Abstract
Recent NMR-based, chemical shift mapping experiments with the minimal DNA-binding domain of XPA (XPA-MBD: M98–F219) suggest that a basic cleft located in the loop-rich subdomain plays a role in DNA-binding. Here, XPA–DNA interactions are further characterized by NMR spectroscopy from the vantage point of the DNA using a single-stranded DNA nonamer, dCCAATAACC (d9). Up to 2.5 molar equivalents of XPA-MBD was titrated into a solution of d9. A subset of 31P resonances of d9 were observed to broaden and/or shift providing direct evidence that XPA-MBD binds d9 by a mechanism that perturbs the phosphodiester backbone of d9. The interior five residues of d9 broadened and/or shifted before 31P resonances of phosphate groups at the termini, suggesting that when d9 is bound to XPA-MBD the internal residues assume a correlation time that is characteristic of the molecular weight of the complex while the residues at the termini undergo a fraying motion away from the surface of the protein on a timescale such that the line widths are more characteristic of the molecular weight of ssDNA. A molecular model of the XPA-MBD complex with d9 was calculated based on the 15N (XPA-MBD) and 31P (d9) chemical shift mapping studies and on the assumption that electrostatic interactions drive the complex formation. The model shows that a nine residue DNA oligomer fully covers the DNA-binding surface of XPA and that there may be an energetic advantage to binding DNA in the 3′→5′ direction rather than in the 5′→3′ direction (relative to XPA-MBD α-helix-3).
INTRODUCTION
Genomic DNA is continuously being damaged. This damage may be induced by endogenous reactive species generated during events of normal cellular metabolism, such as hydrolysis, oxidation and methylation, or by reactive species that are exogenous in origin, such as UV and ionizing radiation (1,2). Mutagenesis, carcinogenesis, aging, genetic diseases and cell death are some of the possible deleterious cellular consequences (3,4). To prevent the potentially harmful outcomes of DNA damage, cells have developed efficient DNA repair mechanisms (2). One such mechanism is nucleotide excision repair (NER), a highly conserved DNA repair pathway found in the three major kingdoms of life (5). An interesting feature of NER is its versatility. Not only is it the primary defense against the carcinogenic effects of solar UV light, but it also acts on a wide variety of bulky, helix-distorting lesions (6,7) and to a lesser extent, more subtle DNA lesions such as AP-sites (8).
Six core NER repair factors coordinate the excision of damaged DNA from the genome in the form of a 24–32 bp oligomer around the lesion (7,9,10). Of the six repair factors (RPA, XPC–hHR23B, TFIIH, XPG, ERCC1–XPF and XPA) both RPA and XPA are essential for NER activity (11–13). XPA interacts with RPA (11,14) and while human XPA has a moderately greater affinity for damaged DNA over undamaged DNA (15,16), an XPA–RPA co-complex has yet a greater affinity for damaged DNA (17,18). XPA also interacts with other NER proteins including ERCC1–XPF (19), TFIIH (20) and XPC–hHR23B (21), and RPA interacts with the nucleases XPG and ERCCI–XPF (17,22). It has been suggested that XPA, in association with RPA, plays a central, multifunctional role in NER, with the two proteins recognizing DNA damage and then recruiting and organizing the other NER proteins into position to remove the lesion (23,24).
Human XPA is a 31 kDa metalloprotein of 273 amino acid residues and one molecule of zinc (25,26). A 122 amino acid region between M98 and F219 (XPA-MBD) is the shortest known region of XPA that binds DNA (27). The solution structure for XPA-MBD has been determined using NMR-based methods: it consists of two subdomains joined together by a linker sequence, a C-terminal zinc-binding core and an N-terminal loop-rich subdomain (28–30). Because the 1H and 15N chemical shifts of the amide resonances of the polypeptide backbone are sensitive to the chemical environment of the nuclei at protein–ligand interfaces (31,32), it has been possible to map the surface of XPA-MBD which interacts with DNA. While the zinc in XPA is essential for binding DNA and for NER activity (33,34), the chemical shift perturbation studies indicate that the zinc-binding core does not play a direct role in DNA-binding (28–30). Instead, the DNA-binding region of XPA is located in the loop-rich subdomain along a surface of basic amino acid residues (28–30). Cisplatin-treated DNA (30), DNA containing dihydrothymidine or 6-4-thymidine-cytidine and undamaged single-stranded (ss)DNA (29) all produced a similar chemical shift perturbation pattern with 15N-enriched XPA-MBD indicating that XPA binds all DNA on the same surface with a low DNA-binding specificity (35). Such chemical shift mapping studies followed the DNA–XPA interactions from the vantage point of the protein’s amide backbone using 15N-labeled XPA-MBD. Here, we use 31P NMR spectroscopy to follow the DNA–XPA interactions from the vantage point of the DNA phosphodiester backbone using a nine residue, ssDNA oligomer, dCCAATAACC (d9). This undamaged, ssDNA oligomer was used because the results of previous chemical shift perturbation studies with XPA-MBD and single-stranded, double-stranded, damaged and undamaged DNA were similar and because it is generally believed that one of the key features XPA and/or RPA recognizes in damaged DNA is single-stranded character (10,13). The 31P chemical shifts of the DNA are highly sensitive to the conformation of the phosphodiester backbone (36) and, hence, provide further insight into the structural basis for DNA recognition by XPA. To test if electrostatic interactions between the negatively charged phosphodiester backbone of DNA and the positively charged basic cleft in the loop-rich subdomain of XPA could account for XPA–DNA interactions, model calculations were performed by docking d9 onto the DNA-binding surface of XPA-MBD.
MATERIALS AND METHODS
Preparation of XPA-MBD and d9
Uniformly 15N-labeled XPA-MBD was expressed in Escherichia coli bacterial strain BL21(DE3)pLysS (Novagen Inc., Madison, WI) as previously described (26,29) and the protein purified by a modified protocol. Frozen cells (–80°C) from a 750 ml culture were resuspended in 40 ml of 50 mM potassium phosphate, pH 7.5. The cell suspension was made 0.2 µM in phenylmethylsulfonyl fluoride immediately prior to three passes through a French Press (SLM Instruments Inc., Rochester, NY). Following 1 min of sonication the cell debris was centrifuged at 17 500 r.p.m. for 45 min in a JA-20 rotor in a Beckman Avanti J-25 centrifuge (Palo Alto, CA). After centrifugation, 10–20 ml supernatant was applied to a Bio-Rad hydroxyapatite column (Bio-Rad, Hercules, CA) attached to a BioCAD Sprint Perfusion Chromatography System (PerSeptive Biosystems, Framingham, MA). The column was washed with 10 mM potassium phosphate, 1.0 mM DTT pH 7.1 and eluted with a linear gradient of 0–400 mM potassium phosphate, 1.0 mM DTT, pH 7.1, over 10 column volumes. XPA-MBD eluted with ∼150 mM potassium phosphate. The peak containing XPA-MBD was collected, pooled and concentrated to ∼2 mM with a Centriprep-10 (Amicon Inc., Beverly, MA). The protein was further purified on a Superdex75 HiLoad column (Pharmacia, Piscataway, NJ) that, at the same time, exchanged XPA-MBD into NMR buffer (20 mM potassium phosphate, 100 mM KCl, 5 mM DTT and 50 µM NaN3, pH 7.3). SDS–PAGE and Coomassie Blue staining showed the NMR samples to be >95% pure.
The ssDNA nonamer, dCCAATAACC (d9), was synthesized on an Applied Biosystems 392 DNA/RNA synthesizer (Foster City, CA) as described previously (29). NMR samples (1–2 mM) of d9 were prepared in 600 µl NMR buffer in D2O. All the chemicals used were purchased from the Sigma Chemical Company (St Louis, MO) except for the D2O (Cambridge Isotope Laboratories, Andover, MA). Protein concentrations were determined using the Bradford assay (Bio-Rad) and DNA concentrations were determined using UV absorbance at 260 nm (1 A260nm ssDNA = 37 µg/ml).
NMR spectroscopy
NMR data were collected on Varian 750- 600- or 500-Unityplus spectrometers. Phosphorus spectra were collected exclusively at a 31P resonance frequency of 202 MHz. Standard phase sensitive (TPPI) homonuclear two-dimensional DQF-COSY (37), NOESY (38) and TOCSY (39,40) spectra were collected for d9 to assign the 1H resonances. HeteroCOSY (41) and heteroTOCSY (42) two-dimensional spectra were collected for d9 to verify the assignment of the 1H resonances and to assign the 31P resonances. For the 15N/1H HSQC titration experiments, aliquots of desalted d9 (0.03 µmol/µl) were added to 600 µl of 0.4 mM XPA-MBD. Two-dimensional 15N/1H HSQC (43,44) spectra (25°C) were recorded at d9:XPA-MBD molar ratios of 0.25, 0.50, 0.75, 1.0, 1.5, 2.0 and 2.5 (molar ratio = moles d9/moles XPA-MBD). For the 31P titration experiments, aliquots of XPA-MBD in 99% D2O (0.0025 µmol/µl) were added to 500 µl of 0.9 mM d9 in 99% D2O. One-dimensional proton decoupled 31P spectra (25°C) were recorded at d9:XPA-MBD molar ratios of 4.0, 2.7, 2.0, 1.0, 0.8, 0.67, 0.57, 0.50 and 0.44. To assist following the movement of the d9 31P resonances with the addition of XPA-MBD, two-dimensional 31P/1H heteroCOSY spectra (15°C) were recorded on a 2.0 mM sample of d9 in 99% D2O at d9:XPA-MBD molar ratios of 10, 5, 3.3, 2.5 and 0.5. Both the 1H and 31P chemical shifts were referenced by the indirect referencing method (45,46).
Modeling calculations
A prominent feature observed in the solution structure of XPA-MBD is a basic cleft in the loop-rich subdomain (29,30). The charged head groups of these basic residues are distributed on the surface of the protein with a spacing of 5–12 Å, a distance that is approximately equal to one or two times the distance between phosphorous atoms in helical DNA (5–8 Å). Because of this correlation and the observation that ssDNA containing purine bases has a tendency to adopt elements of helical structure in solution (47), the starting structure of d9 used for the docking calculations was generated using NAMOT-2 (48) with d9 loosely restrained in a right-handed B-form helix. The XPA-MBD used for the docking calculations was the average cobalt-refined solution structure deposited in the RCSB Protein Data Base (PDB) (1D4U). Residues K141, K148, K168, K179 and K204 form a nearly continuous row in the basic cleft and, consequently, these residues were chosen as anchor points for docking the DNA onto the protein. Both the protein and the DNA were treated as rigid bodies. To prevent unfavorable contacts between the two molecules, a van der Waals penalty was used where the energy between the two molecules was calculated with the AMBER parameters (49). A constraint energy term, Ec, was also applied to aid docking with Ec equal to [Σ(i)]k × (d – d0)2 when d > d0 and equal to 0 when d > d0. The force constant, k, was arbitrarily set to 100 kcal/mol Å2 and d was the distance between the α-carbon of the anchoring residue in the protein and the corresponding phosphorous atom in the DNA. The distance d0 was set to 6 Å corresponding to the range in values (5.0–6.4 Å) observed in lysine residues that formed hydrogen bonds with the phosphate backbone of DNA in the crystal structure of nucleosome (PDB code 1AOI) (50). The Metropolis Monte Carlo simulation method (51) was used to dock the DNA on to the protein. The docked complex was subjected to a short run (1000 cycles) of energy minimization using an AMBER force field (49). Hydrogen donor–acceptor pairs were identified and distance constraints were applied between these donor–acceptor pairs while subjecting the complex to 10 ps molecular dynamic simulation. The resulting complex was energy minimized (1000 cycles) using an AMBER force field. The calculations were performed with d9 orientated in the 3′→5′ and 5′→3′ direction (relative to XPA-MBD α-helix-3) and with and without right-handed, B-form helical restraints on the d9 starting structure during the docking calculations.
RESULTS AND DISCUSSION
Chemical shift mapping study with 15N-labeled XPA-MBD
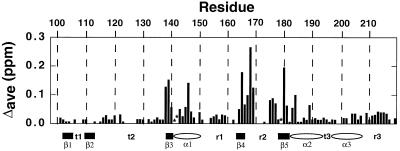
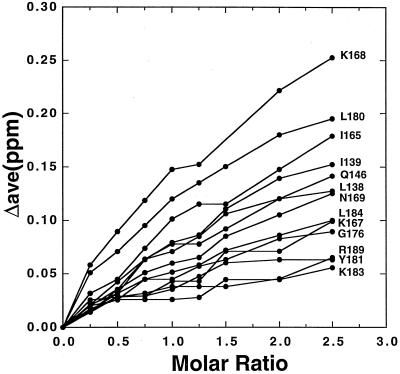
The 1H and 15N amide resonances in the 15N/1H HSQC spectrum of XPA-MBD have been previously assigned (29,30) and the average amide chemical shifts changes in XPA-MBD observed upon the addition of an equimolar quantity of d9 have been previously reported (29). Figure 1 plots the average chemical shift changes Δave = {[(Δ1HN)2 + (Δ15N/5)2]/2}1/2 in the 1HN and 15N resonances of XPA-MBD with the addition of excess DNA, at a d9:XPA-MBD molar ratio of 2.5. XPA-MBD residues marked with an asterisk in Figure 1 are 15N/1H HSQC cross peaks that disappear due to extensive line broadening upon the addition of d9. At 2.5 molar excess of DNA to protein no additional amide residues were significantly perturbed relative to those effected at an approximately equal molar ratio of d9:XPA-MBD. In Figure 2 the average chemical shift changes in the 1HN and 15N resonances of the XPA-MBD residues most perturbed by the addition of DNA are plotted as a function of the d9:XPA-MBD molar ratio. For most of the residues the average 1HN and 15N chemical shift change continued to increase with the addition of DNA suggesting that at 2.5 molar excess of DNA to protein, XPA-MBD was still not saturated with DNA. The most likely explanation for such an observation is that DNA binding to XPA is weak, as reported previously for larger DNA fragments (16,18,35).
Figure 1.
Average chemical shift changes in the 1HN and 15N resonances of XPA-MBD, at a d9:XPA-MBD molar ratio of 2.5, plotted against residue number. The average chemical shift change = Δave = {[(Δ1HN)2 + (Δ15N/5)2]/2}1/2. Asterisks identify 1HN/15N HSQC cross peaks that disappear upon the addition of d9. The elements of secondary structure of XPA-MBD are identified on the x-axis: β = β-strand (rectangle), α = α-helix (oval), r = random coil, t = turn.
Figure 2.
Average chemical shift changes in the 1HN and 15N resonances of XPA-MBD residues most affected by the binding of d9 plotted as a function of the molar ratio of DNA:protein. At a DNA:protein molar ratio of 2.5, the average chemical shift changes in the 1HN and 15N resonances of XPA-MBD are still increasing, indicating that a saturated state has not yet been reached.
NMR chemical shift assignments of d9
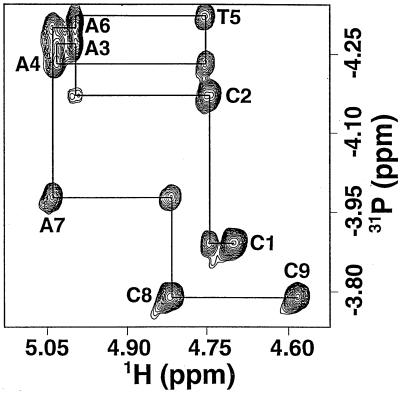
The phosphorus and proton resonances in d9 are labeled following the IUPAC-IUBMB-IUPAB nomenclature recommendation (46). For example, in the dinucleotide T(i-1)pC(i) the phosphorus is labeled (i). The d9 proton chemical shifts were assigned by inspection of the NOESY, TOCSY and DQF-COSY spectra (52,53). The H5′ was assigned deshielded relative to H5″ according to Remin and Shugar (54) whereas the H2′/H2″ assignments were based on coupling constant arguments (55,56). The d9 phosphorus chemical shifts were assigned by inspection of the heteroCOSY and heteroTOCSY spectra on the basis of the 1H chemical shift assignments (Table 1). In the heteroCOSY experiment, antiphase multiplet correlations are observed between 31P and any three- or four-bond coupled proton. In the heteroTOCSY experiment, absorptive in-phase correlations are observed between 31P and any three- or four-bond scalar coupled proton as well as additional correlations due to homonuclear 1H coherences. The power of this method is illustrated in Figure 3, an expansion of the heteroTOCSY spectrum of d9 in the H3′ region at 15°C, a temperature where the chemical shifts of all eight 31P atoms are non-degenerate and the residual HDO signal is not coincident with any of the H3′ resonances. For each phosphorus resonance two cross peaks to sequential 3′-protons were observed; a strong interresidue cross peak to the H3′(i-1) three bonds away and a weak intraresidue cross peak to the H3′(i) five bonds away (42,57). The latter cross peak is due to homonuclear coherence transfer between the intraresidue H4′ that is coupled to the phosphorus four bonds away. As illustrated by the solid line in Figure 3, it is possible to follow the sequential 31P to H3′ connectivities through the d9 sequence.
Table 1. Proton chemical shift assignments for dCCAATAACC at 15°C in D2O determined at a 500 MHz 1H frequency.
| Base |
H8/H6 |
H1′ |
H2′ |
H2′′ |
H3′ |
H4′ |
H5′ |
H5′′ |
H2 |
H5 |
Methyl |
P |
| C1 | 7.69 | 6.14 | 2.20 | 2.50 | 4.68 | 4.12 | 3.78 | 3.71 | – | 5.95 | – | – |
| C2 | 7.50 | 6.02 | 1.72 | 2.24 | 4.73 | 4.13 | 3.96 | 3.96 | – | 5.90 | – | –3.90 |
| A3 | – | 5.98 | 2.61 | 2.74 | 4.98 | 4.36 | 4.08 | 3.98 | 8.20 | – | – | –4.19 |
| A4 | – | 6.11 | 2.66 | 2.71 | 5.02 | 4.44 | 4.22 | 4.22 | 8.28 | – | – | –4.29 |
| T5 | 7.02 | 5.74 | 1.55 | 2.02 | 4.73 | 4.11 | 4.14 | 4.05 | – | – | 1.59 | –4.26 |
| A6 | – | 5.95 | 2.58 | 2.73 | 4.98 | 4.35 | 4.07 | 3.96 | 8.13 | – | – | –4.35 |
| A7 | – | 6.15 | 2.70 | 2.70 | 5.02 | 4.45 | 4.23 | 4.23 | 8.23 | – | – | –4.33 |
| C8 | 7.52 | 6.12 | 2.19 | 2.46 | 4.80 | 4.27 | 4.24 | 4.15 | – | 5.71 | – | –4.00 |
| C9 | 8.85 | 6.24 | 2.28 | 2.40 | 4.57 | 4.15 | 4.13 | 4.04 | – | 6.04 | – | –3.79 |
Figure 3.
A two-dimensional 31P/1H heteroTOCSY spectrum of d9 at 15°C. The interresidues H3′(i–1) to P(i) cross peaks for C1–C8 and the intraresidue H3′(i) to P(i) cross peak for C9 are labeled. All eight phosphorus resonances have cross peaks to both inter- and intraresidue 3′ protons. The solid line is the construct following the sequential 31P to H3′ connectivities through the sequence.
Chemical shift mapping of the phosphodiester backbone of d9
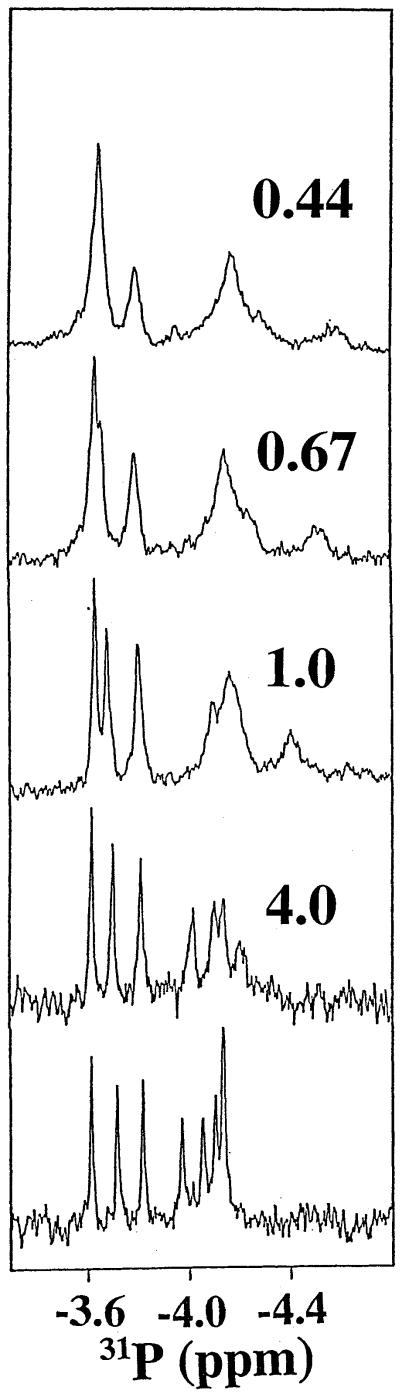
The surface of XPA-MBD that interacts with DNA can be mapped by following perturbations to the 15N/1H HSQC cross peaks with the addition of DNA. In a similar manner, the regions of the phosphodiester backbone of d9 that interact with XPA-MBD can be mapped by following perturbations to the 31P NMR spectrum upon the addition of protein. Figure 4 shows the one-dimensional 31P spectra for d9 in the absence of XPA-MBD and in the presence of increasing amounts of protein. At 25°C, in the absence of XPA-MBD, seven sharp phosphorus resonances were observed for the eight phosphorus backbone atoms in d9. The most shielded resonance was an overlap of the chemical shifts of two 31P atoms, A6 and A7. The group of four most shielded resonances represents the five interior 31P atoms A3 to A7. At a d9:XPA-MBD molar ratio of 4.0 the line shape of the three most deshielded 31P resonances (C2, C8 and C9) were essentially unchanged while the four shielded resonances of the five interior 31P atoms decreased in intensity and became broader. Such signal losses are consistent with the formation of a large molecular weight complex, or with exchange between the free and ligand-bound states with a rate similar to the chemical shift difference between the two states (58,59). When the molar amounts of d9 and XPA-MBD were equal, the three deshielded 31P resonances started to become broad and there was a further broadening of the group of shielded 31P resonances. Furthermore, a new, broad, shielded signal appeared near –4.4 p.p.m. With further additions of protein to d9 that decreased the d9:XPA-MBD molar ratio, there was a continuous increase in the line widths of all the 31P resonances and the most shielded resonance became more shielded. Such observations follow the trends observed in the 15N/1H HSQC titration experiments where it was observed that the average chemical shifts of the 15N and 1H resonances for the amide residues at, or near, the DNA/XPA-MBD interface were still increasing at a d9:XPA-MBD molar ratio of 2.5. The explanation for such an observation is likely to be the same one proposed to explain the 15N/1H titration data (Fig. 2); DNA-binding to XPA-MBD is weak (16,18,35) and the addition of excess DNA or protein helps push the equilibrium towards complex formation.
Figure 4.
Proton-decoupled 31P NMR spectra (1 mM, 25°C, 202 MHz) for d9 in the absence of XPA-MBD (bottom spectrum) and in the presence of various molar ratios of XPA-MBD. The numbers above each spectrum show the molar ratio of d9:XPA-MBD. The data were all apodized similarly. As the d9:XPA-MBD molar ratio decreased, more transients were collected to obtain a similar signal-to-noise level (128 transients with no XPA-MBD, 20 000 transients at 0.44).
Figure 4 shows that the line widths of interior 31P resonances (A3 to C7) broaden before and to an extent greater than the terminal 31P resonances (C2, C8 and C9). This was also observed in heteroCOSY spectra of d9 collected at successively smaller d9:XPA-MBD molar ratios (data not shown). The A6 31P heteroCOSY cross peaks disappeared first, followed by cross peaks for the T5 resonance and then the A4 and A7 resonances. At a d9:XPA-MBD molar ratio of 0.5 no heteroCOSY cross peaks were observed. Furthermore, although the data in Figure 4 were apodized similarly, the number of transients that needed to be collected in order to acquire a spectrum with similar signal-to-noise and line-shape features increased as the molar ratio of d9 to XPA-MBD decreased. The bottom-most phosphorus spectrum in Figure 4, d9 collected in the absence of XPA-MBD, required 128 scans. At a DNA:XPA-MBD molar ratio of 0.44, 20 000 transients were required. The observation that all the 31P resonances became broader with the addition of XPA-MBD is most likely to be due to a shift in the equilibrium towards the complex in which the 31P resonance lines take on the correlation time (and broad lines) characteristic of the molecular weight of the complex. One consequence of a low DNA binding affinity is that even in the presence of excess protein, there is a population of free and bound d9. If the exchange rate between the free and bound forms of d9 is in the ‘intermediate’ regime on the NMR time scale, then the line widths of the 31P resonances will all broaden (58,59). The observation that the resonances of interior phosphate groups of d9 broaden before and to an extent greater than the resonances of phosphate groups at the ends of the sequence is likely to be due to different degrees of mobility between the two regions of d9 when bound to XPA-MBD. The phosphate groups in the interior of d9 take on the correlation time of the large molecular weight complex and, hence, the 31P resonances become broad. Relative to the phosphate groups in the interior of d9, the phosphate groups at the termini have more dynamic freedom, fraying away from the surface of the protein in a manner analogous to the fraying observed at the ends of DNA double-stranded (ds) helices and, hence, these 31P resonances appear sharper. Note that factors other than chemical exchange and motion restrictions may also contribute to the observed line shapes. Because DNA binding is weak, there may be some shifting of the specific contacts between d9 and XPA-MBD that would result in further broadening of the 31P resonances. The DNA may also bind to XPA-MBD in two orientations, 3′→5′ and 5′→3′, that could also result in additional line broadening in the 31P resonances since the phosphorus atoms would be in slightly different chemical environments when bound in different directions.
The 31P chemical shifts of individual phosphate diesters in oligonucleotides are most sensitive to differences in the torsional angles (α, β, ɛ and ζ) and the O–P–O bond angle. Ring current effects, hydrogen bonds and salt bridges generally have a weaker effect on the 31P chemical shifts (36). Figure 4 shows that at least one d9 31P resonance is substantially shielded upon binding to XPA-MBD (signal at about –4.5 p.p.m. at a d9:XPA-MBD molar ratio of 0.67). While efforts to identify this shielded 31P resonance by heteroCOSY or heteroTOCSY experiments were unsuccessful (no heteroCOSY or heteroTOCSY cross peaks were observed at a d9:XPA-MBD molar ratio of 0.5), Figure 4 suggests that the signal is from one of the interior backbone 31P resonances because the C2, C8 and C9 resonances appear to only get broader with each XPA-MBD addition. The shielding of at least one interior 31P resonance indicates that there must be some distortion of the phosphodiester backbone of d9 upon binding to XPA.
Through the analysis of the 31P chemical shifts of many short dsDNA sequences a general trend has been observed; that the 31P chemical shifts of the phosphates at the end of the helix tend to be more deshielded than the 31P chemical shifts of phosphates in the interior of the helix (36,60,61). The latter observations have been interpreted in terms of two different types of B-DNA geometries, BI and BII, with different torsional and bond angles at the phosphorus atom. The phosphates in the interior of the DNA double helix are primarily in the BI (approximately –4.5 p.p.m.) conformation while those at the ends of the helix are a mixture of BI and BII (approximately –3.0 p.p.m.) due to greater terminal conformational freedom associated with ‘fraying’ (36,59). While d9 is a ss random-coil DNA oligomer, it is interesting to note that the two most deshielded 31P resonances are C2 and C9 phosphates at the 5′ and 3′ ends of d9, respectively.
Molecular modeling: docking of d9 on to the DNA-binding surface of XPA-MBD
The lysine and arginine side chains in the loop-rich subdomain of XPA form a nearly continuous line with an interresidue distance of 5–12 Å between adjacent positively charged head groups, a distance compatible with the 5–8 Å distance between phosphate groups on the backbone of helical DNA. To determine if electrostatic interactions between the positively charged surface of XPA with the negatively charged phosphodiester backbone of DNA is a practical mechanism to account for DNA-binding, d9 was modeled onto the surface of the NMR-derived solution structure of XPA-MBD. The maximal number of electrostatic contacts between the DNA and protein were allowed. To assess if there was any directionality to the DNA-binding, the DNA was modeled onto the surface of XPA-MBD in both the 3′→5′ and 5′→3′ directions. The results of the molecular modeling are displayed in Figures 5 and 6.
Figure 5.
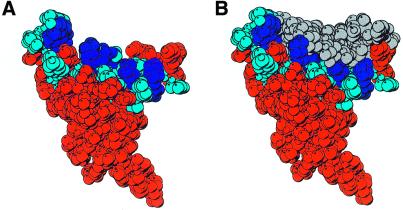
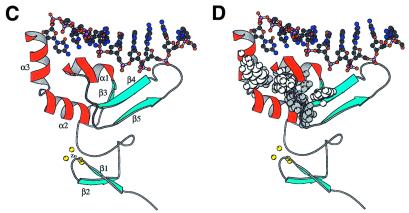
(A) Space-filled model of the side chain residues of XPA-MBD summarizing the results of chemical shift perturbation experiments with d9 and DNA oligomers containing dihydrothymidine and the 6-4-thymine-cytidine photoproduct (29). The side chains of residues whose 1HN and 15N chemical shifts are perturbed most by the addition of DNA are colored blue with the positively charged Lys and Arg side chains colored dark blue. (B) Space-filled model of the results of the molecular modeling of the binding of d9 to XPA-MBD. The DNA is colored grey and is orientated in the 3′→5′ direction. (C) MOLSCRIPT (62) rendition of the structure in (A) and (B) with the β-strands colored light blue, α-helices colored red, loops colored gray and the zinc-coordinated sulphur atoms of the cysteine side chains colored yellow. The DNA is represented by a ball and stick diagram. (D) The side chains that form a hydrophobic core in the loop-rich subdomain are highlighted by space-filling the atoms white and gray. The hydrophobic side chains of residues whose 1HN and 15N chemical shifts were perturbed by the addition of DNA are colored white.
Figure 6.
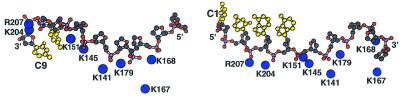
Expansions of the interfacial region between d9 and XPA-MBD. The DNA is orientated in the 3′→5′ (left) and 5′→3′ (right) direction relative to α-helix-3. The positively charged head groups of XPA-MBD side chains of amide 1HN and 15N chemical shifts that were perturbed by the addition of DNA are illustrated as blue spheres. The DNA is illustrated with a ball and chain diagram and bases near the hydrophobic pocket (Fig. 5D) are colored yellow.
Figure 5A is a space-filled representation of the average solution structure determined for XPA-MBD (PDB code 1D4U) (29) and shows that XPA-MBD adopts an extended structure in the shape of a cone. The XPA-MBD residues whose amide 15N and 1HN resonances were most perturbed by the addition of undamaged ssDNA (d9, Fig. 1), undamaged dsDNA and DNA containing the lesions dihydrothymidine and the 6-4-thymidine-cytidine photoproduct (29) are highlighted in blue with the effected, positively charged residues colored dark blue. Figure 5A shows that the blue colored residues are all clustered in the loop-rich subdomain of XPA-MBD at the top of the cone. Figure 5B is a space-filled rendition of the result of modeling d9 bound to XPA-MBD orientated in the 3′→5′ direction (using α-helix-3 of XPA-MBD as the reference point). The DNA, colored grey, fits nicely onto the exterior of XPA-MBD with the phosphodiester backbone near the surface of the protein. A similar arrangement of the protein and DNA is observed when the modeling is performed with the DNA orientation reversed to the 5′→3′ direction. In both instances a nine residue nucleotide sequence appears to fully cover the surface of XPA-MBD.
Figure 5C is a MOLSCRIPT (62) rendition of Figure 5B that illustrates how the elements of secondary structure are organized in relation to the DNA. The DNA essentially sits on top of the triple-strand β-sheet and α-helix-1 in the loop-rich subdomain. There is likely to be no major change in the relative orientation of the units of secondary structure upon DNA-binding because, as reported previously, the NOE patterns observed in the three dimensional 15N-edited NOESY-HSQC spectra of XPA-MBD and XPA-MBD bound to d9 and other DNAs were identical (29). Ikegami et al. (30) recognized that the loop-rich subdomain of XPA-MBD contained a hydrophobic pocket in addition to the basic cleft on the surface of the protein. The side chains of the residues that form the hydrophobic pocket are highlighted in Figure 5D with the residues whose backbone amide chemical shifts were perturbed by the addition of DNA colored white instead of gray. It has been proposed that DNA-binding to XPA might be stabilized by hydrophobic interactions between the bases of ssDNA (damaged or undamaged bases) with the hydrophobic pocket in the loop-rich subdomain (30,63). Figure 5D shows that the bases on the 3′ end of d9 are in a position to potentially interact with the basic cleft. However, no NOEs between the DNA bases of d9 (and other DNA oligomers with and without lesions) and the side chain residues of XPA have been observed experimentally, and consequently, no hydrophobic restraints were introduced into the modeling calculations. Note that the reason(s) such experiments were unsuccessful may be due to the use of non-ideal DNA sequences and/or because DNA-binding to XPA is weak.
Figure 6 is a more detailed illustration of the interactions at the DNA–protein interface as observed by modeling the DNA onto the surface of XPA-MBD in the 3′→5′ and 5′→3′ directions. The positively charged head groups of the basic residues are drawn as blue spheres and the DNA bases nearest the hydrophobic pocket are shown in yellow. In both models, K167 is too distant from the DNA to make contact with the phosphodiester backbone of the DNA. Note that K168 was chosen over K167 in the modeling because (i) the chemical shifts of the K168 backbone amide resonances are effected more by the addition of DNA (Fig. 1) and (ii) K168 lined up better with the other basic residues. A few differences were observed in the results of the docking calculations with the starting DNA orientated in different directions relative to α-helix-3. Most noticeably, one fewer salt bridge (11 versus 10) can form between the positively charged basic XPA side chains and the negatively charged DNA phosphodiester backbone with the DNA orientated in the 5′→3′ direction. Salt bridges may provide up to 15 kcal/mol of stabilization energy per salt bridge (64) and, as illustrated in Figure 6, K141 is too distant from the DNA to form such a salt bridge when the DNA is orientated 5′→3′. In addition to salt bridges, DNA–protein associations may be stabilized by 2–10 kcal/mol per hydrogen bond (65). Although not illustrated in Figure 6, 17 hydrogen bonds can form between the phosphodiester backbone of DNA and side chain residues of XPA-MBD in the model with the DNA positioned in the 3′→5′ direction. On the other hand, only nine hydrogen bonds can form with the DNA positioned in the 5′→3′ direction. Docking calculations performed in the absence of restraints on the conformation of the DNA also showed the 3′→5′ orientation to be energetically more favorable; nine versus eight salt bridges and 19 versus 15 hydrogen bonds. In all models seven of the eight phosphate groups of d9 participate in salt bridge formation and all eight phosphate groups participate in hydrogen bond formation with XPA-MBD.
It has been suggested that hydrophobic contacts between the DNA and XPA also contribute stabilizing energy to the protein–DNA complex (30,63,66). If hydrophobic interactions do contribute to stabilize the DNA–protein complex, then the DNA bases are in a better position to interact with the hydrophobic core of the loop-rich subdomain in the 3′→5′ orientation than in the 5′→3′ orientation. As illustrated in Figure 6, the DNA bases are exposed away from the hydrophobic core when modeled onto XPA in the 5′→3′ direction. Note that such differences largely remain if helical restraints on the confirmation of d9 are removed during the docking calculations.
At d9:XPA-MBD molar ratios <1.0, one of the interior 31P resonances of d9 was observed to become more shielded (Fig. 4) suggesting that the phosphodiester backbone of the DNA was being distorted when bound to XPA-MBD. Such a change in the phosphorus chemical shift is usually due to changes in the torsional angles (α, β, ɛ and ζ) and the O–P–O bond angle (36). Measurement of these angles in the model structures for d9 bound to XPA-MBD indicate that many of these angles do change substantially in all the calculated models (data not shown).
CONCLUSION
Although XPA has been shown to be essential to NER, its precise role in DNA damage recognition and/or verification is still unclear. Wakasugi and Sancar (18) propose that XPA and RPA are responsible for the initial DNA damage recognition prior to the formation of a preincision complex. On the other hand, Sugasawa et al. (21) propose that an XPC–hHR23B complex is responsible for DNA damage recognition and XPA and/or RPA verify the damage during the formation of a preincision complex. While the precise role of XPA in NER remains unresolved, it is clear that XPA interacts directly with DNA either during DNA damage recognition or the formation of the preincision complex. In the preincision complex, XPA is most likely to direct the positioning of the 5′-endonuclease ERCCI–XPF heterodimer (19). The data presented here provide both direct evidence that XPA binds DNA and insight into the basis for the interaction. The 15N/1H HSQC and the 31P NMR titration data follow DNA-binding from the vantage points of the protein and the DNA, respectively. Both experiments showed that XPA-MBD binds d9 and the latter experiment showed that XPA-MBD binds d9 in a way that perturbs the phosphodiester backbone of DNA. At concentrations between 0.5 and 2.0 mM, the NMR spectra of both ligands, d9 and XPA-MBD, continued to change when one ligand was in >2-fold molar excess, indicating that DNA-binding to XPA-MBD is in the millimolar range. 31P resonances in the interior of d9 broadened and/or shifted before 31P resonances at the termini, suggesting that when d9 is bound to XPA-MBD the motion of the DNA is more restricted in the interior of the sequence than at the termini. The shielding of one of the interior 31P resonances of d9 indicates that the phosphodiester backbone became distorted upon association with XPA-MBD. Molecular modeling of the complex between d9 and XPA-MBD showed that a nine residue oligomer fully covers the DNA-binding surface of XPA-MBD. DNA-binding is possible through electrostatic interactions between seven negatively charged phosphate groups on the backbone of d9 and positively charged side chains of residues in the loop-rich subdomain of XPA. Regardless of the restraints placed on the DNA, modeling calculations suggest that there may be an energetic advantage to binding in the 3′→5′ direction rather than the 5′→3′ direction (relative to XPA-MBD α-helix-3). With or without restrictions on the conformations of the DNA, the potential for hydrophobic interactions between the hydrophobic core of the loop-rich subdomain of XPA and the DNA bases is greatest when the d9 is modeled in the 3′→5′ orientation.
Acknowledgments
ACKNOWLEDGEMENTS
The research was performed in the Environmental Molecular Sciences Laboratory (a national scientific user facility sponsored by the DOE Biological and Environmental Research) located at Pacific Northwest National Laboratory and operated for DOE by Battelle. This work was performed under the auspices of the US Department of Energy (Contract DE-AC06-76RLO1830) and was supported by the Department of Energy Office of Biological and Environmental Research Program under Grant 249311 KP11-01-01. L.D.S. also acknowledges support from the National Institutes of Health (GM 41829). G.W.B. was a visiting scientist from Duke University at PNNL.
References
- 1.Cadet J. (1994) DNA damage caused by oxidation, deamination, ultraviolet radiation and photoexcited psoralens. In Hemminki,K., Dipple,A., Shuker,D.E.G., Kadlubar,F.F., Sergerback,D. and Bartsch,H. (eds), DNA Adducts: Identification and Biological Significance. IARC Scientific Publications, Lyon, France, pp. 245–276. [PubMed]
- 2.Friedberg E.C.,Walker,G.C. and Siede,W. (1994) DNA Repair and Mutagenesis. American Society for Microbiology, Washington, DC, pp. 283–365.
- 3.Ames B.N. and Gold,L.S. (1991) Endogenous mutagens and the cause of aging and cancer. Mutat. Res., 250, 3–16. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrest B.A. and Bohr,V.A. (1997) Aging processes, DNA damage and repair. FASEB J., 11, 322–330. [DOI] [PubMed] [Google Scholar]
- 5.Petit C. and Sancar,A. (1999) Nucleotide excision repair: from E.coli to man. Biochimie, 81, 15–25. [DOI] [PubMed] [Google Scholar]
- 6.Gunz D., Hess,M.T. and Naegeli,H. (1996) Recognition of DNA adducts by human nucleotide excision repair. J. Biol. Chem., 271, 25089–25098. [DOI] [PubMed] [Google Scholar]
- 7.Wood R.D. (1997) Nucleotide excision repair in mammalian cells. J. Biol. Chem., 272, 23465–23468. [DOI] [PubMed] [Google Scholar]
- 8.Sancar A. (1996) DNA excision repair. Annu. Rev. Biochem., 65, 43–81. [DOI] [PubMed] [Google Scholar]
- 9.Huang J.C., Svoboda,D.L., Reardon,J.T. and Sancar,A. (1992) Human nucleotide excision nuclease removes thymine dimers from DNA by incising the 22nd phosphodiester bond 5′ and the 6th phosphodiester bond 3′ to the photodimer. Proc. Natl Acad. Sci. USA, 89, 3664–3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancar A. (1995) Excision repair in mammalian cells. J. Biol. Chem., 270, 15915–15918. [DOI] [PubMed] [Google Scholar]
- 11.Li L., Lu,X.Y., Peterson,C.A. and Legerski,R.A. (1995) An interaction between the DNA repair factor XPA and replication protein-A appears essential for nucleotide excision repair. Mol. Cell Biol., 15, 1993–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mu D., Hsu,D.S. and Sancar,A. (1996) Reaction mechanism of human DNA repair excision nuclease. J. Biol. Chem., 271, 8285–8294. [DOI] [PubMed] [Google Scholar]
- 13.Evans E., Moggs,J.G., Hwang,J.R., Egly,J.-M. and Wood,R.D. (1997) Mechanism of open complex and dual incision formation by human nucleotide excision repair factors. EMBO J., 16, 6559–6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuda T., Saijo,M., Kuraoka,I., Kobayashi,T., Nakatsu,Y., Nagai,A., Enjoji,T., Masutani,C., Sugasawa,K., Hanoaka,F. et al. (1995) DNA repair protein XPA binds replication protein A (RPA). J. Biol. Chem., 270, 4152–4157. [DOI] [PubMed] [Google Scholar]
- 15.Robins P., Jones,C.J., Biggerstaff,M., Lindahl,T. and Wood,R.D. (1991) Complementation of DNA repair in Xeroderma pigmentosum group A cell extracts by a protein with affinity for damaged DNA. EMBO J., 10, 3913–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones C.J. and Wood,R.D. (1993) Preferential binding of the Xeroderma pigmentosum group A complementing protein to damaged DNA. Biochemistry, 32, 12096–12104. [DOI] [PubMed] [Google Scholar]
- 17.He Z., Henrickson,L.A., Wold,M.S. and Ingles,C.J. (1995) RPA involvement in the damage recognition and incision steps of nucleotide excision repair. Nature, 374, 566–569. [DOI] [PubMed] [Google Scholar]
- 18.Wakasugi M. and Sancar,A. (1999) Order of assembly of human DNA repair excision nuclease. J. Biol. Chem., 274, 18759–18768. [DOI] [PubMed] [Google Scholar]
- 19.Li L., Elledge,S.J., Peterson,C.A., Bales,E.S. and Legerski,R.J. (1994) Specific association between the human DNA repair proteins XPA and ERCC1. Proc. Natl Acad. Sci. USA, 91, 5012–5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park C.-H., Mu,D., Reardon,J.T. and Sancar,A. (1995) The general transcription-repair factor TFIIH is recruited to the excision repair complex by the XPA protein independent of the TFIIE transcription factor. J. Biol. Chem., 270, 4896–4901. [DOI] [PubMed] [Google Scholar]
- 21.Sugasawa K., Ng,J.M.Y., Masutani,C., Iwai,S., van der Spek,P.J., Eker,A.P.M., Hanaoka,F., Bootsma,D. and Hoeijmakers,J.H.J. (1998) Xeroderma pigmentosum group C protein complex is the initiator of global genome nucleotide excision repair. Mol. Cell, 2, 223–232. [DOI] [PubMed] [Google Scholar]
- 22.Bessho T., Sancar,A., Thompson,L.H. and Thelan,M.P. (1997) Reconstitution of human excision nuclease with recombinant XPF–ERRC1 complex. J. Biol. Chem., 272, 3833–3837. [DOI] [PubMed] [Google Scholar]
- 23.de Laat W.L., Apeldoorn,E., Sugasawa,K., Weterings,E., Jaspers,N.G.J. and Hoeigmakers,J.H.J. (1998) DNA-binding polarity of human replication protein-A positions nucleases in nucleotide excision repair. Genes Dev., 12, 2598–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cleaver J.E. and States,J.C. (1997) The DNA damage-recognition problem in human and other eukaryotic cells: the XPA damage binding protein. Biochem. J., 328, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanaka K., Miura,N., Satokata,I., Miyamoto,I., Yoshida,M.C., Satoh,Y., Kondo,S., Yasui,A., Okayama,H. and Okada,Y. (1990) Analysis of a human DNA excision repair gene involved in group A Xeroderma pigmentosum and containing a zinc-finger domain. Nature, 348, 73–76. [DOI] [PubMed] [Google Scholar]
- 26.Hess N.J., Buchko,G.W., Conradson,S.D., Espinosa,F.J., Ni,S., Thrall,B.D. and Kennedy,M.A. (1998) Human nucleotide excision repair protein XPA: extended X-ray absorption fine-structure evidence for a metal-binding domain. Protein Sci., 7, 1970–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuraoka I., Morita,E.H., Saijo,M., Matsuda,T., Morikawa,K., Shirakawa,M. and Tanaka,K. (1996) Identification of a damaged-DNA binding domain of the XPA protein. Mutat. Res., 362, 87–95. [DOI] [PubMed] [Google Scholar]
- 28.Buchko G.W., Ni,S., Thrall,B.D. and Kennedy,M.A. (1998) Structural features of the minimal DNA binding domain (M98–F219) of human nucleotide excision repair protein XPA. Nucleic Acids Res., 26, 2779–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchko G.W., Daughdrill,G.W., de Lorimier,R., Sudha,R.B.K., Isern,N.G., Lingbeck,J., Taylor,J.-S., Wold,M.S., Gochin,M., Spicer,L.D., Lowry,D.F. and Kennedy,M.A. (1999) Interactions of human nucleotide excision repair protein XPA with DNA and RPA70ΔC327: chemical shift mapping and 15N NMR relaxation studies. Biochemistry, 38, 15116–15128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ikegami T., Kuraoka,I., Saijo,M., Kodo,N., Kyogoku,Y., Morikawa,K., Tanaka,K. and Shirakawa,M. (1998) Solution structure of the DNA- and RPA-binding domain of the human repair factor XPA. Nat. Struct. Biol., 5, 701–706. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y., Reizer,J., Saier,M.H.,Jr, Fairbrother,W.J. and Wright,P.E. (1993) Mapping of the binding interfaces of the proteins of the bacterial phosphotransferase system. Biochemistry, 32, 32–37. [DOI] [PubMed] [Google Scholar]
- 32.Lee A.L., Volkman,B.F., Robertson,S.A., Rudner,S.A., Barbash,D.Z., Cline,T.W., Kanaar,R., Rio,D.C. and Wemmer,D.E. (1997) Chemical shift mapping of the RNA-binding interface of the multiple-RBD protein sex-lethal. Biochemistry, 36, 14306–14317. [DOI] [PubMed] [Google Scholar]
- 33.Asahina H., Kuraoka,I., Shirakawa,M., Morita,E.H., Miura,N., Miyamoto,I., Ohtsuka,E., Okada,Y. and Tanaka,K. (1994) The XPA protein is a zinc metalloprotein with an ability to recognize various kinds of DNA damage. Mutat. Res., 315, 229–238. [DOI] [PubMed] [Google Scholar]
- 34.Miyamoto I., Miura,N., Niwa,H., Miyazaki,J. and Tanaka,K. (1992) Mutational analysis of the structure and function of the Xeroderma pigmentosum group A complementing protein. J. Biol. Chem., 67, 12182–12187. [PubMed] [Google Scholar]
- 35.Morikawa K. and Shirakawa,M. (2000) Three-dimensional structural views of damaged-DNA recognition: T4 endonuclease V, E. coli Vsr protein and human nucleotide excision repair factor XPA. Mutat. Res., 460, 257–275. [DOI] [PubMed] [Google Scholar]
- 36.Gorenstein D.G. (1994) Conformation and dynamics of DNA and protein–DNA complexes by 31P NMR. Chem. Rev., 94, 1315–1338. [Google Scholar]
- 37.Rance M., Sørensen,O.W., Bodenhausen,G., Wagner,G., Ernst,R.R. and Wüthrich,K. (1983) Improved spectral resolution in COSY 1H NMR spectra of proteins via double-quantum filtering. Biochem. Biophys. Res. Commun., 117, 479–485. [DOI] [PubMed] [Google Scholar]
- 38.Jeener J., Meier,B.H., Bachmann,P. and Ernst,R.R. (1979) Investigation of exchange processes by two-dimensional NMR spectroscopy. J. Chem. Phys., 71, 546–4553. [Google Scholar]
- 39.Braunschweiler L. and Ernst,R.R. (1983) Coherence transfer by isotropic mixing: application to proton correlation spectroscopy. J. Magn. Reson., 53, 521–528. [Google Scholar]
- 40.Bax A. and Davis,D.G. (1985) MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J. Magn. Reson., 65, 355–360. [Google Scholar]
- 41.Sklenár V., Miyashiro,H., Zon,G., Miles,H.T. and Bax,A. (1986) Assignment of the 31P and 1H resonances in oligonucleotides by two-dimensional NMR spectroscopy. FEBS Lett., 208, 94–98. [DOI] [PubMed] [Google Scholar]
- 42.Kellogg G.W. and Schweitzer,B.I. (1993) Two- and three-dimensional 31P-driven NMR procedures for complete assignment of backbone resonances in oligodeoxyribonucleotides. J. Biomol. NMR, 3, 577–595. [DOI] [PubMed] [Google Scholar]
- 43.Kay L.E., Keifer,P. and Saarinen,T. (1992) Pure absorption gradient enhanced heteronuclear single quantum correlated spectroscopy with improved sensitivity. J. Am. Chem. Soc., 114, 10663–10665. [Google Scholar]
- 44.Zhang O., Kay,L.E., Olivier,J.P. and Forman-Kay,J.D. (1994) Backbone 1H and 15N resonance assignments of the N-terminal SH3 domain of drk in folded and unfolded states using enhanced-sensitivity pulsed field gradient NMR techniques. J. Biomol. NMR, 4, 845–858. [DOI] [PubMed] [Google Scholar]
- 45.Wishart D.S., Bigam,C.G., Yao,J., Abildgaard,F., Dyson,H.J., Oldfield,E., Markley,J.L. and Sykes,B.D. (1995) 1H, 13C and 15N chemical shift referencing in biomolecular NMR. J. Biomol. NMR, 6, 135–140. [DOI] [PubMed] [Google Scholar]
- 46.Markley J.L., Bax,A., Arata,Y., Hilbers,C.W., Kaptein,R., Sykes,B.D., Wright,P.E. and Wüthrich,K. (1998) Recommendations for the presentation of NMR structures of proteins and nucleic acids. J. Biomol. NMR, 12, 1–23. [DOI] [PubMed] [Google Scholar]
- 47.Pelmenschikov A., Yin,X. and Leszczynski,J. (2000) Revealing the role of water in the acid–base interaction between the phosphate groups of DNA and the amino acid side chains of proteins: a density functional theory study of molecular models. J. Phys. Chem. B, 104, 2148–2153. [Google Scholar]
- 48.Carter E. and Tung,C.S. (1996) NAMOT2-A redesigned nucleic acid modeling tool: construction of non-canonical DNA structures. Comput. Appl. Biosci., 12, 25–30. [DOI] [PubMed] [Google Scholar]
- 49.Weiner S.J., Kollman,P.A., Nguyen,D.T. and Case,D.A. (1986) An all atom force field for simulations of proteins and nucleic acids. J. Computational Chem., 7, 230–252. [DOI] [PubMed] [Google Scholar]
- 50.Luger K., Mader,A.W., Richmond,R.K., Sargent,D.F. and Richmond,T.J. (1997) Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature, 389, 251–260. [DOI] [PubMed] [Google Scholar]
- 51.Metropolis N., Rosenbluth,A.W., Rosenbluth,M.N., Teller,A.H. and Teller,E. (1953) Equation of state calculations by fast computing machines. J. Chem. Phys., 21, 1087–1092. [Google Scholar]
- 52.Feigon J., Leupin,W., Denny,W.A. and Kearns,D.R. (1983) Two-dimensional proton nuclear magnetic resonance investigation of the synthetic deoxyribonucleic acid decamer d(ATATCGATAT)2. Biochemistry, 22, 5943–5951. [DOI] [PubMed] [Google Scholar]
- 53.Hare D.R., Wemmer,D.E., Chou,S., Drobny,G. and Reid,B.R. (1983) Assignment of the non-exchangeable proton resonances of d(CGCGAATTCGCG) using two-dimensional nuclear magnetic resonance methods. J. Mol. Biol., 171, 319–336. [DOI] [PubMed] [Google Scholar]
- 54.Remin M. and Shugar,D. (1972) Conformation of the exocyclic 5′CH2OH in nucleosides and nucleotides aqueous solution from specific assignments of the H5′ and H5′ signals in the NMR spectra. Biochem. Biophys. Res. Commun., 48, 636–642. [DOI] [PubMed] [Google Scholar]
- 55.Wood D.J., Hruska,F.E. and Ogilvie,K.K. (1974) Proton magnetic resonance studies of 2′-deoxythymidine, its 3′- and 5′-monophosphates and 2′-deoxythymidylyl-(3′,5′)-2-deoxythymidine in aqueous solution. Can. J. Chem., 52, 3353–3366. [Google Scholar]
- 56.Davies D.B. and Danyluk,S.S. (1974) Nuclear magnetic resonance studies of 5′-ribo and deoxyribonucleotide structures in solution. Biochemistry, 13, 4417–4434. [DOI] [PubMed] [Google Scholar]
- 57.Kellogg G.W. (1992) Proton-detected hetero-TOCSY experiments with applications to nucleic acids. J. Magn. Reson., 98, 176–182. [Google Scholar]
- 58.Torizawa T., Kata,K., Kimura,Y., Asada,T., Kobayashi,H., Komatsu,Y., Morioka,H., Nikaido,O., Ohtsuka,E. and Shimada,I. (1998) 31P NMR study of the interactions between oligodeoxynucleotides containing (6-4) photoproduct and Fab fragments of monoclonal antibodies specific for (6-4) photoproduct. FEBS lett., 429, 157–161. [DOI] [PubMed] [Google Scholar]
- 59.Castagné C., Murphy,E.C., Gronenborn,A.M. and Delepierre,M. (2000) 31P NMR analysis of the DNA conformation induced by protein binding SRY/DNA complexes. Eur. J. Biochem., 267, 1223–1229. [DOI] [PubMed] [Google Scholar]
- 60.Roongta V.A., Jones,C.R. and Gorenstein,D.G. (1990) Effect of distortions in the deoxyribose phosphate backbone conformation of duplex oligodeoxyribonucleotide dodecamers containing GT, GG, GA, AC and GU bp mismatches on 31P NMR spectra. Biochemistry, 29, 5245–5258. [DOI] [PubMed] [Google Scholar]
- 61.Schweitzer B.I., Gardner,K.H. and Tucker-Kellogg,G. (1995) HeteroTOCSY-based experiments for measuring heteronuclear relaxation in nucleic acids and proteins. J. Biomol. NMR, 6, 180–188. [DOI] [PubMed] [Google Scholar]
- 62.Kraulis P.J. (1991) MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallog., 24, 946–950. [Google Scholar]
- 63.Wood R.D. (1999) DNA damage recognition during nucleotide excision repair in mammalian cell. Biochimie, 81, 39–44. [DOI] [PubMed] [Google Scholar]
- 64.Mills B.M., Vacano,E. and Hagerman,P.J. (1999) Flexibility of single-stranded DNA: use of gapped duplex helices to determine the persistence lengths of poly(dT) and poly(dA). J. Mol. Biol., 285, 245–257. [DOI] [PubMed] [Google Scholar]
- 65.Creighton T.E. (1993) In Proteins: Structure and Molecular Properties. W.H. Freeman & Company, New York, NY, pp. 147–148 and 355–360.
- 66.Buschta-Hedayat N., Buterin,T., Hess,M.T., Nissura,M. and Naegeli,H. (1999) Recognition of nonhybridizing base pairs during nucleotide excision repair of DNA. Proc. Natl Acad. Sci. USA., 96, 6090–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]