Abstract
Hypoxia-induced miR-210 displays a pro-survival, cytoprotective and pro-angiogenic role in several in vitro systems. In vivo, we previously found that miR-210 inhibition increases ischemic damage. Here we describe the generation of a versatile transgenic mouse model allowing the evaluation of miR-210 therapeutic potential in ischemic cardiovascular diseases. We generated a Tet-On miR-210 transgenic mouse strain (TG-210) by targeted transgenesis in the ROSA26 locus. To functionally validate miR-210 transgenic mice, hindlimb ischemia was induced by femoral artery dissection. Blood perfusion was evaluated by power Doppler while tissue damage and inflammation were assessed by histological evaluation. We found that miR-210 levels were rapidly increased in TG-210 mice upon doxycycline administration. miR-210 overexpression was maintained over time and remained within physiological levels in multiple tissues. When hindlimb ischemia was induced, miR-210 overexpression protected from both muscular and vascular ischemic damage, decreased inflammatory cells density and allowed to maintain a better calf perfusion. In conclusion, we generated and functionally validated a miR-210 transgenic mouse model. Albeit validated in the context of a specific cardiovascular ischemic disease, miR-210 transgenic mice may also represent a useful model to assess the function of miR-210 in other physio-pathological conditions.
Introduction
Ischemic diseases are characterized by diminished oxygen concentration in the involved tissues, a condition named hypoxia. Hypoxia induces an articulate program of responses aimed at relieving the effects of decreased oxygen tension and removing irreversibly damaged cells1. These responses include the expression of a specific subset of miRNAs, named hypoxia-induced miRNAs (hypoxamiRs)2–4. HypoxamiRs regulate cell response to decreased oxygen tension modulating the expression of several target genes 2. miR-210 can be considered the master hypoxamiR, since it has been found upregulated by hypoxia in all cells and tissues tested to date, carrying out a variety of functions3–7. Indeed, miR-210 is a target of Hypoxia-inducible factor 1-α (HIF1α), which directly activates its transcription under low oxygen tension5. miR-210, in turn, by targeting several transcripts involved in multiple aspects of cellular response to hypoxia, can inhibit apoptosis8, 9, support stem-cell survival10, repress mitochondrial metabolism promoting the shift from mitochondria respiration to glycolysis11 and induce angiogenesis8, 12–14.
Preclinical and clinical evidence confirms the crucial role of miR-210 in the regulation of cell response to hypoxia. Indeed, miR-210 has been found up-regulated in a variety of ischemic conditions and tissues such as hindlimb ischemia in mice9, human arteriosclerosis obliterans and atherosclerotic plaques15–18, ischemic wounds19, after brain transient focal ischemia in rats20, 21, upon myocardial infarction in humans22 and mice14, in failing hearts of diabetic patients23, in solid tumors6, and in pre-eclampsia24.
In a previous study, we identified a prominent role of miR-210 in a mouse model of hindlimb ischemia. miR-210 was inhibited by LNA-antisense oligonucleotides administration followed by femoral artery dissection. We found that miR-210 blocking increased apoptosis and necrosis of both muscular and vascular tissue in the ischemic limb9. Indeed, miR-210 blocking counteracted the physiological decrease of oxidative metabolism observed upon hypoxia, inducing mitochondrial dysfunction. This, in turn, led to increased reactive oxygen species (ROS) production, causing apoptosis and tissue damage.
Given miR-210 pro-survival, cytoprotective and pro-angiogenic role in several ischemic conditions, it has been proposed as a potential therapeutic target9, 12, 14, 18, 25. In order to obtain an in vivo model allowing the evaluation of miR-210 therapeutic potential in ischemic cardiovascular diseases, we generated a miR-210 transgenic mouse strain in which miR-210 expression is under the transcriptional control of a tet-repressor/tet-operator system26, 27. This study aims to characterize and functionally validate our transgenic mouse model for further investigations in the area of ischemic diseases. To this aim, we took advantage of our previous observation that miR-210 blocking increased ischemic damage9, and tested whether miR-210 transgenic mice exhibited lower muscle damage upon hindlimb ischemia.
Results
Generation of transgenic mice conditionally overexpressing miR-210
To generate a conditional miR-210 transgenic mouse strain (TG-210), mouse miR-210 was inserted into the ROSA26 locus, by using a targeting strategy that allowed the overexpression of the miRNA either conditionally, upon administration of an inducer (doxycycline), or constitutively, upon recombination of the loxP sites. Figure 1a shows that miR-210 gene was under the control of a promoter containing the tetracycline Operator (H1tetO), followed by a cassette containing an Improved Tetracycline Repressor (iTetR) controlled by CAG promoter. In the absence of doxycycline, the Tet-repressor protein binds to the tet-operator control element, inhibiting the miRNA expression from the H1 promoter (Fig. 1b). When doxycycline is administered, it binds to the tet-repressor protein and blocks its interaction with the H1 promoter26, 27. Alternatively, the whole iTetR cassette can be excised by CRE-mediate recombination of the loxP sites, yielding a constitutive activation. Mouse genotype was assessed by PCR (Fig. 1c and Supplementary Fig. 1).
Figure 1.
Generation of transgenic mice conditionally overexpressing miR-210. (a) The miR-210 coding region, flanked by 110 base pairs of its genomic sequence on each side, was inserted into the ROSA26 mouse locus using a RMCE targeting vector containing the indicated elements. The cassette containing a CAG promoter and an Improved Tetracycline Repressor (iTetR) can be excised by CRE mediated recombination of the loxP sites (red triangles), yielding constitutive overexperession of miR-210. (b) Schematic representation of miR-210 regulation in TG-210 mice. In the absence of the doxycycline inducer, the tet-repressor protein binds to the tet-operator control element and inhibits miR-210 expression from the H1 promoter. When doxycycline is administered, it binds to the tet-repressor protein and blocks its interaction with the H1 promoter, allowing miR-210 expression. (c) Genotyping strategy. Transgenic mice were genotyped by PCR using the primers indicated in panel a (red primers for WT and green primers for TG-210). The representative gel shows a 299 base pair WT band in all mice and a 656 base pair band present in heterozygous TG-210 mice. Excised lanes are indicated by dashes. Full-length gel is presented in Supplementary Figure 1.
Conditional overexpression of miR-210
In order to verify whether miR-210 was inducible upon doxycycline administration in our transgenic mouse model, miR-210 expression levels were measured in TG-210 mice treated with doxycycline (TG-210Doxy) for 5 days, compared to WT and TG-210 untreated mice (TG-210 UT); RNAs derived from bone marrow, gastrocnemius muscle, heart, kidney and brain were analyzed (Fig. 2a). TG-210 UT mice expressed increased levels of the transgene in absence of doxycycline, displaying a certain degree of leakiness in the model. Nevertheless, when doxycycline was administrated, TG-210Doxy mice showed significantly higher level of miR-210 compared to both WT untreated and TG-210 UT mice in each tissue/organ.
Figure 2.

miR-210 induction in TG-210 transgenic mice. (a) Ubiquitous increase of miR-210 upon doxycycline treatment. Bone marrow (BM), gastrocnemius muscle (Gas), heart, kidney and brain of WT mice fed with doxycyclin (WT Doxy), TG-210 untreated mice (TG-210 Ut) and TG-210 mice treated with doxycycline-containing food (TG-210 Doxy) for 5 days, were analyzed. The box plot shows miR-210 level measured by qPCR and expressed as fold induction versus WT Doxy. Box plots represent data divided in quartiles (n = 5–6; One-way Anova with Tukey’s multiple comparisons *P < 0.0001). (b) Time course of miR-210 overexpression in gastrocnemius muscles. Gastrocnemius muscles were harvested from non-ischemic TG-210Doxy mice after the indicated time of doxycycline administration, or from TG-210 UT mice. The bar graph shows miR-210 level measured by qPCR and expressed as fold induction versus TG-210 UT (n = 3–5; mean ± SE, Student’s t-test two-tailed ***P < 0.0001; **P < 0.003).
Next, we evaluated miR-210 expression level over time. Figure 2b shows that doxycycline administration led to a rapid and significant induction of miR-210 in the gastrocnemius of TG-210Doxy mice compared to TG-210 UT mice already after one day of treatment. This overexpression was time dependent, since miR-210 levels increased over time. Interestingly, miR-210 induction observed in TG-210Doxy mice was similar to that observed in physio-pathologically relevant conditions8, 9.
Taken together, these data indicate that, TG-210Doxy mice conditionally overexpressed miR-210, although some leakiness was observed.
miR-210 overexpression decreased tissue damage after acute hindlimb ischemia
To functionally validate miR-210 transgenic mice, the effect of miR-210 overexpression was evaluated in a mouse model of hindlimb ischemia. To this aim, TG-210 mice were fed with pellets of food containing doxycycline, starting from day 5 before ischemia. Control WT mice were also fed with the same food (WTDoxy), to exclude side effects of the drug. Since TG-210 UT mice showed weakly increased levels of miR-210 in each tissue tested, they were also used as control. Hindlimb ischemia was induced by femoral artery dissection in all experimental groups and mice were analyzed at day 3. First, tissue damage was assayed by in vivo systemic administration of Evans Blue Dye (EBD)28. EBD allows identifying damaged skeletal myofibers that lost cell membrane integrity, becoming permeable to EBD entry. This way, in fluorescence microscopy, damaged tissue emits a red fluorescence, while healthy myofibers remain dark28 (Fig. 3a). Figure 3b shows that EBD associated fluorescence was significantly lower in TG-210Doxy gastrocnemius muscles compared to WTDoxy muscles, highlighting less extensive damage. This result suggests that miR-210 overexpression protected skeletal myofibers from ischemic muscle damage. TG-210 UT mice seemed to display damage levels between WTDoxy and TG-210Doxy mice, but differences did not reach statistical significance.
Figure 3.

miR-210 overexpression in TG-210Doxy mice decreases tissue damage after ischemia. (a) Representative EBD stained sections of ischemic gastrocnemius muscles of WTDoxy, TG-210 UT and TG-210Doxy mice, 3 days after ischemia. EBD was administrate intraperitoneally 16 hours before the sacrifice. Red fluorescence indicates damaged myofibers, permeable to EBD; cells with an intact cell membrane remain dark. Nuclei were stained by Hoechst 33342 (blue fluorescence). Magnification 200x, calibration bar 20 µm. (b) The box plot shows EBD associated fluorescence expressed as Arbitrary Units (AU) and data are divided in quartiles (n = 9–10; One-way Anova with Tukey’s multiple comparisons **P < 0.0065).
Necrotic cell death stimulates a host inflammatory response involving the recruitment of specific myeloid cell populations29. To further confirm the differences observed in tissue damage among the three groups of mice, the presence of neutrophil granulocytes was investigated. Indeed, neutrophils are the first population recruited during the acute inflammatory response30, 31. Their density was evaluated in ischemic gastrocnemius muscles by immunofluorescence staining for GR-1. As shown in Fig. 4, significantly lower density of GR-1 positive cells was observed in TG-210Doxy mice compared to WTDoxy controls, according to the lower tissue damage observed. Also in this case, TG-210 UT mice seemed to show an intermediate phenotype.
Figure 4.

miR-210 overexpression in TG-210Doxy mice decreases neutrophil granulocytes in ischemic tissues. Neutrophil granulocytes density was evaluated by GR-1 immunofluorescence staining of gastrocnemius muscle sections, 3 days after ischemia. The box plot shows GR-1 positive cells/area of WTDoxy, TG-210 UT and TG-210Doxy mice. Data are divided in quartiles, (n = 6–7; One-way Anova with Tukey’s multiple comparisons **P < 0.007).
To further strengthen these observations, we evaluated vascular damage that follows hindlimb ischemia both by measuring residual calf perfusion and by morphometric analysis in histological sections. Residual calf perfusion was assessed by power Doppler ultrasound (Fig. 5a), measuring the vascularity ratio32 (Fig. 5b). The plot shows that residual calf perfusion was significantly higher in TG-210Doxy mice compared to WTDoxy controls, suggesting that miR-210 overexpression allows to maintain a better perfusion in the ischemic limb. Also in this case, TG-210 UT mice seemed to show a phenotype intermediate between WTDoxy and TG-210Doxy mice, suggesting a partial protection, but differences were not statistically significant.
Figure 5.

miR-210 overexpression in TG-210Doxy mice increases residual calf perfusion after ischemia. (a) Ultrasound perfusion images of mouse calf muscles 3 days after ischemia. (b) The box plot shows quantification of vascularity ratio (left ischemic/right non-ischemic), an index of calf perfusion. Box plots represent data divided in quartiles, (n = 10–11; One-way Anova with Tukey’s multiple comparisons **P < 0.04).
Next, arteriolar length density (ALD) was quantified in both contralateral non ischemic and ischemic gastrocnemius muscles (Fig. 6). Controlateral non ischemic gastrocnemius muscles of WT and Tg-210Doxy mice showed similar ALD; upon ischemia, TG-210Doxy gastrocnemius muscles conserved a significantly higher ALD compared to both WTDoxy and TG-210 UT mice (Fig. 6b). Of note, ALD differences were in agreement with blood perfusion data (Fig. 5), indicating their functional relevance.
Figure 6.

TG-210Doxy mice overexpressing miR-210 maintain a higher arteriolar density after ischemia. (a) Representative α-SMA immunofluorescence of WTDoxy, TG-210 UT and TG-210Doxy gastrocnemius muscles 3 days after ischemia. Magnification 400x, calibration bar 20 µm. (b) The box plot shows ALD quantification and data are divided in quartiles, (n = 5–9; One-way Anova with Tukey’s multiple comparisons *P < 0.05; **P ≤ 0.007).
Capillary density was quantified in hematoxylin/eosin stained sections of ischemic (Fig. 7a) and non-ischemic gastrocnemius muscles. In contralateral non-ischemic muscles, no differences were observed among the three groups of mice. As expected, three days after ischemia capillary density decreased in all experimental group. Nevertheless, TG-210Doxy muscles seem to retain a higher capillary density compared to control groups (Fig. 7b). However, this difference was not statistically significant in comparison with controls, possibly due to insufficient sample numerosity.
Figure 7.
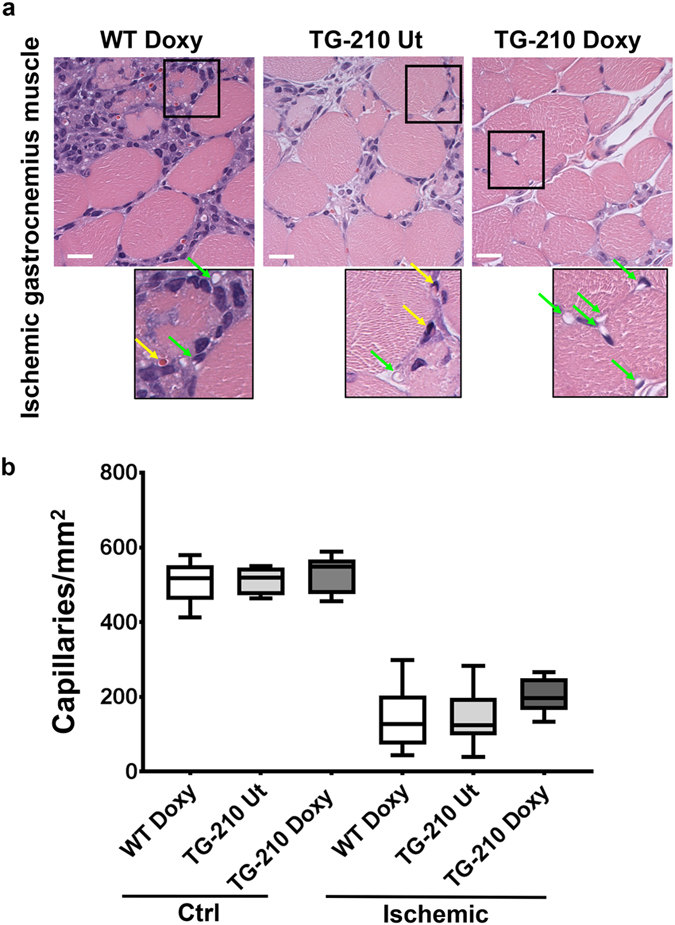
Capillary density after ischemia in miR-210 overexpressing mice. (a) Representative Hematoxylin/Eosin stained sections of ischemic gastrocnemius muscles of WTDoxy, TG-210 UT and TG-210Doxy mice, 3 days after ischemia. Magnification 400x. Calibration bar 20 µm. Inset shows capillaries at higher magnification. Green arrows indicate capillaries, yellow arrows indicate capillaries with a trapped erythrocyte. (b) The box plot shows quantification of capillaries/mm2 and data are divided in quartiles, (n = 11).
Taken together these results suggest that miR-210 overexpression in TG-210Doxy mice protects from ischemic damage, allowing to maintain a better perfusion in the tissue.
Discussion
In this work, we characterized a novel transgenic mouse model conditionally overexpressing miR-210. To this aim, we took advantage of the TetOn technology that has been successfully used in the past for conditional overexpression of different siRNA and miRNAs33–35. We generated this model with the purpose of obtaining a tool by which testing miR-210 therapeutic potential in ischemic cardiovascular diseases. Indeed, we previously demonstrated an anti-apoptotic, pro-survival role of miR-210 both in endothelial cells exposed to hypoxia and in mouse skeletal muscles exposed to acute ischemia8, 9. Our findings are also in keeping with several reports indicating that miR-210 inhibition increases apoptosis and cell death in a variety of cell culture systems4, 5, 36, 37.
Here, we demonstrated that, administrating an inducer, in this case doxycycline, transgenic mice overexpressed miR-210 efficiently in different tissues and organs. When miR-210 levels were analyzed in gastrocnemius muscles, we observed that they were significantly higher compared to TG-210 UT, although still compatible with physiological upregulation observed under hypoxia8 or ischemia9. Our analysis also showed that TG-210 uninduced mice expressed miR-210 at levels weakly but significantly higher compared to WT control mice, highlighting a certain degree of leakiness in the system. This problem was previously described by different authors as possibly due to incomplete or limited tetR-mediated suppression of the transgene33, 38–42. Thus, to understand if miR-210 could have a cytoprotective role even when weakly increased over basal levels, TG-210 uninduced mice were tested along with the induced ones. We observed that tissue damage, blood flow and inflammation levels were intermediate between those of WT and induced TG-210 mice, suggesting a possible biological function, conceivably via a preconditioning mechanism10, 25. However, these trends never reached statistical significance. This indicates that, if present, miR-210 potential preconditioning was not sufficient for a full ischemia protection in the adopted experimental system. More powered experiments with a higher number of samples are necessary to clarify this issue.
For a functional validation of miR-210 transgenic model, we took advantage of the characterization of the effects of miR-210 inhibition in hindlimb ischemia, previously described by our group9. For all parameters tested, by increasing miR-210 levels, we obtained results that were inverse to those observed by miR-210 blocking, i.e. lower tissue damage and lower decrease of blood flow and blood vessel density.
Of note, our observations are also in agreement with an in vivo study in which a gain of function approach was used after acute myocardial infarction in mice. It was found that miR-210 overexpression, induced by injection of minicircle non-viral vectors in the peri-infarct region, can inhibit apoptosis, increase angiogenesis, and improve cardiac functions14.
However, miR-210 function is complex and might be highly context- and time-dependent. In a model of perinatal hypoxic-ischemic encephalopathy, miR-210 blocking by local administration of LNA-anti-miR-210, reduces brain infarct size, improving neurological function recovery43. On the other hand, lentiviral brain delivery of miR-210 in mice, before the induction of focal cerebral ischemia, enhances microvessel density, the number of neural progenitor cells and improves neurobehavioral outcomes of the ischemic mouse44.
Interestingly, no significant differences were observed in both ALD and capillary density upon miR-210 expression in the non ischemic limbs, indicating no overt induction of angiogenesis in physiological conditions and suggesting a potential therapeutic applicability of miR-210 administration.
To further strengthen our data, the presence of inflammatory cells in the ischemic tissues was also evaluated. Indeed, necrotic cell death stimulates a host inflammatory response that involves the recruitment of specific myeloid cell populations within the injured area29. Specifically, neutrophils represent the first inflammatory myeloid cells that invade the area of injury with a peak of expression from 1 day to 5-day post-injury30, 31. As expected, TG-210Doxy mice showed significantly lower density of neutrophil granulocytes, according to the decreased tissue damage observed. Specific studies are needed to investigate miR-210 function in the inflammatory response.
Taken together, these results demonstrated that miR-210 overexpression in TG-210Doxy mice protects gastrocnemius muscle from muscular and vascular ischemic damage and allows to maintain a better calf perfusion, identifying miR-210 as potential therapeutic target in ischemic cardiovascular diseases12, 14, 18, 25.
Limitations of this study include increased basal miR-210 levels in TG-210 mice, somewhat complicating data analysis. Moreover, the present study should be interpreted as a proof of principle, meant to stimulate further investigations on miR-210 role in ischemia. More studies are also necessary to clarify the mechanisms of miR-210 action, as both direct and indirect mechanisms might underpin miR-210 protective function. miR-210 targeting of oxidative phosphorylation genes, allowing the control of ROS formation upon ischemia, is a likely mechanism; nevertheless, other indirect effects might be at work as well 2–5, 7, 9, 11.
Albeit validated in the context of a specific cardiovascular ischemic disease, TG-210 mice may also represent a useful model to assess the function of miR-210 in other physio-pathological conditions, such as cancer6, 45 pre-eclampsia46 and immune system dysfunctions15, 47, 48.
Material and Methods
Mouse model
All experimental procedures complied with the Guidelines of the Italian National Institutes of Health and with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Academy of Sciences, Bethesda, Md) and were approved by the institutional Animal Care and Use Committee: Ministero della Salute, Direzione Generale della Sanità Animale e dei Farmaci Veterinari, authorization no. 96/2015-PR (IACUC 666).
Two months old Doxycycline-inducible transgenic C57BL/6NTac-Gt(ROSA)26Sor tm3720(Mir210)Tac (TG-210) male mice or C57BL/6N littermate (Wild Type, WT) were used for all experiments. TG-210 mice were generated by Taconic Artemis (Germany). To generate TG-210 mice, the mouse miR-210 coding region flanked by 110 base pair of its genomic sequence on each side, was inserted into the ROSA26 locus using the Recombination-Mediated Cassette Exchange (RMCE) technique 49. The targeting vector included (Fig. 1A):
A promoter and a tetracycline Operator (H1tetO) with a 5xT transcription termination signal, driving miR-210 expression;
a cassette flanked by loxP sites, containing a CAG promoter, an Improved Tetracycline Repressor (iTetR) and a polyadenylation signal;
a Neomycin resistance (NeoR) cassette for positive clone selection following successful RMCE.
The targeting vector was co-transfected with the recombinase expressing vector pCAG-Flpe pA into TaconicArtemis C57BL/6 embryonic stem (ES) cell line carrying RMCE docking sites in the ROSA26 locus. Recombinant clones were isolated using positive (NeoR) selection. Correct RMCE events were confirmed by Southern-blot analysis using a standard protocol. Validated ES cells were injected into F1 blastocysts. After recovery, 8 injected blastocysts were transferred to each uterine horn of 2.5 days post-coitum pseudopregnant NMRI females. Chimerism was measured in chimeras (G0) by coat color contribution of ES cells to the BALB/c host (black/white). Highly chimeric mice were bred with C57BL/6 females. Germline transmission was identified by the presence of black, C57BL/6 strain, offspring (G1). Tg-210 heterozygous male mice were bred with two WT females each in order to establish and amplify a colony. Mice were housed in groups of 3–5 mice at 22 ± 2 °C, using a 12 h light-12 h dark cycle. Unless otherwise stated, animals were fed normal chow diet (SDS, irradiate VRF1).
Genotyping analysis
Genomic DNA was extracted from tail biopsies by using DirectPCR Lysis Reagent (Viagen Biotech) according to the manufacturer’s protocol. PCR reactions were performed to identify the presence of the transgenic miR-210 coding region, using the following primers: forward primer 5′-CCTGCAATATTTGCATGTCG-3′ and reverse primer 5′-GTCCCTATTGGCGTTACTATGG-3′. The unmodified ROSA26 locus was amplified as a control and to determine the zygosity of the locus, using the following primers: forward primer 5′-CTCTTCCCTCGTGATCTGCAACTCC-3′ and reverse primer 5′CATGTCTTTAATCTACCTCGATGG-3′. PCR conditions were as follows: pre-denaturation at 95 °C for 5 min, followed by denaturation at 95 °C for 30 s, primer annealing at 60 °C for 30 s, and extension at 72 °C for 1 min, and finally an additional extension at 72 °C for 10 min.
Reactions were analysed on 1.5% agarose gel containing ethidium bromide.
Induction of miR-210 transgene overexpression
In order to induce miR-210 overexpression, mice were fed with food pellets containing doxycycline 2 g/kg (Mucedola) ad libitum. Doxycycline was administrated 5 days before all surgical and experimental procedures.
To confirm the efficacy of miR-210 induction in hindlimb ischemia experiments, miR-210 expression was evaluated in non-ischemic contralateral quadriceps femoris muscles of each mouse, at 3 days of ischemia.
miR-210 quantification
Total RNA was extracted from each tissue using TRIzol (Invitrogen) and the TissueLyser system (Qiagen). miRNA levels were analyzed using TaqMan quantitative real-time PCR (qPCR; 1 ng per assay) and quantified with 7900HT Fast Real Time PCR system (Applied Biosystems).
Primers for miR-210, miR-16, U6 and the reagents for reverse transcriptase and qPCR reactions were all purchased from Applied Biosystems. miR-210 level in each sample was normalized to miR-16 and U6 average expression, as previously described9, 50.
Surgical and perfusion procedures
Before all surgical and perfusion procedures, mice were anesthetized with an intraperitoneal injection of 10 mg/kg Xylazine (Intervet Farmaceutici) and 100 mg/kg Ketamine (Ketavet 100; Intervet Farmaceutici). Dissection of the left femoral artery was previously described51.
Perfusion in ischemic calves was measured with Ultrasound device VEVO 2100 (Visual Sonics), using a 2100 transducer in power Doppler mode (transmit Power 100%; center frequency 32 MHz; gate 2; pulse repetition frequency; beam angle 0; Doppler gain 35 dB; dynamic range 35 dB) 3 days after ischemia. Residual calf perfusion was assessed by measuring the vascularity ratio (left ischemic/right non ischemic) as previously described32.
For evaluation of myofibers permeability by Evans Blue Dye (EBD), 1% EBD solution (Sigma-Aldrich) in phosphate-buffered saline (PBS, pH 7.5) was prepared, sterilized by Millex-GP 0.22 µm filtration (Millipore) and stored at 4 °C. EBD solution (1% volume relative to body mass) was injected into the right side of the peritoneal cavity 16 hours before sacrifice28. Thereafter gastrocnemius muscle samples were harvested and frozen in OCT embedding medium.
For histological analysis, mice were perfused via left ventricle with PBS pH 7.5, followed by 10% buffered formalin, at 100 mm/Hg for 10 min51. Next, gastrocnemius muscles were harvested, fixed and paraffin embedded.
Histology and morphometric analysis
For tissue damage quantification, 10 µm thick frozen sections from EBD samples were cut at –21 °C by a cryostat (Leica, Cryocut 1800), air-dried at room temperature, fixed in cold acetone (–20 °C) for 10 min and washed in PBS. Nuclei were stained by Hoechst 33342 (Sigma-Aldrich) and sections were mounted with fluorescence mounting medium (Dako, S3023)9. EBD associated fluorescence was quantified in-10–12 random fields/section at 200x magnification by ImageJ software52. The presence of myofibers in areas of the section not stained/dark was identified by both nuclear staining with Hoechst and morphological criteria by phase contrast microscopy. For capillary density quantification, Hematoxylin/Eosin stained sections of paraffin embedded gastrocnemius muscles were prepared as previously described51. Capillary density was measured counting the number of capillary profiles in 30–40 random fields/section, at 1000x magnification9. α-smooth muscle actin (α-SMA) labeling was used to identify arterioles as previously described 51. Briefly, the following antibodies were used: α-SMA antibody (α-SMA clone 1A4; Sigma) and anti-mouse IgG Fab specific FITC conjugate secondary antibody (F5262, Sigma). Arterioles with at least one layer of stained smooth muscle cells were visualized at 400x magnification. Images were acquired on the whole section and arterioles with a minimum internal diameter between 4 and 40.99 μm were considered. Arteriolar length density (LD) was determined by the following formula: LD (mm/mm3) = Σ(a/b)/M, where a and b are the maximum and minimum internal arteriolar diameters, respectively, and M is the solid tissue area53. For immunofluorescence of neutrophil granulocytes, paraffin embedded gastrocnemius muscles sections were incubated with the following antibodies: rat anti-mouse GR-1 (BD PharmigenTM) diluted 1:50 in PBS containing 5% BSA and goat anti-rat 488 Alexa Fluor diluted 1:100 in PBS.
A Zeiss Axioimager 2 fluorescence microscope equipped with Axiovision image analyzer software was used to acquire images and to measure areas. All histological and morphometric analyses were carried out by two blinded readers with comparable results.
Statistical analysis
Continuous variables were analyzed by two-tailed Student’s t-test or one-way ANOVA approach with Tukey’s multiple comparisons. All statistical tests were performed 2-sided and a p < 0.05 was considered as statistically significant. Continuous variables were expressed in bar graphs as mean ± standard error (SE) or in box plots representing data divided in quartiles. Outliers were identified by Tukey’s test. GraphPad Prism v.4.03 software (GraphPad Software Inc.) was used for statistical analysis.
Electronic supplementary material
Acknowledgements
This study was supported by Ministero della Salute (Ricerca Corrente, RF-2011-02347907 and PE-2011-02348537), Telethon-Italy (n. GGP14092), AFM-Telethon (n. 18477) and Cariplo Foundation (n. 2013-0887). L. Perani, M. Venturini and A. Esposito, Preclinical Imaging Facility, Experimental Imaging Center, San Raffaele Scientific Institute, Milan are acknowledged for mouse ultrasound imaging.
Author Contributions
G.Z., B.M. and F.M. designed the study, analyzed data and wrote the manuscript; G.Z., B.M., P.F., D.M., performed the experiments; G.S. and C.G. analyzed data and revised the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09763-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Semenza GL. Targeting hypoxia-inducible factor 1 to stimulate tissue vascularization. J Investig Med. 2016;64:361–363. doi: 10.1097/JIM.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greco S, Gaetano C, Martelli F. HypoxamiR regulation and function in ischemic cardiovascular diseases. Antioxid Redox Signal. 2014;21:1202–1219. doi: 10.1089/ars.2013.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–223. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devlin C, Greco S, Martelli F, Ivan M. miR-210: More than a silent player in hypoxia. IUBMB Life. 2011;63:94–100. doi: 10.1002/iub.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee HE, Ivan C, Calin GA, Ivan M. HypoxamiRs and cancer: from biology to targeted therapy. Antioxid. Redox Signal. 2014;21:1220–1238. doi: 10.1089/ars.2013.5639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuschi, P., Maimone, B., Gaetano, C. & Martelli, F. Noncoding RNAs in the vascular system response to oxidative stress. Antioxid Redox Signal (2017). [DOI] [PubMed]
- 8.Fasanaro P, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zaccagnini G, et al. Hypoxia-induced miR-210 modulates tissue response to acute peripheral ischemia. Antioxid Redox Signal. 2014;21:1177–1188. doi: 10.1089/ars.2013.5206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan SY, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alaiti MA, et al. Up-regulation of miR-210 by vascular endothelial growth factor in ex vivo expanded CD34+cells enhances cell-mediated angiogenesis. J Cell Mol Med. 2012;16:2413–2421. doi: 10.1111/j.1582-4934.2012.01557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, et al. Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res. 2012;35:182–191. doi: 10.1159/000331054. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–31. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M, et al. Up-regulation of microRNA-210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol. 2014;150:22–30. doi: 10.1016/j.clim.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Li T, et al. Identification of miR-130a, miR-27b and miR-210 as serum biomarkers for atherosclerosis obliterans. Clin Chim Acta. 2011;412:66–70. doi: 10.1016/j.cca.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 17.Raitoharju E, et al. miR-21, miR-210, miR-34a, and miR-146a/b are up-regulated in human atherosclerotic plaques in the Tampere Vascular Study. Atherosclerosis. 2011;219:211–217. doi: 10.1016/j.atherosclerosis.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Eken, S. M. et al. MicroRNA-210 Enhances Fibrous Cap Stability in Advanced Atherosclerotic Lesions. Circ Res (2016). [DOI] [PubMed]
- 19.Biswas S, et al. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci U.S.A. 2010;107:6976–6981. doi: 10.1073/pnas.1001653107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y, et al. miR-210 mediates vagus nerve stimulation-induced antioxidant stress and anti-apoptosis reactions following cerebral ischemia/reperfusion injury in rats. J. Neurochem. 2015;134:173–181. doi: 10.1111/jnc.13097. [DOI] [PubMed] [Google Scholar]
- 22.Bostjancic E, Zidar N, Glavac D. MicroRNA microarray expression profiling in human myocardial infarction. Dis. Markers. 2009;27:255–268. doi: 10.1155/2009/641082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greco S, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61:1633–1641. doi: 10.2337/db11-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li JYZ, Yong TY, Michael MZ, Gleadle JM. MicroRNAs: are they the missing link between hypoxia and pre-eclampsia? Hypertens. pregnancy. 2014;33:102–114. doi: 10.3109/10641955.2013.832772. [DOI] [PubMed] [Google Scholar]
- 25.Kim HW, Jiang S, Ashraf M, Haider KH. Stem cell-based delivery of Hypoxamir-210 to the infarcted heart: implications on stem cell survival and preservation of infarcted heart function. J Mol Med (Berl) 2012;90:997–1010. doi: 10.1007/s00109-012-0920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthess Y, et al. Conditional inhibition of cancer cell proliferation by tetracycline-responsive, H1 promoter-driven silencing of PLK1. Oncogene. 2005;24:2973–2980. doi: 10.1038/sj.onc.1208472. [DOI] [PubMed] [Google Scholar]
- 27.Kappel S, Matthess Y, Kaufmann M, Strebhardt K. Silencing of mammalian genes by tetracycline-inducible shRNA expression. Nat Protoc. 2007;2:3257–3269. doi: 10.1038/nprot.2007.458. [DOI] [PubMed] [Google Scholar]
- 28.Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelosi L, Coggi A, Forcina L, Musaro A. MicroRNAs modulated by local mIGF-1 expression in mdx dystrophic mice. Front Aging Neurosci. 2015;7 doi: 10.3389/fnagi.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shireman PK, Contreras-Shannon V, Reyes-Reyna SM, Robinson SC, McManus LM. MCP-1 parallels inflammatory and regenerative responses in ischemic muscle. J Surg Res. 2006;134:145–157. doi: 10.1016/j.jss.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Kulkarni OP, Lichtnekert J, Anders H-J, Mulay SR. The Immune System in Tissue Environments Regaining Homeostasis after Injury: Is ‘Inflammation’ Always Inflammation? Mediators Inflamm. 2016;2016 doi: 10.1155/2016/2856213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babu M, et al. Differential Promoter Methylation of Macrophage Genes Is Associated With Impaired Vascular Growth in Ischemic Muscles of Hyperlipidemic and Type 2 Diabetic Mice: Genome-Wide Promoter Methylation Study. Circ Res. 2015;117:289–299. doi: 10.1161/CIRCRESAHA.115.306424. [DOI] [PubMed] [Google Scholar]
- 33.Seibler J, et al. Reversible gene knockdown in mice using a tight, inducible shRNA expression system. Nucleic Acids Res. 2007;35 doi: 10.1093/nar/gkm122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jordan SD, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13:434–446. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 35.Kornfeld J-W, et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494:111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–4368. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 37.Huang X, Le Q-T, Giaccia AJ. MiR-210–micromanager of the hypoxia pathway. Trends Mol Med. 2010;16:230–237. doi: 10.1016/j.molmed.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mizuguchi H, Hayakawa T. Characteristics of adenovirus-mediated tetracycline-controllable expression system. Biochim Biophys Acta. 2001;1568:21–29. doi: 10.1016/S0304-4165(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 39.Meyer-Ficca ML, et al. Comparative analysis of inducible expression systems in transient transfection studies. Anal Biochem. 2004;334:9–19. doi: 10.1016/j.ab.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Marquis J, Kampfer SS, Angehrn L, Schumperli D. Doxycycline-controlled splicing modulation by regulated antisense U7 snRNA expression cassettes. Gene Ther. 2009;16:70–77. doi: 10.1038/gt.2008.138. [DOI] [PubMed] [Google Scholar]
- 41.Delerue F, White M, Ittner LM. Inducible, tightly regulated and non-leaky neuronal gene expression in mice. Transgenic Res. 2014;23:225–233. doi: 10.1007/s11248-013-9767-7. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z, Ma B, Homer RJ, Zheng T, Elias JA. Use of the tetracycline-controlled transcriptional silencer (tTS) to eliminate transgene leak in inducible overexpression transgenic mice. J Biol Chem. 2001;276:25222–25229. doi: 10.1074/jbc.M101512200. [DOI] [PubMed] [Google Scholar]
- 43.Ma Q, et al. Inhibition of microRNA-210 provides neuroprotection in hypoxic-ischemic brain injury in neonatal rats. Neurobiol Dis. 2016;89:202–212. doi: 10.1016/j.nbd.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng L-L, et al. Lentivirus-Mediated Overexpression of MicroRNA-210 Improves Long-Term Outcomes after Focal Cerebral Ischemia in Mice. CNS Neurosci Ther. 2016;22:961–969. doi: 10.1111/cns.12589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noman MZ, et al. Hypoxia-inducible miR-210 regulates the susceptibility of tumor cells to lysis by cytotoxic T cells. Cancer Res. 2012;72:4629–4641. doi: 10.1158/0008-5472.CAN-12-1383. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, et al. Elevated levels of hypoxia-inducible microRNA-210 in pre-eclampsia: new insights into molecular mechanisms for the disease. J Cell Mol Med. 2012;16:249–259. doi: 10.1111/j.1582-4934.2011.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song G, et al. Studying the association of microRNA-210 level with chronic hepatitis B progression. J Viral Hepat. 2014;21:272–280. doi: 10.1111/jvh.12138. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, et al. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat Immunol. 2014;15:667–675. doi: 10.1038/ni.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Du Z-W, Hu B-Y, Ayala M, Sauer B, Zhang S-C. Cre recombination-mediated cassette exchange for building versatile transgenic human embryonic stem cells lines. Stem Cells. 2009;27:1032–1041. doi: 10.1002/stem.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spinetti G, et al. MicroRNA-15a and microRNA-16 impair human circulating proangiogenic cell functions and are increased in the proangiogenic cells and serum of patients with critical limb ischemia. Circ Res. 2013;112:335–346. doi: 10.1161/CIRCRESAHA.111.300418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaccagnini G, et al. Magnetic Resonance Imaging Allows the Evaluation of Tissue Damage and Regeneration in a Mouse Model of Critical Limb Ischemia. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh VB, Singh MV, Gorantla S, Poluektova LY, Maggirwar SB. Smoothened Agonist Reduces Human Immunodeficiency Virus Type-1-Induced Blood-Brain Barrier Breakdown in Humanized Mice. Sci Rep. 2016;6 doi: 10.1038/srep26876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y-F, et al. Improvement of left ventricular remodeling after myocardial infarction with eight weeks L-thyroxine treatment in rats. J Transl Med. 2013;11 doi: 10.1186/1479-5876-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



