −0.35, p = 0.0002), 15.9-week males (slope = −0.49, p = 0.0002), 9.9-week females (slope = −0.57, p < 0.0001), and 15.9- week females (slope = −0.92, p < 0.0001). As in Phase 1, the terminal data suggested that PHO cholinergic effects increased linearly with dose, and that females were more strongly affected. However in Fig. 7, Inset B, baseline- normalized values indicated no gender- or age-dependent enhanced effects of PHO, as all four regression lines were basically parallel and indistinguishable. Likewise in Fig. 7, Inset C, absolute terminal BTC hydrolysis rate was significantly correlated with PHO challenge dose for all four groups: 9.9-week males (slope = −0.08, p = 0.0009), 15.9-week males (slope = −0.11, p = 0.0005), 9.9-week females (slope = −0.17, p < 0.0020), and 15.9-week females (slope = −0.33, p < 0.0003). In Fig. 7, Inset D, baseline-normalized BTC hydrolysis rates reinforced that PHO cholinergic effects increased linearly with dose, and indicated no gender- or age-dependent enhanced effects of PHO, as all four regression lines were basically parallel and indistinguishable.
Fig. 7.
Phase 2 terminal blood, males and females: Inset A, acetylthiocholine hydrolysis, absolute values; Inset B, acetylthiocholine hydrolysis, baseline-normalized values; Inset C, butyrylthiocholine hydrolysis, absolute values; Inset D, butyrylthiocholine hydrolysis, baseline-normalized values.
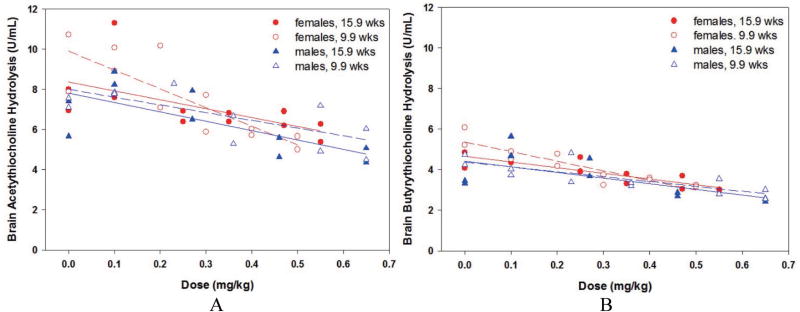
Phase 2, brain ATC and BTC hydrolysis rates are presented in Fig. 8, Insets A (absolute ATC hydrolysis) and B (absolute BTC hydrolysis). Naïve levels of brain BTC hydrolysis were approximately 60% of brain ATC hydrolysis. Two-sided t tests comparing four male and four female brain levels indicated no statistically significant age- or gender-related effects for either ATC or BTC hydrolysis rates from terminal brains. Similar to the blood results, linear regression of brain ATC hydrolysis was significantly correlated with PHO challenge dose in all four groups: 9.9-week males (slope = −3.8, p = 0.0120), 15.9-week males (slope = −4.7, p = 0.0221), 9.9-week females (slope = −9.3 p = 0.0016), and 15.9-week females (slope = −4.4, p = 0.0339). Although not shown, overlap of the regression 95% fiducial limits indicated no significant gender- or age-related effects. Again, linear regression of brain BTC hydrolysis was significantly correlated with PHO challenge dose in all four groups: 9.9-week males (slope = −2.4, p = 0.0027), 15.9-week males (slope = −2.8 p = 0.0433), 9.9-week females (slope = −4.8 p = 0.0002), and 15.9-week females (slope = −2.8, p = 0.0008). Although not shown, overlap of the regression 95% fiducial limits indicated no significant gender-or age-related effects.
Fig. 8.
Phase 2 brain, males and females, absolute values: Inset A, acetylthiocholine hydrolysis; Inset B, butyrylthiocholine hydrolysis.
In conclusion, in the ingestion lethality studies, the oral LD50 (95% fiducial limits) for PHO in corn oil was 0.88 (0.79, 1.04) mg/kg in males and 0.55 (0.46, 0.63) mg/kg in females with a common probit slope of 15. The oral LD01 was 0.61 mg/kg in males and 0.38 mg/kg in females. Female rats had higher baseline blood cholinesterase titers, but male rats were significantly (p < 0.0001) more tolerant of oral PHO as measured by cholinesterase inhibition slopes and dose/lethality responses. From Phase 2, higher titers of blood AChE and BChE were present in female rats, and 9.9-week rats generally had lower levels of both circulating enzymes. However, the rate of decreasing baseline-normalized activity levels in response to increasing PHO challenges was similar among all groups. In brain tissue, the BChE and especially AChE inhibition response to increasing PHO challenges in 9.9-week females was slightly more pronounced than that in either older females or 9.9- and 15.9-week old males. Enhanced tolerance in male rats may have been due to differences in gender-specific physiology, such as hepatic carboxylesterase titers that increase with age. However, the Phase 1 males were approximately half-way between the two groups of Phase 2 males in body weight, and there was no apparent explanation why the Phase 1 males were more tolerant of oral PHO than males in Phase 2. Females reacted to oral PHO challenges similarly between the two phases, even though the Phase 1 females likely had been approximately 25 weeks old.
In summary, both study phases found the female rat, especially older females, to have higher levels of blood cholinesterase titers relative to males, but that in terms of absolute cholinesterase inhibition slopes and 24-hr lethality rates, females rats were less tolerant than males at equivalent oral challenges of PHO. There were no consistent age- or gender-related effects when blood or brain ATC or BTC hydrolysis rates were normalized to baseline levels. Additional studies, such as gender-based PHO toxicokinetics (which were beyond the scope of the current contracted study), may shed light on these observations.
Acknowledgments
The authors wish to recognize the excellent technical assistance of Nichole Carpenter, Megan Lallier, Brent McCracken, Leslie Wannamaker, and Patrick DeArmond. The authors also wish to thank Countermeasures Against Chemical Threats (CounterACT) Program Steering Committee (CPSC) members for their expertise and guidance in the design of this study.
This work was supported by the National Institutes of Health (NIH) Office of the Director through an interagency agreement (OD#: Y1- OD-0387-01) between the National Institute of Allergy and Infectious Diseases (NIAID) and Department of Defense (DoD) and prepared under the auspices of the NIH, NIAID, NINDS, and the DoD Defense Technical Information Center (DTIC) under the Chemical, Biological, Radiological & Nuclear Defense Information Analysis Center (CBRNIAC) program, Contract No. SP0700-00-D-3180, Delivery Order Number 0794, CBRNIAC Task 689/CB-13-0689.
The views expressed in this article are those of the authors and do not reflect the official policy of the NIH, Department of Health and Human Services, or the U.S. Government. No official support or endorsement of this article by the NIAID, NINDS, or NIH is intended or should be inferred. The experimental protocol was approved by the Institutional Animal Care and Use Committee at Battelle. All procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended. The sponsor developed the concept of the study and contributed to its design and the interpretation of the data as well as the preparation of the manuscript and the decision to submit it for publication. The sponsor also made similar contributions to other studies occurring at Battelle during the same time frame.
Footnotes
Conflict of Interest---- The authors have no known conflicts of interest.
References
- Baburaj S. Experts ring alarm bell over drop in number of crows. [Accessed 16 Feb., 2016];2013 http://timesofindia.indiatimes.com/city/bhopal/Experts-ring-alarm-bell-over-drop-in-number-of-crows/articleshow/26812349.cms.
- De Bleecker J, Lison D, Van Den Abeele K, Willems J, De Reuck J. Acute and subacute organophosphate poisoning in the rat. Neurotoxicology. 1994;15:341–348. [PubMed] [Google Scholar]
- Ellman G, Courtney K, Andres V, Jr, Feather-Stone R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Finney D. Probit Analysis. Third. Cambridge University Press; 1971. [Google Scholar]
- Holme F, Thompson B, Holte S, Vigoren W, Espinoza N, Ulrich A, Griffith W, Faustman E. The role of diet in children’s exposure to organophosphate pesticides. Environ. Res. 2016;147:133–140. doi: 10.1016/j.envres.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han D. Airborne concentrations of organophosphorus pesticides in Korean pesticide manufacturing/formulation workplaces. Ind. Health. 2011;49:703–713. doi: 10.2486/indhealth.ms1304. [DOI] [PubMed] [Google Scholar]
- https://pubchem.ncbi.nlm.nih.gov/compound/phorate (Accessed 10 Feb., 2016).
- http://pmep.cce.cornell.edu/profiles/insect-mite/mevinphospropargite/phorate/insect-prof-phorate.html (Accessed 10 Feb., 2016).
- http://www.pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC33402 (Accessed 10 Feb., 2016).
- http://www.inchem.org/documents/jmpr/jmpmono/v077pr17.gif (Accessed 10 Feb., 2016).
- Kashyap S, Jani J, Saiyed H, Gupta S. Clinical effects and cholinesterase activity changes in workers exposed to Phorate (Thimet) J. Environ. Sci. Health B. 1984;19:479–489. doi: 10.1080/03601238409372445. [DOI] [PubMed] [Google Scholar]
- Khatiwada S, Tripathi M, Pokharel K, Acharya R, Subedi A. Measurement of organophosphate pesticides, organochlorine pesticides, and polycyclic aromatic hydrocarbons in household dust from two rural villages in Nepal. J. Nepal Med. Assoc. 2012;52:49–51. [PubMed] [Google Scholar]
- Lisker E, Ensminger M, Gill S, Goh K. Detections of eleven organophosphorus insecticides and one herbicide threatening pacific salmonids, oncorhynchus spp., in California, 1991–2010. Bull. Environ. Contam. Toxicol. 2011;87:355–360. doi: 10.1007/s00128-011-0351-7. [DOI] [PubMed] [Google Scholar]
- McGarry K, Bartlett R, Machesky N, Snider T, Moyer R, Yeung D, Brittain M. Evaluation of HemogloBind™ treatment for preparation of samples for cholinesterase analysis. Adv. Biosci. Biotechnol. 2013;4:1020–1023. doi: 10.4236/abb.2013.412136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mission K. Pesticide Spray Proves Disastrous In Salkiana Village, Jalandhar. [Accessed 10 Feb., 2016];2006 http://www.countercurrents.org/en-kvm040806.htm.
- Moser V, Chanda S, Mortensen S, Padilla S. Age-and gender-related differences in sensitivity to chlorpyrifos in the rat reflect developmental profiles of esterase activities. Toxicol. Sci. 1998;46:211–222. doi: 10.1006/toxs.1998.2526. [DOI] [PubMed] [Google Scholar]
- Natoff I. Influence of the route of administration on the toxicity of some cholinesterase inhibitors. J. Pharm. Pharmacol. 1967;19:612–616. doi: 10.1111/j.2042-7158.1967.tb09598.x. [DOI] [PubMed] [Google Scholar]
- Newmark J. Therapy for nerve agent poisoning. Arch. Neurol. 2004;61:649–652. doi: 10.1001/archneur.61.5.649. [DOI] [PubMed] [Google Scholar]
- Oehlert G. A note on the delta method. Amer. Statist. 1992;46:27–29. [Google Scholar]
- Puschner B, Gallego S, Tor E, Wilson D, Holstege D, Galey F. The Diagnostic Approach and Public Health Implications of Phorate Poisoning In a California Dairy Herd. [Accessed 16 Feb., 2016];2013 http://www.omicsonline.org/the-diagnostic-approach-and-pub-lic-health-implications-of-phorate-poisoning-in-a-california-dairy-herd-2161-0495.S13-001.pdf.
- Rusyniak D, Nañagas K. Organophosphate poisoning. Semin. Neurol. 2004;24:197–204. doi: 10.1055/s-2004-830907. [DOI] [PubMed] [Google Scholar]
- Salas J, González M, Noa M, Pérez N, Díaz G, Gutiérrez H, Zazueta R, Osuna I. Organophosphorus pesticide residues in Mexican commercial pasteurized milk. J. Agric. Food Chem. 2003;51:4468–4471. doi: 10.1021/jf020942i. [DOI] [PubMed] [Google Scholar]
- Snider T, Wilhelm C, Babin M, Platoff G, Jr, Yeung D. Assessing the therapeutic efficacy of oxime therapies against percutaneous organophosphorus pesticide and nerve agent challenges in the Hartley guinea pig. J. Toxicol. Sci. 2015;40:759–775. doi: 10.2131/jts.40.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultatos L, Minor L, Murphy S. Metabolic activation of phosphorothioate pesticides: role of the liver. J. Pharmacol. Exp. Ther. 1985;232:624–628. [PubMed] [Google Scholar]
- Sterri S, Berge G, Fonnum F. Esterase activities and soman toxicity in developing rat. Acta Pharmacol. Toxicol. 1985;57:136–140. doi: 10.1111/j.1600-0773.1985.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Vale A, Lotti M. Organophosphorus and carbamate insecticide poisoning. Handb. Clin. Neurol. 2015;131:149–168. doi: 10.1016/B978-0-444-62627-1.00010-X. [DOI] [PubMed] [Google Scholar]
- Young R, Jung F, Ayer H. Phorate intoxication at an insecticide formulating plant. Am. Ind. Hyg. Assoc. J. 1979;40:1013–1016. doi: 10.1080/15298667991430631. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhang Z, Chen F, Zhang H, Hu X. Effect of sonication on eliminating of phorate in apple juice. Ultrason. Sonochem. 2012;19:43–48. doi: 10.1016/j.ultsonch.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhao X. Susceptibility of nine organophosphorus pesticides in skimmed milk towards inoculated lactic acid bacteria and yogurt starters. J. Sci. Food Agric. 2015;95:260–266. doi: 10.1002/jsfa.6710. [DOI] [PubMed] [Google Scholar]