Abstract
Many established inflammation- and nutrition-related factors have been investigated as potential independent prognostic factors in various cancers, including the C-reactive protein/albumin ratio (CAR), lymphocyte/monocyte ratio (LMR), modified Glasgow prognostic score (mGPS), body mass index (BMI), and prognostic nutritional index (PNI). This study was performed to estimate the prognostic value of these factors in predicting survival and platinum resistance in ovarian cancer (OC), especially according to stage. Kaplan-Meier and multivariate analyses were performed to plot the survival curve and determine the independent prognostic factors. Additionally, the area under the receiver operating characteristic curve (AUC) was used to predict platinum resistance and prognosis by comparing the predictive ability of PNI and cancer antigen (CA)-125. In all patients, decreased PNI was significantly associated with platinum resistance and poor overall survival (OS) and progression-free survival (PFS). Regarding tumor stage, decreased PNI was significantly associated with poor PFS and OS only in stage III OC. Furthermore, the PNI also showed a significantly higher AUC value than CA-125 for predicting mortality and platinum resistance in all OC patients, but not in stage III patients. In conclusion, decreased PNI is a powerful predictor of a poor prognosis in OC, and especially for stage III cases.
Introduction
In the United States, ovarian cancer (OC) is the leading cause of death from gynecologic cancer and is the fifth most common cause of cancer mortality in women. It has been estimated that there will be 22,440 new cases of, and 14,080 deaths from, OC in the United States in 2017, and less than 40% of affected women will be cured1. Recent data suggest that hormone therapy and pelvic inflammatory disease may increase the risk of OC2,3. Although most patients undergo primary cytoreductive surgery followed by chemotherapy, they always have a poor prognosis because of high chemotherapy resistance and advanced disease. Approximately 80% of OC patients will show tumor progression and relapse after first-line chemotherapy due to drug resistance or therapeutic failure within 1–2 years4,5. However, to our knowledge, the mechanism of chemoresistance remains unclear. It is still a major clinical challenge to clarify the relationships among inflammation, metabolism, drug resistance, and cancer, and to find an optimal prognostic factor for predicting chemotherapy resistance and survival of OC patients.
It has been found that preoperative nutritional and immunological condition, as well as systemic inflammatory response markers, are associated with the postoperative prognosis and overall survival (OS) of patients with malignant tumors6. Immune function can be affected by nutritional condition and inflammation status, which in turn affect the C-reactive protein (CRP) level and concentration of lymphocytes. Serum albumin, the synthesis of which is suppressed by malnutrition and inflammation, is generally used to assess nutritional status, severity of disease, disease progression, and prognosis7. In addition, albumin concentrations can be influenced by CRP concentrations, and this relationship is similar across various tumor types8. Therefore, in recent years, some inflammation- and nutrition-based factors have been investigated as possible prognostic and predictive markers in various cancers. Details of these factors are all easily available from peripheral blood samples, including the C-reactive protein/albumin ratio (CAR), lymphocyte/monocyte ratio (LMR), albumin and lymphocyte count combined into the prognostic nutritional index (PNI), and CRP- and albumin-related factors of the modified Glasgow prognostic score (mGPS)9–12. As an efficient, simple, and convenient novel prognostic factor, the PNI is calculated according to the following formula: serum albumin value (g/L) + 0.005 × lymphocyte count (per mm3) in peripheral blood11. Recently, PNI has been reported to be an independent prognostic factor for survival in different malignant carcinomas, including colorectal cancer, gastric cancer, lung cancer, and pancreatic cancer13–16. However, the prognostic importance of PNI for OC still needs to be elucidated, especially according to tumor stage. Although Miao et al.17 reported that PNI was an independent prognostic factor in OC patients, they did not assess the combination of PNI with other established prognostic factors, such as CAR, LMR, and mGPS. Thus, it is meaningful to combine the PNI and other established nutrition- and inflammation-related prognostic factors to obtain optimal independent prognostic scores for predicting the chemoresistance and clinical outcomes of OC patients at different stages.
Results
Patient characteristics
In total, 237 patients who were diagnosed with OC and underwent cytoreductive surgery followed by platinum-based chemotherapy were evaluated. Their median age was 50 years (range: 24–76 years). Among these patients, 131 (55.3%) were defined as platinum-sensitive and 106 (44.7%) were platinum-resistant. The majority of patients presented with serous ovarian carcinoma (n = 123, 51.9%) and were classified Federation of Gynecologists and Obstetricians (FIGO) stage III (n = 140, 59.1%) at initial diagnosis. The baseline characteristics of all patients are listed in Table 1.
Table 1.
Correlations of PNI and clinicopathological characteristics of ovarian cancer patients.
| Variable | n (%) | PNI | P value | |
|---|---|---|---|---|
| <47.2, n (%) | ≥47.2, n (%) | |||
| Age (years) | 0.066 | |||
| ≤50 | 125 (52.7) | 65 (47.4) | 60 (60.0) | |
| >50 | 112 (47.3) | 72 (52.6) | 40 (40.0) | |
| Stage | <0.001 | |||
| FIGO I | 28 (11.8) | 8 (5.8) | 20 (20.0) | |
| FIGO II | 39 (16.5) | 15 (10.9) | 24 (24.0) | |
| FIGO III | 140 (59.1) | 92 (67.2) | 48 (48.0) | |
| FIGO IV | 30 (12.7) | 22 (16.1) | 8 (8.0) | |
| Residual tumor mass | <0.001 | |||
| ≤2 cm | 132 (55.7) | 62 (45.3) | 70 (70.0) | |
| >2 cm | 105 (44.3) | 75 (54.7) | 30 (30.0) | |
| Histological subtype | 0.001 | |||
| Serous | 123 (51.9) | 61 (44.5) | 62 (62.0) | |
| Non-serous | 86 (36.3) | 63 (45.9) | 23 (23.0) | |
| Histological grade | 0.237 | |||
| G1 | 79 (33.3) | 40 (30.0) | 39 (39.0) | |
| G2 | 52 (21.9) | 30 (21.9) | 22 (22.0) | |
| G3 | 88 (37.1) | 56 (40.9) | 32 (32.0) | |
| Ascites | <0.001 | |||
| Negative | 102 (43.0) | 34 (24.8) | 68 (68.0) | |
| Positive | 135 (57.0) | 103 (75.2) | 32 (32.0) | |
| CA-125 (U/ml) | <0.001 | |||
| <35 | 26 (11.0) | 5 (3.6) | 21 (21.0) | |
| ≥35 | 183 (77.2) | 117 (85.4) | 66 (66.6) | |
| Chemosensitivity | <0.001 | |||
| Sensitive | 131 (55.3) | 61 (44.5) | 70 (70.0) | |
| Resistant | 106 (44.7) | 76 (55.5) | 30 (30.0) | |
| CAR | <0.001 | |||
| <0.5 | 142 (59.9) | 59 (43.1) | 83 (83.0) | |
| ≥0.5 | 95 (40.1) | 78 (56.9) | 17 (17.0) | |
| LMR | <0.001 | |||
| <3.82 | 132 (55.7) | 106 (77.4) | 26 (26.0) | |
| ≥3.82 | 105 (44.3) | 31 (22.6) | 74 (74.0) | |
| mGPS | <0.001 | |||
| 0 | 97 (40.9) | 27 (19.7) | 70 (70.0) | |
| 1 | 76 (32.1) | 46 (33.6) | 30 (30.0) | |
| 2 | 64 (27.0) | 64 (46.7) | 0 (0.0) | |
| BMI (kg/m2) | 0.460 | |||
| <18.5 | 31 (13.1) | 20 (14.6) | 11 (11.0) | |
| ≥18.5 | 206 (86.9) | 117 (85.4) | 89 (89.0) | |
CAR, C-reactive protein/albumin ratio; BMI, body mass index; FIGO, Federation of Gynecologists and Obstetricians; LMR, lymphocyte/monocyte ratio; mGPS, modified Glasgow prognostic score; PNI, prognostic nutritional index.
Cutoff point for determining the PNI
The median and mean levels of the PNI were 45.9 and 45.68 ± 7.14 (range: 26.60–65.60), respectively. Analyses using the biostatistical tool Cutoff Finder showed that a wide range of cutoff points for PNI were significant (Fig. 1). For OS, the optimal cutoff point of PNI was 47.2; patients were divided into two groups based on this cutoff value (PNI ≥ 47.2, n = 100, 42.2%; PNI < 47.2, n = 137, 57.8%).
Figure 1.
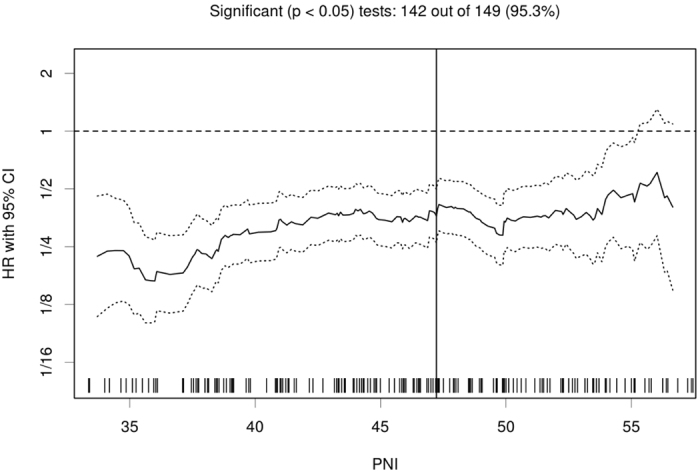
Hazard ratio (HR) for overall survival (OS) independent of the cutoff point for the prognostic nutritional index (PNI) in patients with ovarian cancer. The vertical line denotes the optimal cutoff point. The plots were generated using Cutoff Finder.
Correlation between PNI and clinicopathological parameters
The relation between preoperative PNI and the clinicopathological characteristics of patients with OC is shown in Table 1. Decreased PNI was significantly associated with advanced FIGO tumor stage (P < 0.001), maximum residual tumor (P < 0.001), histological subtype (P = 0.001), malignant ascites (P < 0.001), cancer antigen (CA)-125 ≥ 35 U/ml (P < 0.001), platinum resistance (P < 0.001), lower LMR (P < 0.001), and higher CAR (P < 0.001) and mGPS (P < 0.001). However, there were no significant associations between PNI and age (P = 0.066), grade (P = 0.237), or body mass index (BMI) (P = 0.460). Among tumor stage III patients, decreased PNI was also significantly associated with residual tumor mass (P = 0.023), histological subtype (P = 0.005), malignant ascites (P < 0.001), CA-125 ≥ 35U/ml (P = 0.006), lower LMR (P < 0.001), and higher CAR (P < 0.001) and mGPS (P < 0.001), but not with platinum resistance (P = 0.095).
Survival and prognostic factors
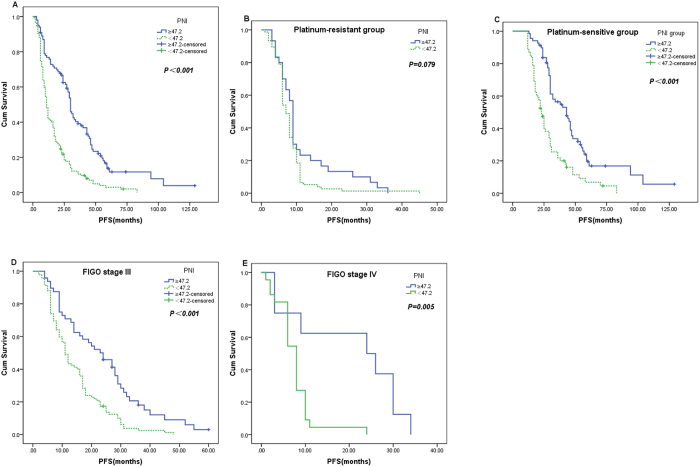
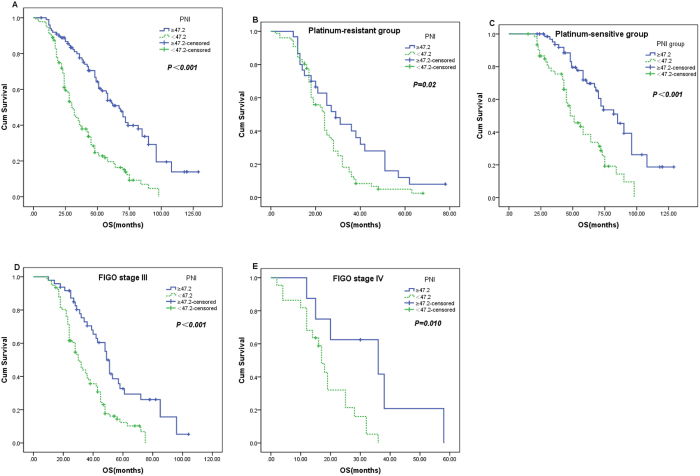
The median progression-free survival (PFS) and OS in the whole study population were 17 months and 36 months, respectively. Patients in the low-PNI group had significantly shorter PFS (17.3 vs. 37.8 months, P < 0.001) and OS (38.7 vs. 68.8 months, P < 0.001) than those in the high-PNI group (Figs 2A and 3A). When the patients were divided into platinum-sensitive and -resistant groups, significant differences in PFS and OS between high- and low-PNI patients were observed only in the platinum-sensitive group (49.4 vs. 28.9 months, P < 0.001 and 55.7 vs. 82.7 months, P < 0.001 respectively), and not in the platinum-resistant group (11.3 vs. 8.0 months, P = 0.079 and 34.0 vs. 24.8 months, P = 0.020, respectively) (Figs 2B,C and 3B,C).
Figure 2.
Kaplan–Meier progression-free survival curves showing the difference between the high- and low-PNI groups. (A) in all patients; (B,C) in platinum-resistant and platinum-sensitive subgroups; (D,E) in stage III and IV cases.
Figure 3.
Kaplan–Meier OS curves showing the difference between the high-PNI and low-PNI groups. (A) in all patients; (B,C) in platinum-resistant and platinum-sensitive subgroups; (D,E) in stage III and IV cases.
Univariate analyses revealed that FIGO tumor stage, residual tumor mass, massive ascites, CA-125 level, chemosensitivity, mGPS, PNI, CAR, LMR, and BMI were significantly associated with both PFS and OS (Tables 2 and 3). To assess the independent prognostic factors of OC, multivariate Cox proportional hazards were also assessed. In the multivariate Cox regression model, the FIGO tumor stage (hazard ratio [HR] = 1.478, 95% confidence interval [CI]: 1.170–1.868, P = 0.001), residual tumor mass (HR = 1.471, 95% CI: 1.009–2.144, P = 0.045), platinum resistance (HR = 7.427, 95% CI: 4.891–11.278, P < 0.001), mGPS (HR = 1.327, 95% CI: 1.053–1.673, P = 0.017), PNI (HR = 2.096, 95% CI: 1.380–3.185, P = 0.001), and BMI (HR = 1.828, 95% CI: 1.155–2.895, P = 0.010) were significantly associated with PFS (Table 2). However, only FIGO tumor stage (HR = 1.933, 95% CI: 1.461–2.556, P < 0.001), platinum resistance (HR = 4.832, 95% CI: 3.213–7.266, P < 0.001), and PNI (HR = 2.544, 95% CI: 1.761–3.675, P < 0.001) were also independently and significantly associated with shortened OS (Table 3).
Table 2.
Univariate and multivariate Cox proportional hazards analysis of progression-free survival.
| Variable | HR | Univariate | P value | HR | Multivariate | P value |
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||
| Age (years) (≤50 vs. >50) | 0.990 | 0.756–1.298 | 0.945 | |||
| FIGO stage (I/II/III/IV) | 2.390 | 1.966–2.907 | <0.001 | 1.478 | 1.170–1.868 | 0.001 |
| Histological grade (G1/G2/G3) | 1.146 | 0.973–1.350 | 0.102 | |||
| Histological subtype (serous vs. non-serous) | 1.075 | 0.802–1.441 | 0.629 | |||
| Residual tumor mass (≤2 cm vs. >2 cm) | 3.500 | 2.582–4.744 | <0.001 | 1.471 | 1.009–2.144 | 0.045 |
| Ascites (yes vs. no) | 2.127 | 1.603–2.821 | <0.001 | |||
| CA-125 (U/ml) (<35 vs. ≥35) | 2.025 | 1.267–3.236 | 0.003 | |||
| Chemosensitivity (sensitive vs. resistant) | 8.390 | 6.066–11.605 | <0.001 | 7.427 | 4.891–11.278 | <0.001 |
| mGPS (0/1/2) | 1.750 | 1.470–2.083 | <0.001 | 1.327 | 1.053–1.673 | 0.017 |
| PNI (<47.2 vs. ≥ 47.2) | 2.411 | 1.807–3.216 | <0.001 | 2.096 | 1.380–3.185 | 0.001 |
| CAR (<0.5 vs. ≥ 0.5) | 1.751 | 1.326–2.312 | <0.001 | |||
| LMR (<3.82 vs. ≥ 3.82) | 2.271 | 1.702–3.030 | <0.001 | |||
| BMI (<18.5 vs. ≥ 18.5) | 2.615 | 1.746–3.917 | <0.001 | 1.828 | 1.155–2.895 | 0.010 |
CAR, C-reactive protein/albumin ratio; BMI, body mass index; FIGO, Federation of Gynecologists and Obstetricians; LMR, lymphocyte/monocyte ratio; mGPS, modified Glasgow prognostic score; PNI, prognostic nutritional index.
Table 3.
Univariate and multivariate Cox proportional hazards analysis of overall survival.
| Variables | HR | Univariate | P value | HR | Multivariate | P value |
|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||
| Age (years) ≤50 vs. >50) | 1.002 | 0.742–1.353 | 0.990 | |||
| FIGO stage (I/II/III/IV) | 2.781 | 2.183–3.543 | <0.001 | 1.933 | 1.461–2.556 | <0.001 |
| Histological grade (G1/G2/G3) | 1.151 | 0.955–1.388 | 0.139 | |||
| Histological subtype (serous vs. non-serous) | 1.276 | 0.923–1.762 | 0.140 | |||
| Residual tumor mass (≤2 cm vs. >2 cm) | 3.349 | 2.405–4.664 | <0.001 | |||
| Ascites (negative vs. positive) | 2.331 | 1.693–3.211 | <0.001 | |||
| CA-125 (U/ml) (<35 vs. ≥35) | 2.299 | 1.299–4.067 | 0.004 | |||
| Chemosensitivity (sensitive vs. resistant) | 5.965 | 4.235–8.402 | <0.001 | 4.832 | 3.213–7.266 | <0.001 |
| mGPS (0/1/2) | 1.652 | 1.360–2.008 | <0.001 | |||
| PNI (<47.2 vs. ≥47.2) | 2.701 | 1.946–3.749 | <0.001 | 2.544 | 1.761–3.675 | <0.001 |
| CAR (<0.5 vs. ≥0.5) | 1.763 | 1.300–2.390 | <0.001 | |||
| LMR (<3.82 vs. ≥3.82) | 2.670 | 1.938–3.678 | <0.001 | |||
| BMI (<18.5 vs. ≥18.5) | 1.842 | 1.181–2.874 | 0.007 |
CAR, C-reactive protein/albumin ratio; BMI, body mass index; FIGO, Federation of Gynecologists and Obstetricians; LMR, lymphocyte/monocyte ratio; mGPS, modified Glasgow prognostic score; PNI, prognostic nutritional index.
When patients were stratified by FIGO tumor stage, high-PNI patients had significantly longer PFS than low-PNI patients only for cases of FIGO tumor stages III (P < 0.001) and IV (P = 0.005) (Fig. 2D,E). Similarly, high-PNI patients had significantly longer OS than low-PNI patients only in stages III (P < 0.001) and IV (P = 0.010) (Fig. 3D,E). However, the multivariate Cox regression model demonstrated that the PNI was an independent predictive factor of poor PFS (HR 1.815, 95% CI 1.113–2.958, P = 0.017) and OS (HR 1.699, 95% CI 1.035–2.789, P = 0.036) only in FIGO tumor stage III OC patients, as were residual tumor mass and chemosensitivity. All these findings show that the PNI is an independent risk factor for poor PFS and OS in OC patients, especially those at stage III.
Comparison of predictive ability
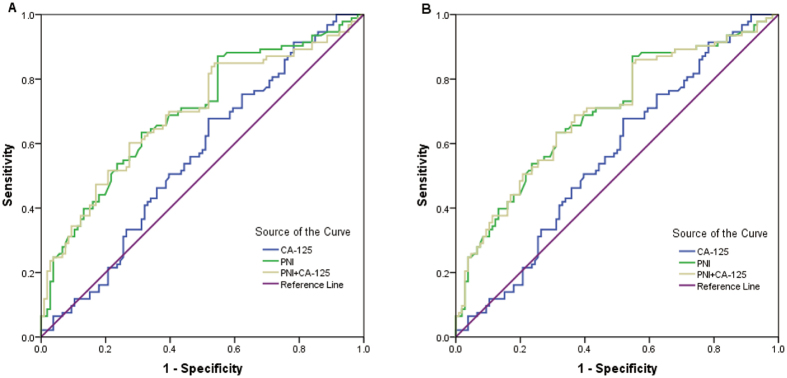
The receiver operating characteristic curve (ROC) and area under the receiver operating characteristic curve (AUC) values were used to compare the predictive ability among CA-125, PNI, and their combination for OS and platinum resistance (Fig. 4 and Table 4). With respect to predicting mortality, the PNI had a significantly higher AUC value than the CA-125 (0.677 vs. 0.567, P = 0.044). The combination of the PNI and CA-125 had a higher AUC value than either alone, although the difference was not significant (P > 0.05). Regarding platinum resistance, the PNI showed a higher AUC value than CA-125 (0.699 vs. 0.560, P = 0.006) and their combination (0.699 vs. 0.692, P = 0.847). The combination of the PNI and CA-125 had a significantly higher AUC value than CA-125 (P = 0.007). However, the PNI did not have a significantly higher AUC than CA-125 with respect to OS (0.649 vs. 0.547, P = 0.388) or platinum resistance (0.618 vs. 0.520, P = 0.094) among FIGO tumor stage III patients. Furthermore, the combination of PNI and CA-125 also did not have significantly higher AUC value than either one alone.
Figure 4.
Receiver operating characteristic curves of PNI, CA-125, and both in combination with respect to the prediction of OS (A) and platinum resistance (B).
Table 4.
Comparison of the diagnostic performance in predicting mortality and chemoresistance.
| Variable | Overall survival | Platinum resistance | ||
|---|---|---|---|---|
| AUC(95% CI) | P | AUC(95% CI) | P | |
| CA-125 | 0.567 (0.466–0.669) | 0.152 | 0.560 (0.480–0.639) | 0.146 |
| PNI | 0.677 (0.598–0.755) | <0.001 | 0.699 (0.632–0.765) | <0.001 |
| CA-125 + PNI | 0.688 (0.600–0.776) | <0.001 | 0.696 (0.623–0.770) | <0.001 |
AUC, area under the receiver operating characteristic curve; PNI, prognostic nutritional index.
Discussion
To date, no widespread nutrition- or inflammation-related factor has been found to index chemoresistance or prognosis in OC patients, especially according to tumor stage. Although the association between PNI and prognosis has been clarified in other cancers18, the impact of PNI on platinum resistance and clinical outcomes in OC, especially according to tumor stage, has not been clarified.
Laky et al.19 showed that about 20% of newly diagnosed gynecologic cancer patients have malnutrition. More than 20% of cancer patients die from malnutrition rather than the cancer itself20. Due to the metabolic effects of tumor mass, malignant ascites, and small bowel obstruction, OC patients are more likely to present with malnutrition and cachexia21. Furthermore, the tumor is more prone to develop chemoresistance in malnourished OC patients22. Recently, Matassa et al.23 also observed that oxidative metabolism drives inflammation-induced platinum resistance in OC. Lymphocytes were also reported to play a major role in immune responses by mediating the immunologic damage caused by various cancers24. As components of the PNI, both the albumin count and the lymphocyte count are closely related to inflammatory responses in cancer patients, which are independent predictors of long-term outcomes in OC25,26. According to the prognostic association between PNI and albumin and lymphocyte counts, it seems that PNI is a reflection of systemic inflammation, which may influence cancer growth and metastasis17. Thus, both inflammation- and malnutrition-related prognostic factors may induce chemotherapy resistance and predict the OS of OC patients.
Consistent with previous studies, our study demonstrated that FIGO tumor stage was an independent prognostic factor in OC patient27. To estimate the clinical outcomes of OC patients better, many inflammation- and malnutrition-based markers, such as BMI, LMR, mGPS, and CAR, have been investigated as potentially important prognostic and predictive factors in OC patients28–30. Similar to the study by Miao et al.17 our study demonstrated that the independent prognostic factor best predicting the OS of OC patients was PNI rather than BMI, CAR, LMR, or mGPS. The chi-square test determined that a PNI < 47.2 was not only associated with advanced FIGO tumor stage, maximum residual tumor, malignant ascites, platinum resistance, and lower LMR but also with higher CAR and mGPS. However, our study further showed that when patients were stratified by FIGO tumor stage, stage III patients showed the most significant association between PNI level and the outcome of the disease. Furthermore, ROC and AUC analyses showed that PNI was significantly superior to CA-125 in predicting mortality and platinum resistance in all-stage OC patients, but not in stage III cases. These results suggest that as an easily available laboratory hematological marker, PNI is superior to other nutrition- and inflammation-related prognostic factors in predicting survival in OC patients, especially for FIGO tumor stage III patients. Furthermore, PNI may also predict the platinum-based chemotherapeutic response of all-stage OC patients.
This study provides further support for the proposition that elevated preoperative PNI is associated with a good prognosis in OC patients. A study by Liu et al.31 showed that the CAR had superior prognostic ability compared to other established inflammation-related prognostic indices, such as the PNI, mGPS, neutrophil/lymphocyte ratio (NLR), and platelet/lymphocyte ratio (PLR) in 200 OC patients. The reason for this difference may be that our study included LMR, BMI, and platinum resistance. In addition, the current study not only further assessed the correlation between PNI and tumor stage but also compared the predictive ability of CA-125, the PNI, and their combination with respect to OS and platinum resistance, according to ROC and AUC values. Nevertheless, both Liu et al.31 and the present study used retrospective, single-center studies, and the number of patients was small in both. Therefore, more studies are needed to confirm these results. Furthermore, the mechanisms linking the PNI, poor prognosis and platinum resistance must be clarified.
Materials and Methods
Patients
In total, 237 newly diagnosed OC patients, treated with cytoreductive surgery and platinum-based chemotherapy between January 2007 and December 2015 at Nanfang Hospital of Southern Medical University, were identified. Pathological parameters, clinical data, and survival times were extracted from medical records. Patients who had active infection, coexisting hematologic malignancies, or other hematologic or autoimmune disorders were excluded. The primary endpoint of the study was PFS, which was calculated from the date of treatment to the date of recurrence or progression. OS was defined as the time from treatment to the date of death or last follow-up. All OC patients were followed up every 2–4 months for the first 2 years, and every 3–6 months thereafter until December 2016. At each visit, the patients were assessed by clinical and imaging examinations and the serum levels of CA-125 of patients were assessed. This study was approved by the medical ethics committee of Southern Medical University. All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from each patient.
All of the following data were obtained from medical records: age, BMI, FIGO stage, massive ascites, surgery, residual tumor mass, tumor (histology, grade), chemosensitivity, and clinical characteristics (CA-125, CRP, albumin, lymphocyte, and monocyte levels). Based on previous studies, optimal debulking was defined as a maximum diameter of residual tumor after surgery of ≤2 cm32,33. Patients were defined as platinum-resistant if the disease progressed within 6 months after completing first-line platinum-based chemotherapy, while all other patients were defined as platinum-sensitive34. PNI was calculated according to the following formula: serum albumin (g/L) + 0.005 × lymphocyte count (per mm3) in the peripheral blood11. CAR was calculated by CRP (mg/L)/albumin (g/L) ratio35. LMR was defined as the absolute lymphocyte count/absolute monocyte ratio36. The mGPS encompassed both the CRP and albumin concentrations. Patients with both CRP > 10 mg/L and albumin < 35 g/L were allocated a score of 2. Patients with both CRP ≤ 10 mg/L and albumin ≥ 35 g/L were allocated a score of 0. Patients with only one of these abnormal levels were given a score of 112. BMI, CAR, and LMR were categorized into two groups according to the cutoff values of ≥18.5 kg/m2, ≥0.5, and ≥3.82, respectively9,37,38.
Statistical analysis
Statistical analyses were performed with SPSS software (ver. 20.0; IBM Corp., Armonk, NY, USA). Comparisons between categorical variables were performed using the chi-square test. The optimal cutoff value for PNI was determined via a web-based application, programmed in R by Budczies et al. (http://molpath.charite.de/cutoff/)39. Significant prognostic variables in univariate analyses were included in multivariate Cox regression models to determine independent prognostic factors, using a forward stepwise method. Differences in survival among classification groups were analyzed using Kaplan-Meier curves and log-rank tests. ROC curves were calculated for PNI and CA-125, alone and in combination. The AUC values were compared using MedCalc software (ver. 15.2.1; MedCalc Software bvba, Ostend, Belgium). A two-sided P value < 0.05 was considered statistically significant.
Acknowledgements
We thank doctors, nurses, patients and their family members engaged in this study for their kindness to support our study.
Author Contributions
Weiwei Zhang and Yazhou Ren proposed the study, and collected and analyzed the data. Weijiang Liang and Bin Ye wrote the manuscript. All authors discussed the results and contributed to this manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
A correction to this article is available online at https://doi.org/10.1038/s41598-018-27841-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/22/2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
Contributor Information
Weijiang Liang, Email: wjliang22@126.com.
Yazhou Ren, Email: Yazhou.ren@uestc.edu.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Morch LS, Lokkegaard E, Andreasen AH, Kjaer SK, Lidegaard O. Hormone therapy and different ovarian cancers: a national cohort study. Am J Epidemiol. 2012;175:1234–1242. doi: 10.1093/aje/kwr446. [DOI] [PubMed] [Google Scholar]
- 3.Lin HW, et al. Risk of ovarian cancer in women with pelvic inflammatory disease: a population-based study. The Lancet. Oncology. 2011;12:900–904. doi: 10.1016/S1470-2045(11)70165-6. [DOI] [PubMed] [Google Scholar]
- 4.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet (London, England) 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 5.Pinato DJ, Graham J, Gabra H, Sharma R. Evolving concepts in the management of drug resistant ovarian cancer: dose dense chemotherapy and the reversal of clinical platinum resistance. Cancer Treatment Reviews. 2013;39:153–160. doi: 10.1016/j.ctrv.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Schwegler I, et al. Nutritional risk is a clinical predictor of postoperative mortality and morbidity in surgery for colorectal cancer. The British Journal of Surgery. 2010;97:92–97. doi: 10.1002/bjs.6805. [DOI] [PubMed] [Google Scholar]
- 7.Ballmer PE, Ochsenbein AF, Schutz-Hofmann S. Transcapillary escape rate of albumin positively correlates with plasma albumin concentration in acute but not in chronic inflammatory disease. Metabolism: Clinical and Experimental. 1994;43:697–705. doi: 10.1016/0026-0495(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 8.McMillan DC. The systemic inflammation-based Glasgow prognostic score: a decade of experience in patients with cancer. Cancer Treatment Reviews. 2013;39:534–540. doi: 10.1016/j.ctrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Xu XL, Yu HQ, Hu W, Song Q, Mao WM. A novel inflammation-based prognostic score, the C-reactive protein/albumin ratio predicts the prognosis of patients with operable esophageal squamous cell carcinoma. PLoS One. 2015;10:e0138657. doi: 10.1371/journal.pone.0138657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu SJ, et al. Lymphocyte to monocyte ratio and prognostic nutritional index predict survival outcomes of hepatitis B virus-associated hepatocellular carcinoma patients after curative hepatectomy. J Surg Oncol. 2016;114:202–210. doi: 10.1002/jso.24297. [DOI] [PubMed] [Google Scholar]
- 11.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85:1001–1005. [PubMed] [Google Scholar]
- 12.Proctor MJ, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow inflammation outcome study. British Journal of Cancer. 2011;104:726–734. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohri Y, et al. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–2692. doi: 10.1007/s00268-013-2156-9. [DOI] [PubMed] [Google Scholar]
- 14.Migita K, et al. The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Annals of Surgical Oncology. 2013;20:2647–2654. doi: 10.1245/s10434-013-2926-5. [DOI] [PubMed] [Google Scholar]
- 15.Kanda M, et al. Nutritional predictors of postoperative outcome in pancreatic cancer. The British Journal of Surgery. 2011;98:268–274. doi: 10.1002/bjs.7305. [DOI] [PubMed] [Google Scholar]
- 16.Shoji F, et al. Predictive impact for postoperative recurrence using the preoperative prognostic nutritional index in pathological stage I non-small cell lung cancer. Lung Cancer. 2016;98:15–21. doi: 10.1016/j.lungcan.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Miao Y, Li S, Yan Q, Li B, Feng Y. Prognostic significance of preoperative prognostic nutritional index in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Oncol Res Treat. 2016;39:712–719. doi: 10.1159/000452263. [DOI] [PubMed] [Google Scholar]
- 18.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. Journal of Cancer Research and Clinical Oncology. 2014;140:1537–1549. doi: 10.1007/s00432-014-1714-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laky B, et al. Malnutrition among gynaecological cancer patients. Eur J Clin Nutr. 2007;61:642–646. doi: 10.1038/sj.ejcn.1602540. [DOI] [PubMed] [Google Scholar]
- 20.Ottery FD. Cancer cachexia: prevention, early diagnosis, and management. Cancer Pract. 1994;2:123–131. [PubMed] [Google Scholar]
- 21.Garcia-Luna PP, Parejo CJ, Pereira CJL. Causes and impact of hyponutrition and cachexia in the oncologic patient. Nutricion Hospitalaria. 2006;21(Suppl 3):10–16. [PubMed] [Google Scholar]
- 22.Laky B, Janda M, Cleghorn G, Obermair A. Comparison of different nutritional assessments and body-composition measurements in detecting malnutrition among gynecologic cancer patients. Am J Clin Nutr. 2008;87:1678–1685. doi: 10.1093/ajcn/87.6.1678. [DOI] [PubMed] [Google Scholar]
- 23.Matassa DS, et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016;23:1542–1554. doi: 10.1038/cdd.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu B, et al. Prognostic value of inflammation-based scores in patients with osteosarcoma. Scientific Reports. 2016;6:39862. doi: 10.1038/srep39862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asher V, Lee J, Bali A. Preoperative serum albumin is an independent prognostic predictor of survival in ovarian cancer. Medical Oncology (Northwood, London, England) 2012;29:2005–2009. doi: 10.1007/s12032-011-0019-5. [DOI] [PubMed] [Google Scholar]
- 26.Milne K, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. Journal of Translational Medicine. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang WW, Liu KJ, Hu GL, Liang WJ. Preoperative platelet/lymphocyte ratio is a superior prognostic factor compared to other systemic inflammatory response markers in ovarian cancer patients. Tumour Biol. 2015;36:8831–8837. doi: 10.1007/s13277-015-3533-9. [DOI] [PubMed] [Google Scholar]
- 28.Bae HS, et al. The effect of body mass index on survival in advanced epithelial ovarian cancer. J Korean Med Sci. 2014;29:793–797. doi: 10.3346/jkms.2014.29.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eo WK, et al. The Lymphocyte-monocyte ratio predicts patient survival and aggressiveness of ovarian cancer. J Cancer. 2016;7:289–296. doi: 10.7150/jca.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu J, Wang H, Liu CC, Lu Y, Tang H. The Glasgow Prognostic Score (GPS) is a novel prognostic indicator in advanced epithelial ovarian cancer: a multicenter retrospective study. Journal of Cancer Research and Clinical Oncology. 2016;142:2339–2345. doi: 10.1007/s00432-016-2228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17:285. doi: 10.1186/s12885-017-3220-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hefler LA, et al. Serum C-reactive protein as independent prognostic variable in patients with ovarian cancer. Clinical Cancer Research: the Official Journal of the American Association for Cancer Research. 2008;14:710–714. doi: 10.1158/1078-0432.CCR-07-1044. [DOI] [PubMed] [Google Scholar]
- 33.Piver MS, et al. The impact of aggressive debulking surgery and cisplatin-based chemotherapy on progression-free survival in stage III and IV ovarian carcinoma. J Clin Oncol. 1988;6:983–989. doi: 10.1200/JCO.1988.6.6.983. [DOI] [PubMed] [Google Scholar]
- 34.Trillsch F, et al. Prognostic and predictive effects of primary versus secondary platinum resistance for bevacizumab treatment for platinum-resistant ovarian cancer in the AURELIA trial. Ann Oncol. 2016;27:1733–1739. doi: 10.1093/annonc/mdw236. [DOI] [PubMed] [Google Scholar]
- 35.Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond) 2009;9:30–33. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou, S. et al. Peripheral blood lymphocyte to monocyte ratio recovery from low levels at diagnosis after completion of first line therapy predicts good clinical outcomes in patients with diffuse large B-cell lymphoma. Oncotarget (2017). [DOI] [PMC free article] [PubMed]
- 37.Jiang N, et al. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20:10537–10544. doi: 10.3748/wjg.v20.i30.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Long W, Li PF, Lin YB, Liang Y. An elevated peripheral blood monocyte-to-lymphocyte ratio predicts poor prognosis in patients with primary pulmonary lymphoepithelioma-like carcinoma. PLoS One. 2015;10:e0126269. doi: 10.1371/journal.pone.0126269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budczies J, et al. Cutoff Finder: a comprehensive and straightforward Web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]