A supramolecular capsule with a polyaromatic shell binds d-sucrose from natural saccharide mixtures with perfect selectivity.
Abstract
Selective recognition of saccharides by artificial receptors in water is a challenging goal due to their strong hydrophilicities and complex molecular structures with subtle regio- and stereochemical differences. We report the selective and efficient encapsulation of d-sucrose within a coordination-driven molecular capsule from natural saccharide mixtures in water (~100% selectivity, >85% yield, and ~103 M−1 binding constant). Unlike previous artificial receptors and natural receptors that rely on multiple hydrogen-bonding interactions, theoretical calculations and control experiments indicate that the observed unique selectivity arises from multiple CH-π interactions between the sucrose hydrocarbon backbone and the shape-complementary polyaromatic cavity (~1 nm in diameter) of the capsule.
INTRODUCTION
Hydrogen bonds are a ubiquitous and indispensable tool in the selective binding of guest substrates by enzymes and biological receptors (1). Even in water, where hydrophilic natural compounds such as saccharides are fully solvated by water molecules through extensive hydrogen bonds (1–3), the protein surfaces and active sites precisely discriminate subtle stereo- and regiochemical differences in the complex structures (Fig. 1A) (4–6). The Davis group demonstrated that isophthalamide-based organic cages can efficiently accommodate monosaccharides such as d-glucose (binding constant: Ka = up to 190 M−1) (7) and disaccharides such as d-cellobiose (Ka = up to 3300 M−1) (8, 9) in water through hydrogen bonds and CH-π interactions. Similar cooperative interactions (10), reversible covalent bonds (11), coordinative interactions (12), and ion-dipole interactions (13) were also used for the recognition of natural saccharides in water. However, strict discrimination of saccharides in water remains an extremely hard task for synthetic molecular receptors (14–16). To further develop artificial receptors as novel sensing devices (17) and to provide mechanistic insights into biological recognition events such as taste (18–20), here we demonstrate a new recognition motif using a molecular cavity enclosed by polyaromatic frameworks (Fig. 1B) that exclusively bind d-sucrose in water. d-Sucrose is one of the most common natural compounds in our daily life, yet the relatively large and bulky structure (a ~1 nm length and ~300 Å3 volume) prevents it from full encapsulation by traditional covalent hosts (7–16). On the other hand, coordination-driven molecular cages and capsules (21–25) are available in a much larger range of cavity sizes and shapes, suitable for large guest substrates. However, because of the lack of effective bonding motifs, the recognition and binding of saccharides within coordination host compounds remain very rare, even in organic media (26, 27).
Fig. 1. Cartoon representation of saccharide recognitions and structures of a polyaromatic nanocapsule and saccharides.
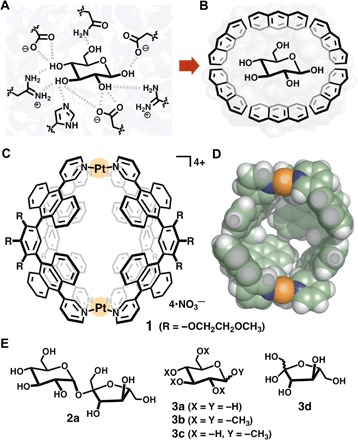
Recognition of d-glucose (A) in a hydrogen-bonding cavity modeled after the binding site of sucrose hydrolase (E322Q-glucose complex) from Xanthomonas axonopodis pv. glycines (6) and (B) in a polyaromatic cavity. (C) Coordination-driven polyaromatic nanocapsule 1 and (D) its slice through the center of the crystal structure [space-filling model; substituents (R) are replaced by hydrogen atoms for clarity]. (E) d-Sucrose (2a), glucose derivatives 3a to 3c, and d-fructose (3d) used as guest molecules.
The encapsulation of polyaromatic fullerene guests by saccharide-based molecular hosts, that is, cyclodextrin dimers, in water has been reported by several groups (28–30). The intriguing host-guest complexes prompted us to invert the relationship and use a polyaromatic-shelled host (31, 32) for the selective recognition of saccharide guests. We used coordination-driven molecular capsule 1, which has a spherical cavity (~1 nm in diameter and ~580 Å3 in volume) surrounded by polyaromatic anthracene panels (Fig. 1, C and D) (33). Although the host capability of 1 toward various hydrophobic compounds (for example, adamantanes, pyrenes, and fullerene C60) is well known (33–36), the potential for binding highly hydrophilic biomolecules in the hydrophobic cavity of 1 was rather unexpected. Nevertheless, we report here that nanocapsule 1 can effectively encapsulate d-sucrose (2a; Ka ≥ 1100 M−1) from mixtures of 2a and other natural disaccharides in water with perfect selectivity. Theoretical host-guest calculations and control binding experiments with d-glucose derivatives 3a to 3c (Fig. 1E) indicate that the observed unique selectivity stems from multiple CH-π interactions between the hydrocarbon framework of 2a and the shape-complementary polyaromatic cavity of 1.
RESULTS
Encapsulation of monosaccharides
We initially examined host-guest interactions between nanocapsule 1 and natural monosaccharides, such as d-glucose (3a), d-fructose (3d), and d-mannose, in water. For example, when a mixture of 1 (0.39 μmol) and slight excess 3a (2.0 μmol) was stirred in D2O (0.5 ml) at 60°C for 30 min (scheme S1), neither new peaks nor peak shifts (expected for host-guest interactions) were observed in the proton nuclear magnetic resonance (1H NMR) and electrospray ionization–time-of-flight (ESI-TOF) mass spectrometry (MS) spectra of the resultant solution (Fig. 2, A and B). No interactions were also observed for the other monosaccharides (figs. S1 and S2). These results are explicable by usual hydrophilic and hydrophobic properties: The highly hydrophilic saccharides preferentially exist in the aqueous phase rather than the hydrophobic cavity of 1.
Fig. 2. Host-guest interactions between capsule 1 and monosaccharides.
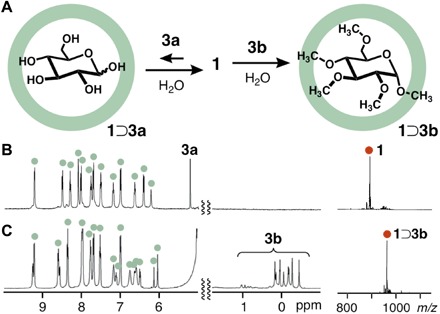
(A) Schematic representation of host-guest interactions between capsule 1 and d-glucose (3a) or pentamethylated α-d-glucose 3b in H2O. 1H NMR spectra (500 MHz, D2O, room temperature; left) and ESI-TOF MS spectra (H2O, room temperature, tetravalent molecular ion peak; right) of (B) a mixture of 1 and 3a, and (C) host-guest complex 1⊃3b.
In contrast, one molecule of pentamethylated α-d-glucose 3b was quantitatively encapsulated within capsule 1 under the same conditions (Fig. 2A, right, and scheme S2) (37), although 3b is very soluble in water (>50 mM). The 1:1 host-guest complex 1⊃3b exhibited 1H NMR signals derived from the methyl groups of encapsulated 3b ranging from −0.45 to 1.03 parts per million (ppm) (Fig. 2C, left, and figs. S3 and S4). These signals are significantly shifted upfield (Δδmax = −3.86 ppm), compared with free 3b in D2O, because of the shielding effects of the nearby anthracene rings. Close contact between 1 (protons Hf,b′) and 3b (CH3 groups) in the cavity was confirmed by one-dimensional (1D) nuclear Overhauser effect spectroscopy (NOESY) NMR studies (fig. S5). The noncovalent host-guest 1⊃3b complex is highly stable in water; we estimated the binding constant at >108 M−1 from 1H NMR and MS analyses under high-dilution conditions (5.0 μM; figs. S6 and S7) (37). The ESI-TOF MS spectrum only displayed prominent peaks from the intact host-guest structure [for example, mass-to-charge ratios (m/z) of 1995.4 for [1⊃3b − 2•NO3−]2+, 1309.6 for [1⊃3b − 3•NO3−]3+, and 966.7 for [1⊃3b − 4•NO3−]4+; Fig. 2C, right, and fig. S8]. The optimized structure of 1⊃3b indicates that the four CH3 groups of 3b are in close proximity (~3.6 Å) to the anthracene panels of 1 (fig. S9). The strong host-guest interactions observed between 1 and 3b are mainly caused by hydrophobic CH-π (polyaromatic) interactions in the confined cavity (38–41). Monomethylated α-d-glucose 3c was also bound by capsule 1 in 81% yield (figs. S1C and S2C). These unusual binding affinities prompted us to further examine host-guest interactions between the polyaromatic capsule and natural disaccharides.
Encapsulation of disaccharides
We next investigated aqueous binding for common disaccharides, d-sucrose, d-lactose, d-maltose, and d-trehalose (scheme S3), and thereby, efficient encapsulation by capsule 1 was observed for only d-sucrose (2a). Upon mixing 1 (0.39 μmol) with 2a (2.0 μmol) in D2O (0.5 ml) for 30 min at 60°C, 1:1 host-guest complex 1⊃2a was formed in 86% yield (Fig. 3A, right, and figs. S10 and S11). In the 1H NMR spectrum, all signals for the host and guest were assigned by 2D NMR studies (Fig. 3B and figs. S12 to S15). The methine signals HA–E,G–I and methylene signals HF,J,K of encapsulated 2a were found in the range of −1.14 to 1.29 ppm due to aromatic shielding (Δδmax = −4.64 ppm). Broadening of the anthracene signals Hc–e of 1 (at 6.5 to 7.2 ppm) suggests restricted motion of the polyaromatic panels upon encapsulation of the relatively large guest 2a. The number of the host proton signals of 1⊃2a is the same as that of empty 1, indicating full inclusion of 2a in the capsule cavity. The 1H diffusion-ordered spectroscopy (DOSY) NMR spectrum revealed the presence of a single host-guest species (Fig. 3C and fig. S16). A 1:1 host-guest composition was unambiguously confirmed by the ESI-TOF MS analysis, which showed prominent peaks at m/z values of 2041.7, 1340.5, and 989.9 assignable to [1⊃2a − n•NO3−]n+ species (n = 2, 3, and 4, respectively; Fig. 3E and fig. S17). Host-guest interactions were undetected for 1 and the other disaccharides in water (fig. S18). Furthermore, the formation of the ternary complex 1⊃(3a•3d) was not observed upon combination of 1 and an equimolar mixture of 3a and d-fructose (3d) (5 eq each), which are components of 2a, even under various conditions (Fig. 3A, left, and D).
Fig. 3. Encapsulation of d-sucrose within capsule 1 in water.
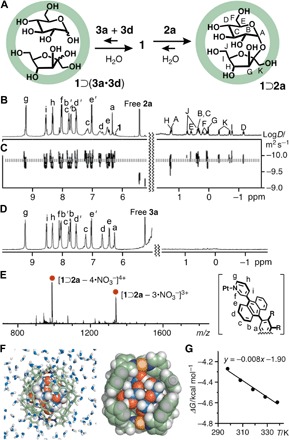
(A) Schematic representation of host-guest interactions between capsule 1, d-glucose (3a), and d-fructose (3d) (left) and the encapsulation of d-sucrose (2a) within 1 (right). (B) 1H NMR and (C) 1H DOSY NMR spectra (500 MHz, D2O, room temperature) of 1⊃2a. (D) 1H NMR spectrum (500 MHz, D2O, room temperature) of a mixture of 3a and 3d with 1. (E) ESI-TOF MS spectrum (H2O, room temperature) of 1⊃2a. (F) Structure of 1⊃2a in water (left) and its slice through the center (right) (substituents and counterions are omitted for clarity). (G) van’t Hoff plot for the thermodynamic parameters of 1⊃2a.
To elucidate detailed host-guest interactions, we estimated theoretical binding free energies of 1⊃2a and the related host-guest complexes in water by comparing the solvation free energies of the saccharide guests and intermolecular host-guest interactions (42). The binding energy of 1⊃2a is lower than that of host-guest complexes such as 1⊃(d-trehalose) and 1⊃(d-lactose) (−10.2 and −37.2 kcal mol−1, respectively) and higher than that of 1⊃3b (11.0 kcal mol−1) (table S1). These results are consistent with the 1H NMR–binding experiments. In the optimized structure (Fig. 3F and figs. S19 and S20), encapsulated 2a adopts a spherical conformation in the spherical cavity of 1. The conformation is supported by the NOESY NMR analysis, where correlation signals are observed between the pyranose and furanose moieties (HE–HJ and HA–HK) of 2a (fig. S13). The three CH2 groups of 2a are in close contact (<3.8 Å) with the polyaromatic panels of 1 in the optimized structure (Fig. 3F). Thermodynamic parameters for the host-guest interactions of 1⊃2a in water were determined by a van’t Hoff plot (Fig. 3G) using temperature-dependent 1H NMR analysis (fig. S21 and table S2). The obtained positive value of entropy (8.00 cal mol–1 K–1) and negative value of enthalpy (−1.90 kcal mol–1) indicate that the encapsulation process is driven mainly by enthalpic stabilization. The binding constant was also calculated to be 1170 ± 120 M−1 at 25°C (fig. S22 and table S3).
Selective recognition of d-sucrose
Nanocapsule 1 is a working sucrose receptor and selectively binds d-sucrose even from mixtures of natural disaccharides in water. To illustrate this instance, the 1⊃2a complex exclusively formed from a D2O solution containing 1 and a mixture of 2a and d-trehalose (2b) (5 eq each) for 30 min at 60°C (Fig. 4A). The upfield region 1H NMR spectrum of the resultant solution displayed only peaks when corresponding to encapsulated 2a (Fig. 4, B and C). The host-guest complex could be isolated as a pale yellow solid (in 80% yield; Fig. 4D and fig. S23) from the aqueous solution by salting out with a grain of KNO3. The sucrose-bound capsule 1⊃2a was selectively obtained from all competitive binding experiments with 2a and other disaccharides [that is, d-lactose (2c), d-maltose (2d), d-cellobiose (2e), and d-lactulose (2f); Fig. 4, E and F], as revealed by 1H NMR analysis (fig. S24). On the basis of the present experimental and theoretical studies, we postulate that the observed strict discrimination stems from an effective steric match and multiple CH-π interactions between the hydrocarbon backbone of sucrose and the polyaromatic interior surface of capsule 1.
Fig. 4. Selective recognition of d-sucrose and structures of disaccharides and artificial sugars.
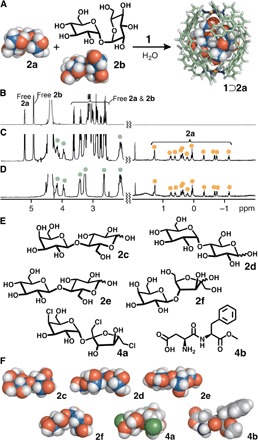
(A) Schematic representation of the selective encapsulation of d-sucrose (2a) from a mixture of 2a and d-trehalose (2b) by capsule 1. 1H NMR spectra (500 MHz, D2O, room temperature) of (B) 2a and 2b, (C) a mixture of 1⊃2a, 2a, and 2b, and (D) isolated 1⊃2a. (E) d-Lactose (2c), d-maltose (2d), d-cellobiose (2e), d-lactulose (2f), sucralose (4a), and aspartame (4b) used as guest molecules and (F) their optimized structures [density functional theory (DFT), B3LYP/6-31G(d); conductor-like polarizable continuum model (CPCM; H2O) level].
Note that the artificial cavity of capsule 1 could quantitatively and strongly bind artificial sugar substitutes such as sucralose (4a) and aspartame (4b) in water (Fig. 4, E and F, figs. S25 to S27, and scheme S4) (42, 43). The competitive encapsulation experiments revealed the binding preference in the order of 4a > 4b >>> 2a (figs. S28 and S29, and schemes S5 and S6). The binding constants of 1 toward 4a and 4b were estimated to be 24,200 and 13,000 M−1, respectively, by 1H NMR studies (fig. S27). The observed order is the same as the degree of their sweetness toward people. Artificial sugar substitutes 4a and 4b are approximately 600 and 200 times sweeter than 2a, respectively (42). Because the size and shape of 4a are comparable to those of 2a, the lower hydrophilicity of 4a, which has three hydrophobic chloro groups, as compared with that of 2a would enhance its affinity toward the hydrophobic cavity of 1.
DISCUSSION
Here, we described the selective encapsulation of d-sucrose in water from natural disaccharide mixtures within a nonfunctionalized polyaromatic cavity of a coordination-driven molecular capsule. Unlike previous synthetic molecular receptors, which rely on multiple hydrogen bonding, covalent bonding, coordinative, or ion-dipole interaction sites, the present capsule binds d-sucrose with perfect selectivity through a combination of shape-complementary and specific CH-π (polyaromatic ring) interactions. These results expand the versatility and utility of artificial polyaromatic nanospaces for the selective recognition and isolation of complex biomolecules in water.
MATERIALS AND METHODS
Chemicals
Capsule 1 (35) and saccharide 3c (44) were synthesized according to previously reported procedures in the literature. Solvents and reagents were purchased from TCI Co. Ltd., Wako Pure Chemical Industries Ltd., Kanto Chemical Co. Inc., Sigma-Aldrich Co., and Cambridge Isotope Laboratories Inc.
General
The NMR spectra were obtained using Bruker AVANCE III 400 (400 MHz) and ASCEND-500 (500 MHz). The 1H NMR and 2D NMR spectra were measured with tetramethylsilane as the internal standard. The ESI-TOF MS data were obtained using Bruker micrOTOF II. The Fourier transform infrared (FT-IR) spectra were recorded using JASCO FT/IR-4200.
Formation of 1⊃2a
Pt capsule 1 (1.5 mg, 0.39 μmol) and d-sucrose (2a; 0.7 mg, 2.0 μmol) were added to a glass test tube containing D2O (0.5 ml). The mixture was stirred for 30 min at 60°C. The formation of 1:1 host-guest complex 1⊃2a was confirmed by NMR and ESI-TOF MS analyses (86% yield based on 1). Thermodynamic parameters of the encapsulation of 2a by capsule 1 were estimated by temperature-dependent 1H NMR analysis. The binding constants (Ka = [1⊃2b]/[1]•[2b]) were determined on the basis of the integral ratios of the host-guest proton signals (fig. S22 and table S3). The thermodynamic parameters (ΔH and ΔS) were calculated from ΔG values obtained at 298 to 338 K (Fig. 3G, fig. S21, and table S2) (45).
1H NMR (500 MHz, D2O, room temperature): δ −1.14 (t, J = 9.5 Hz, 1H, 2a), −0.75 (d, J = 9.5 Hz, 1H, 2a), −0.67 (d, J = 12 Hz, 1H, 2a), −0.32 (d, J = 12 Hz, 1H, 2a), 0.04 to 0.09 (m, 2H, 2a), 0.22 (m, 1H, 2a), 0.34 to 0.45 (m, 2H, 2a), 0.62 (t, J = 9.5 Hz, 1H, 2a), 0.76 (d, J = 14 Hz, 1H, 2a), 1.29 (m, 2H, 2a), 2.43 to 2.48 (s, 24H, 1), 3.07 (m, 16H, 1), 3.40 (s, 12H, 1), 3.88 to 4.20 (m, 24H, 1), 4.47 (m, 4H, 1), 4.62 (m, 4H, 1), 6.33 (s, 4H, 1), 6.45 (br, 8H, 1), 6.73 (br, 8H, 1), 7.01 (d, J = 9.0 Hz, 8H, 1), 7.15 (br, 8H, 1), 7.53 (dd, J = 9.0, 7.0 Hz, 8H, 1), 7.71 (d, J = 9.0 Hz, 8H, 1), 7.77 (br, 8H, 1), 8.09 to 8.27 (m, 16H, 1), 8.32 (dd, J = 8.0, 5.5 Hz, 8H, 1), 8.54 (d, J = 8.0 Hz, 8H, 1), 9.21 (d, J = 5.5 Hz, 8H, 1). 1H NMR (500 MHz, D2O, 60°C): δ −1.12 (t, J = 9.5 Hz, 1H, 2a), −0.73 (d, J = 9.0 Hz, 1H, 2a), −0.66 (d, J = 12 Hz, 1H, 2a), −0.33 (d, J = 12 Hz, 1H, 2a), 0.02 (d, J = 9.0 Hz, 1H, 2a), 0.09 (d, J = 12 Hz, 1H, 2a), 0.19 (m, 1H, 2a), 0.29 to 0.43 (m, 3H, 2a), 0.58 (t, J = 9.5 Hz, 1H, 2a), 0.73 (d, J = 14 Hz, 1H, 2a), 1.27 (t, J = 9.0 Hz, 1H, 2a), 1.30 (s, 1H, 2a), 2.45 to 2.49 (s, 24H, 1) 3.08 (m, 16H, 1), 4.57 to 4.58 (m, 8H, 1), 6.15 to 6.26 (s, 4H, 1), 6.34 to 6.45 (m, 8H, 1), 6.56 to 6.66 (br, 8H, 1), 6.92 (d, J = 8.5 Hz, 8H, 1), 7.05 to 7.21 (br, 8H, 1), 7.48 (dd, J = 9.0, 7.0 Hz, 8H, 1), 7.65 (d, J = 9.0 Hz, 8H, 1), 7.74 (dd, J = 9.0, 7.0 Hz, 8H, 1), 7.95 to 8.04 (m, 8H, 1), 8.30 (dd, J = 9.5, 5.5 Hz, 8H, 1), 8.45 to 8.52 (m, 8H, 1), 9.16 to 9.20 (m, 8H, 1). 1H DOSY NMR (500 MHz, D2O, 25°C): D = 1.48 × 10−10 m2 s−1. FT-IR (KBr, cm−1): 2926, 2883, 2828, 1638, 1384, 1357, 1245, 1195, 1105, 1061, 1031, 945, 821, 768, 706, 671, 639, 617. ESI-TOF MS (H2O, room temperature): m/z 2041.7 [1⊃2a − 2•NO3−]2+, 1340.5 [1⊃2a − 3•NO3−]3+, 989.9 [1⊃2a − 4•NO3−]4+.
Selective encapsulation of 2a by 1 from mixed disaccharides
Pt capsule 1 (1.5 mg, 0.39 μmol), d-sucrose (2a; 0.7 mg, 2.0 μmol), and d-trehalose (2b; 0.7 mg, 2.0 μmol) were added to a glass test tube containing D2O (0.5 ml). The mixture was stirred for 30 min at 60°C. The selective formation of 1:1 host-guest complex 1⊃2a was confirmed by 1H NMR analysis. When a grain of KNO3 was added to the aqueous solution, a yellow precipitate of 1⊃2a was generated. The 1H NMR spectrum of the collected yellow solid (1.3 mg) in D2O (0.5 ml) revealed the isolation of 1⊃2a in 80% yield. The selective formation of 1⊃2a was also observed from mixtures of 2a/2c, 2a/2d, 2a/2e, and 2a/2f under the same conditions.
Theoretical calculation of host-guest complexes
The universal force field (UFF) model (46) was used for intramolecular potential models and nonelectrostatic interactions, unless otherwise specified. The atomic charges of molecular capsule 1 (R = -OCH3) were determined by the charge equilibration method (47) implemented in the FORCITE module at the geometry of the crystal structure, whereas the atomic charges of saccharides [that is, d-sucrose (2a), d-trehalose (2b), d-lactose (2c), and pentamethylated α-d-glucose (3b)] were taken from the GLYCAM06j force field (48). The geometry optimizations of all the molecules were performed with the FORCITE module of Materials Studio and the Sander module of an Amber14 suite (49). The 10-ns-long annealing simulations of the hydroxy groups on the saccharides from 1000 to 10 K were performed to optimize their orientations before the full-geometry optimizations with the Sander module. The final optimized structures of the molecular capsule and the saccharides were obtained by performing the geometry optimizations implemented in the FORCITE program (fig. S19). Relative binding energies were evaluated with gas-phase binding energies and solvation energies of the saccharides in aqueous solutions on the assumption that differences in solvation energies between the host-guest complexes with the different saccharides are negligible. Given that the capsular hosts isolated the sugars from bulk water environment, and conformations of the host frameworks in contact with the bulk water molecules were not greatly altered upon the encapsulations, the solvation energies of the host-guest complexes with the different saccharides can be expected to be almost identical. First, the gas-phase binding energies were calculated with the UFF model. Then, the solvation free energies, ΔGsolv, were calculated by the finite-difference linearized Poisson-Boltzmann method for electrostatic contributions and the solvent-accessible surface area model for nonpolar contributions (50), both of which were implemented in the Amber14 suite. We used default values except for a grid spacing of 0.1 Å and the atomic radii of the modified Bondi ones (51). The molecular dynamics simulation was performed with the modified pmemd module in the Amber14 suite (49), where the intramolecular geometries were fixed at their optimized structures and the capsule and saccharides were treated as a rigid body (52). We used the cubic unit cell of the 44.31 Å edge length, which contained capsule 1 (R = -OCH3), 2a, and 2723 TIP3P (transferable intermolecular potential with 3 points) waters (fig. S20).
Acknowledgments
M. Yamashina thanks the Japan Society for the Promotion of Science (JSPS) for an Overseas Research Fellowship. Funding: This study was supported by the JSPS KAKENHI (grant nos. JP25104011/JP26288033/JP17H05359) and Support for Tokyotech Advanced Researchers (STAR). Author contributions: M. Yamashina and M. Yoshizawa designed the work, carried out the research, analyzed the data, and wrote the paper. M.A., T.H., and S.H. were involved in the work discussion. T.H. and S.H. contributed to the theoretical calculations. M. Yoshizawa is the principal investigator. All authors discussed the results and commented on the manuscript. M. Yoshizawa claims responsibility for all figures in the main text and the Supplementary Materials. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/8/e1701126/DC1
scheme S1. Host-guest interactions between 1 and 3a.
scheme S2. Formation of 1⊃3b.
scheme S3. Host-guest interactions between 1 and 2b.
scheme S4. Formation of 1⊃4a.
scheme S5. Selective encapsulation of 4a by 1 from a mixture of 2a and 4a.
scheme S6. Competitive binding experiment of 4a and 4b by 1.
fig. S1. 1H NMR spectra (500 MHz, D2O, room temperature) of 1 with various monosaccharides.
fig. S2. ESI-TOF MS spectra (H2O, room temperature) of 1 with various monosaccharides.
fig. S3. Temperature-dependent 1H NMR spectra (500 MHz, D2O) of 1⊃3b.
fig. S4. 1H DOSY NMR spectrum (500 MHz, D2O, 25°C) of 1⊃3b.
fig. S5. 1D NOESY spectrum (500 MHz, D2O, room temperature, irradiation at 7.96 ppm) of 1⊃3b.
fig. S6. Concentration-dependent 1H NMR spectra (500 MHz, D2O, room temperature) of 1⊃3b.
fig. S7. ESI-TOF MS spectrum (H2O, room temperature) of 1⊃3b at 5.0 μM.
fig. S8. ESI-TOF MS spectrum (H2O, room temperature) of 1⊃3b.
fig. S9. Optimized structure of 1⊃3b (R = -OCH3).
fig. S10. 1H NMR spectra (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S11. 1H NMR spectra (500 MHz, D2O, 60°C) of 1⊃2a.
fig. S12. 1H-1H Correlation spectroscopy (COSY) spectra (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S13. NOESY spectra (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S14. Homonuclear Hartmann-Hahn (HOHAHA) spectrum (500 MHz, D2O, 60°C) of 1⊃2a.
fig. S15. Heteronuclear single quantum coherence (HSQC) NMR spectrum (500 MHz, D2O, 60°C) of 1⊃2a.
fig. S16. 1H DOSY NMR spectrum (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S17. ESI-TOF MS spectrum (H2O, room temperature) of 1⊃2a.
fig. S18. 1H NMR spectra (500 MHz, D2O, room temperature) of 1 with various disaccharides.
fig. S19. Optimized structure of 1⊃2a (R = -OCH3).
fig. S20. A snapshot of 1⊃2a (R = -OCH3) in water from molecular dynamics simulation.
fig. S21. Temperature-dependent 1H NMR spectra (500 MHz, D2O) of 1⊃2a.
fig. S22. Concentration-dependent 1H NMR spectra (500 MHz, D2O, 0.155 mM based on 1, room temperature) of 1⊃2a.
fig. S23. Selective encapsulation of 2a from a mixture of 2a and 2b by 1.
fig. S24. Selective encapsulation of 2a from a mixture of 2a and various disaccharides by 1.
fig. S25. Encapsulation of 4a within 1.
fig. S26. Encapsulation of 4b within 1.
fig. S27. Concentration-dependent 1H NMR spectra (500 MHz, D2O, 0.8 mM based on 1, room temperature) of 1⊃4a and 1⊃4b.
fig. S28. Competitive binding experiments of 2a and artificial sugars by 1.
fig. S29. Competitive binding experiment of 4a and 4b by 1.
table S1. Theoretical binding energies of host-guest complexes (R = -OCH3).
table S2. Thermodynamic parameters of 1⊃2a.
table S3. Binding constants of 1 toward 2a in water.
REFERENCES AND NOTES
- 1.K. E. Avis, V. L. Wu, Biotechnology and Biopharmaceutical Manufacturing, Processing, and Preservation (CRC Press, 1996). [Google Scholar]
- 2.R. V. Stick, Carbohydrates: The Sweet Molecules of Life (Academic Press, 2001). [Google Scholar]
- 3.T. K. Lindhorst, Essentials of Carbohydrate Chemistry and Biochemistry (John Wiley & Sons, 2007). [Google Scholar]
- 4.H.-J. Böhm, G. Schneider, Protein-Ligand Interactions: From Molecular Recognition to Drug Design (John Wiley & Sons, 2005). [Google Scholar]
- 5.A. B. Hughes, Amino Acids, Peptides and Proteins in Organic Chemistry, Volume 2, Modified Amino Acids, Organocatalysis and Enzymes (John Wiley & Sons, 2009). [Google Scholar]
- 6.Kim M.-I., Kim H.-S., Jung J., Rhee S., Crystal structures and mutagenesis of sucrose hydrolase from Xanthomonas axonopodis pv. glycines: Insight into the exclusively hydrolytic amylosucrase fold. J. Mol. Biol. 380, 636–647 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Rios P., Carter T. S., Mooibroek T. J., Crump M. P., Lisbjerg M., Pittelkow M., Supekar N. T., Boons G.-J., Davis A. P., Synthetic receptors for the high-affinity recognition of O-GlcNAc derivatives. Angew. Chem. Int. Ed. 55, 3387–3392 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrand Y., Crump M. P., Davis A. P., A synthetic lectin analog for biomimetic disaccharide recognition. Science 318, 619–622 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Sookcharoenpinyo B., Klein E., Ferrand Y., Walker D. B., Brotherhood P. R., Ke C., Crump M. P., Davis A. P., High-affinity disaccharide binding by tricyclic synthetic lectins. Angew. Chem. Int. Ed. 51, 4586–4590 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Rauschenberg M., Bomke S., Karst U., Ravoo B. J., Dynamic peptides as biomimetic carbohydrate receptors. Angew. Chem. Int. Ed. 49, 7340–7345 (2010). [DOI] [PubMed] [Google Scholar]
- 11.James T. D., Sandanayake K. R. A. S., Shinkai S., A glucose-selective molecular fluorescence sensor. Angew. Chem. Int. Ed. 33, 2207–2209 (1994). [Google Scholar]
- 12.Striegler S., Gichinga M. G., Disaccharide recognition by binuclear copper(II) complexes. Chem. Commun. 5930–5932 (2008). [DOI] [PubMed] [Google Scholar]
- 13.Jang Y., Natarajan R., Ko Y. H., Kim K., Cucurbit[7]uril: A high-affinity host for encapsulation of amino saccharides and supramolecular stabilization of their α-anomers in water. Angew. Chem. Int. Ed. 53, 1003–1007 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Davis A. P., Wareham R. S., Carbohydrate recognition through noncovalent interactions: A challenge for biomimetic and supramolecular chemistry. Angew. Chem. Int. Ed. 38, 2978–2996 (1999). [PubMed] [Google Scholar]
- 15.Mazik M., Molecular recognition of carbohydrates by acyclic receptors employing noncovalent interactions. Chem. Soc. Rev. 38, 935–956 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Walker D. B., Joshi G., Davis A. P., Progress in biomimetic carbohydrate recognition. Cell. Mol. Life Sci. 66, 3177–3191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X., James T. D., Glucose sensing in supramolecular chemistry. Chem. Rev. 115, 8001–8037 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Nelson G., Hoon M. A., Chandrashekar J., Zhang Y., Ryba N. J. P., Zuker C. S., Mammalian sweet taste receptors. Cell 106, 381–390 (2001). [DOI] [PubMed] [Google Scholar]
- 19.Li X., Staszewski L., Xu H., Durick K., Zoller M., Adler E., Human receptors for sweet and umami taste. Proc. Natl. Acad. Sci. U.S.A. 99, 4692–4696 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chandrashekar J., Hoon M. A., Ryba N. J. P., Zuker C. S., The receptors and cells for mammalian taste. Nature 444, 288–294 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Fujita M., Tominaga M., Hori A., Therrien B., Coordination assemblies from a Pd(II)-cornered square complex. Acc. Chem. Res. 38, 369–378 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Smulders M. M. J., Riddell I. A., Browne C., Nitschke J. R., Building on architectural principles for three-dimensional metallosupramolecular construction. Chem. Soc. Rev. 42, 1728–1754 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Cook T. R., Stang P. J., Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 115, 7001–7045 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Brown C. J., Toste F. D., Bergman R. G., Raymond K. N., Supramolecular catalysis in metal–ligand cluster hosts. Chem. Rev. 115, 3012–3035 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Wang W., Wang Y.-X., Yang H.-B., Supramolecular transformations within discrete coordination-driven supramolecular architectures. Chem. Soc. Rev. 45, 2656–2693 (2016). [DOI] [PubMed] [Google Scholar]
- 26.He C., Lin Z., He Z., Duan C., Xu C., Wang Z., Yan C., Metal-tunable nanocages as artificial chemosensors. Angew. Chem. Int. Ed. 47, 877–881 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Liu Y., Wu X., He C., Jiao Y., Duan C., Self-assembly of cerium-based metal–organic tetrahedrons for size-selectively luminescent sensing natural saccharides. Chem. Commun. 7554–7556 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Andersson T., Nilsson K., Sundahl M., Westman G., Wennerström O., C60 embedded in γ-cyclodextrin: A water-soluble fullerene. Chem. Commun. 604–606 (1992). [Google Scholar]
- 29.Yoshida Z.-i, Takekuma H., Takekuma S.-i., Matsubara Y., Molecular recognition of C60 with γ-cyclodextrin. Angew. Chem. Int. Ed. 33, 1597–1599 (1994). [Google Scholar]
- 30.Murthy C. N., Geckeler K. E., Synthetic approaches for the nanoencapsulation of fullerenes. Curr. Org. Synth. 3, 1–7 (2006). [Google Scholar]
- 31.Yoshizawa M., Klosterman J. K., Molecular architectures of multi-anthracene assemblies. Chem. Soc. Rev. 43, 1885–1898 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Yoshizawa M., Yamashina M., Coordination-driven nanostructures with polyaromatic shells. Chem. Lett. 46, 163–171 (2017). [Google Scholar]
- 33.Kishi N., Li Z., Yoza K., Akita M., Yoshizawa M., An M2L4 molecular capsule with an anthracene shell: Encapsulation of large guests up to 1 nm. J. Am. Chem. Soc. 133, 11438–11441 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Kishi N., Li Z., Sei Y., Akita M., Yoza K., Siegel J. S., Yoshizawa M., Wide-ranging host capability of a PdII-linked M2L4 molecular capsule with an anthracene shell. Chem. Eur. J. 19, 6313–6320 (2013). [DOI] [PubMed] [Google Scholar]
- 35.Yamashina M., Sei Y., Akita M., Yoshizawa M., Safe storage of radical initiators within a polyaromatic nanocapsule. Nat. Commun. 5, 4662 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Yamashina M., Sartin M. M., Sei Y., Akita M., Takeuchi S., Tahara T., Yoshizawa M., Preparation of highly fluorescent host–guest complexes with tunable color upon encapsulation. J. Am. Chem. Soc. 137, 9266–9269 (2015). [DOI] [PubMed] [Google Scholar]
- 37.Gibb C. L. D., Gibb B. C., Well-defined, organic nanoenvironments in water: The hydrophobic effect drives a capsular assembly. J. Am. Chem. Soc. 126, 11408–11409 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Nishio M., Umezawa Y., Honda K., Tsuboyama S., Suezawa H., CH/π hydrogen bonds in organic and organometallic chemistry. CrystEngComm 11, 1757–1788 (2009). [Google Scholar]
- 39.Nishio M., The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 13, 13873–13900 (2011). [DOI] [PubMed] [Google Scholar]
- 40.Yazaki K., Sei Y., Akita M., Yoshizawa M., A polyaromatic molecular tube that binds long hydrocarbons with high selectivity. Nat. Commun. 5, 5179 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Yamashina M., Matsuno S., Sei Y., Akita M., Yoshizawa M., Recognition of multiple methyl groups on aromatic rings by a polyaromatic cavity. Chem. Eur. J. 22, 14147–14150 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Ager D. J., Pantaleone D. P., Henderson S. A., Katritzky A. R., Prakash I., Walters D. E., Commercial, synthetic nonnutritive sweeteners. Angew. Chem. Int. Ed. 37, 1802–1817 (1998). [Google Scholar]
- 43.Masuda K., Koizumi A., Nakajima K.-i., Tanaka T., Abe K., Misaka T., Ishiguro M., Characterization of the modes of binding between human sweet taste receptor and low-molecular-weight sweet compounds. PLOS ONE 7, e35380 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang H., Sun L., Glazebnik S., Zhao K., Peralkylation of saccharides under aqueous conditions. Tetrahedron Lett. 36, 2953–2956 (1995). [Google Scholar]
- 45.Hiraoka S., Harano K., Shiro M., Shionoya M., Quantitative dynamic interconversion between AgI-mediated capsule and cage complexes accompanying guest encapsulation/release. Angew. Chem. Int. Ed. 44, 2727–2731 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Rappe A. K., Casewit C. J., Colwell K. S., Goddard W. A. III, Skiff W. M., UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 114, 10024–10035 (1992). [Google Scholar]
- 47.Rappe A. K., Goddard W. A. III, Charge equilibration for molecular dynamics simulations. J. Phys. Chem. 95, 3358–3363 (1991). [Google Scholar]
- 48.Kirschner K. N., Yongye A. B., Tschampel S. M., González-Outeiriño J., Daniels C. R., Foley B. L., Woods R. J., GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 29, 622–655 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D. A. Case, V. Babin, J. T. Berryman, R. M. Betz, Q. Cai, D. S. Cerutti, T. E. Cheatham III, T. A. Darden, R. E. Duke, H. Gohlke, A. W. Goetz, S. Gusarov, N. Homeyer, P. Janowski, J. Kaus, I. Kolossváry, A. Kovalenko, T. S. Lee, S. LeGrand, T. Luchko, R. Luo, B. Madej, K. M. Merz, F. Paesani, D. R. Roe, A. Roitberg, C. Sagui, R. Salomon-Ferrer, G. Seabra, C. L. Simmerling, W. Smith, J. Swails, R. C. Walker, J. Wang, R. M. Wolf, X. Wu, P. A. Kollman, “AMBER 14” (University of California, San Francisco, 2014). [Google Scholar]
- 50.Sitkoff D., Sharp K. A., Honig B., Accurate calculation of hydration free energies using macroscopic solvent models. J. Phys. Chem. 98, 1978–1988 (1994). [Google Scholar]
- 51.Onufriev A., Bashford D., Case D. A., Exploring protein native states and large-scale conformational changes with a modified generalized born model. Proteins 55, 383–394 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Dullweber A., Leimkuhler B., McLachlan R., Symplectic splitting methods for rigid body molecular dynamics. J. Chem. Phys. 107, 5840–5851 (1997). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/3/8/e1701126/DC1
scheme S1. Host-guest interactions between 1 and 3a.
scheme S2. Formation of 1⊃3b.
scheme S3. Host-guest interactions between 1 and 2b.
scheme S4. Formation of 1⊃4a.
scheme S5. Selective encapsulation of 4a by 1 from a mixture of 2a and 4a.
scheme S6. Competitive binding experiment of 4a and 4b by 1.
fig. S1. 1H NMR spectra (500 MHz, D2O, room temperature) of 1 with various monosaccharides.
fig. S2. ESI-TOF MS spectra (H2O, room temperature) of 1 with various monosaccharides.
fig. S3. Temperature-dependent 1H NMR spectra (500 MHz, D2O) of 1⊃3b.
fig. S4. 1H DOSY NMR spectrum (500 MHz, D2O, 25°C) of 1⊃3b.
fig. S5. 1D NOESY spectrum (500 MHz, D2O, room temperature, irradiation at 7.96 ppm) of 1⊃3b.
fig. S6. Concentration-dependent 1H NMR spectra (500 MHz, D2O, room temperature) of 1⊃3b.
fig. S7. ESI-TOF MS spectrum (H2O, room temperature) of 1⊃3b at 5.0 μM.
fig. S8. ESI-TOF MS spectrum (H2O, room temperature) of 1⊃3b.
fig. S9. Optimized structure of 1⊃3b (R = -OCH3).
fig. S10. 1H NMR spectra (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S11. 1H NMR spectra (500 MHz, D2O, 60°C) of 1⊃2a.
fig. S12. 1H-1H Correlation spectroscopy (COSY) spectra (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S13. NOESY spectra (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S14. Homonuclear Hartmann-Hahn (HOHAHA) spectrum (500 MHz, D2O, 60°C) of 1⊃2a.
fig. S15. Heteronuclear single quantum coherence (HSQC) NMR spectrum (500 MHz, D2O, 60°C) of 1⊃2a.
fig. S16. 1H DOSY NMR spectrum (500 MHz, D2O, room temperature) of 1⊃2a.
fig. S17. ESI-TOF MS spectrum (H2O, room temperature) of 1⊃2a.
fig. S18. 1H NMR spectra (500 MHz, D2O, room temperature) of 1 with various disaccharides.
fig. S19. Optimized structure of 1⊃2a (R = -OCH3).
fig. S20. A snapshot of 1⊃2a (R = -OCH3) in water from molecular dynamics simulation.
fig. S21. Temperature-dependent 1H NMR spectra (500 MHz, D2O) of 1⊃2a.
fig. S22. Concentration-dependent 1H NMR spectra (500 MHz, D2O, 0.155 mM based on 1, room temperature) of 1⊃2a.
fig. S23. Selective encapsulation of 2a from a mixture of 2a and 2b by 1.
fig. S24. Selective encapsulation of 2a from a mixture of 2a and various disaccharides by 1.
fig. S25. Encapsulation of 4a within 1.
fig. S26. Encapsulation of 4b within 1.
fig. S27. Concentration-dependent 1H NMR spectra (500 MHz, D2O, 0.8 mM based on 1, room temperature) of 1⊃4a and 1⊃4b.
fig. S28. Competitive binding experiments of 2a and artificial sugars by 1.
fig. S29. Competitive binding experiment of 4a and 4b by 1.
table S1. Theoretical binding energies of host-guest complexes (R = -OCH3).
table S2. Thermodynamic parameters of 1⊃2a.
table S3. Binding constants of 1 toward 2a in water.


