Abstract
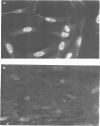
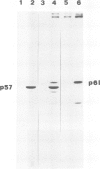
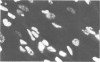
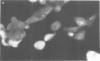
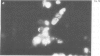
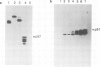
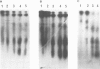
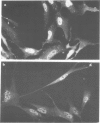
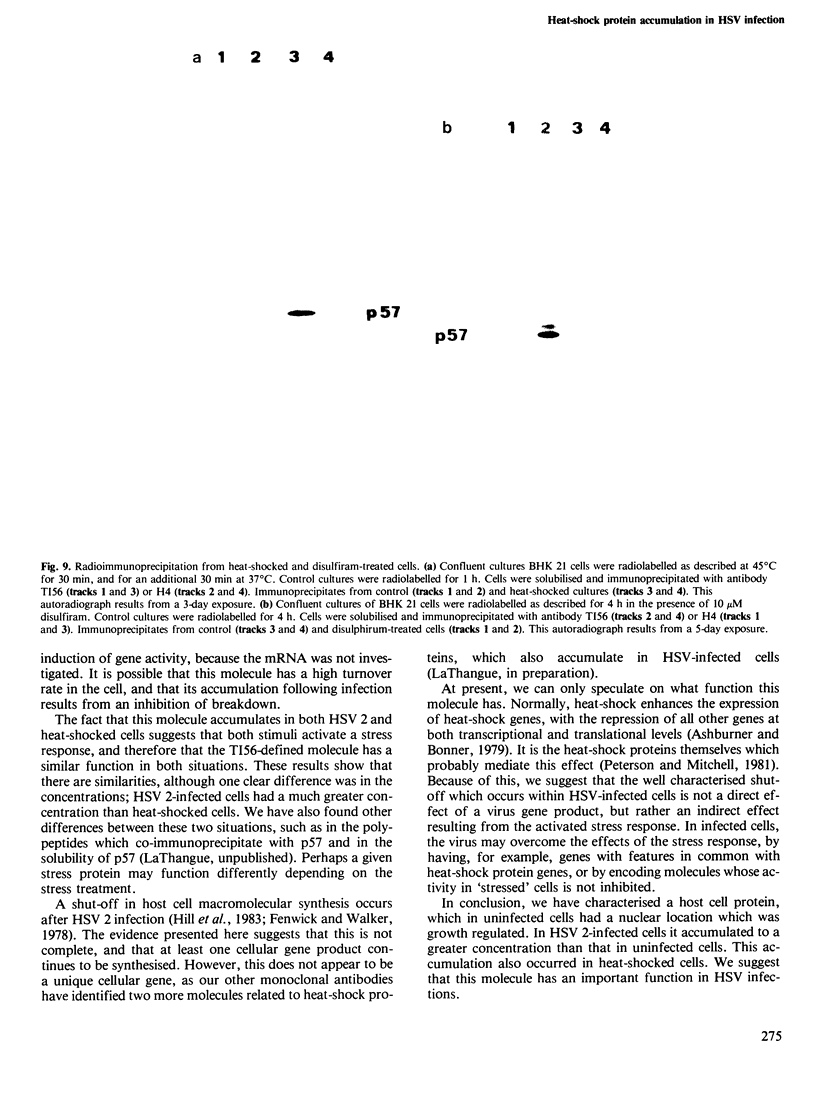
A monoclonal antibody, produced from mice immunized with a herpes simplex virus (HSV)-infected cell extract, reacts with a molecule which is present in uninfected cells and which accumulates in large amounts during HSV 2 infection. In uninfected cells this molecule is growth regulated, in that exponentially growing cells have intense nuclear immunofluorescence, whereas confluent quiescent cells have little. It has a mol. wt. of 57 000 (p57) in exponential cells, and one of 61 000 (p61) in quiescent cells. In HSV 2-infected cells, p57 accumulates and nuclear and cytoplasmic immunofluorescence increases. In uninfected cells, p57 also accumulates during heat-shock treatment, and this is associated with a new immunofluorescence throughout the cytoplasm. We suggest that HSV 2 infection induces a cellular stress response which is involved in the shut-off of host cell polypeptide synthesis.
Full text
PDF
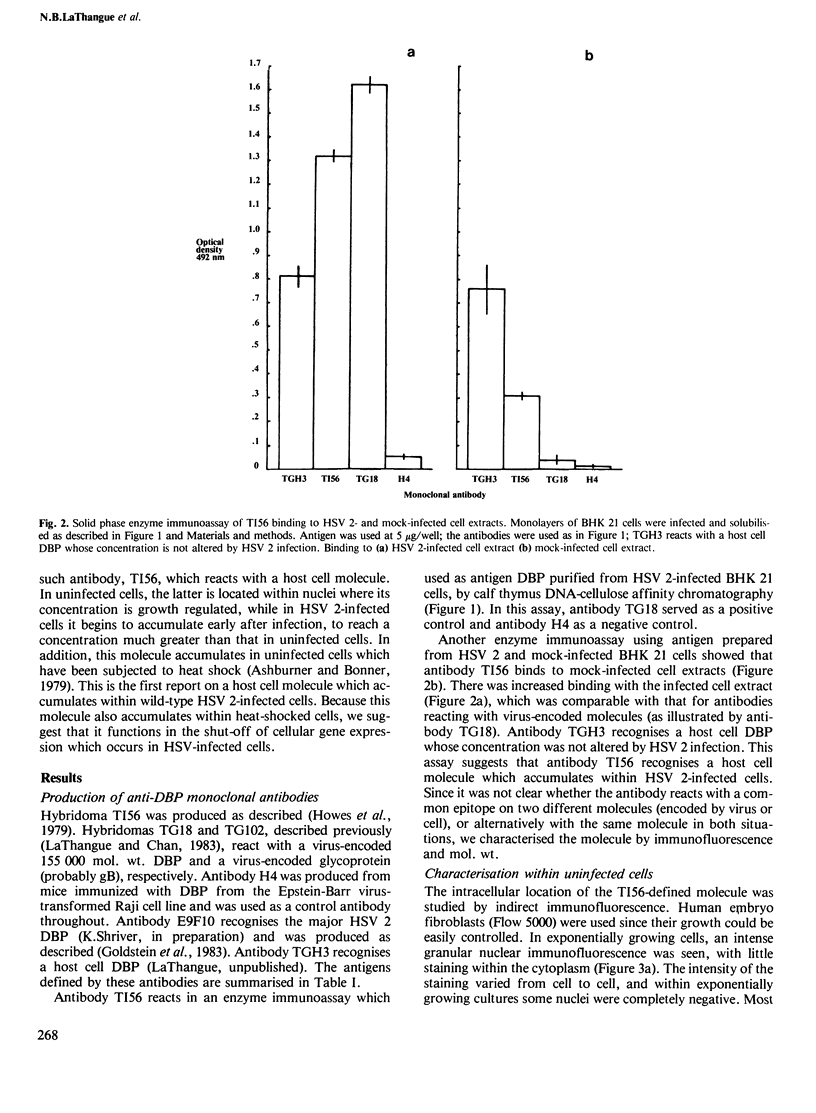
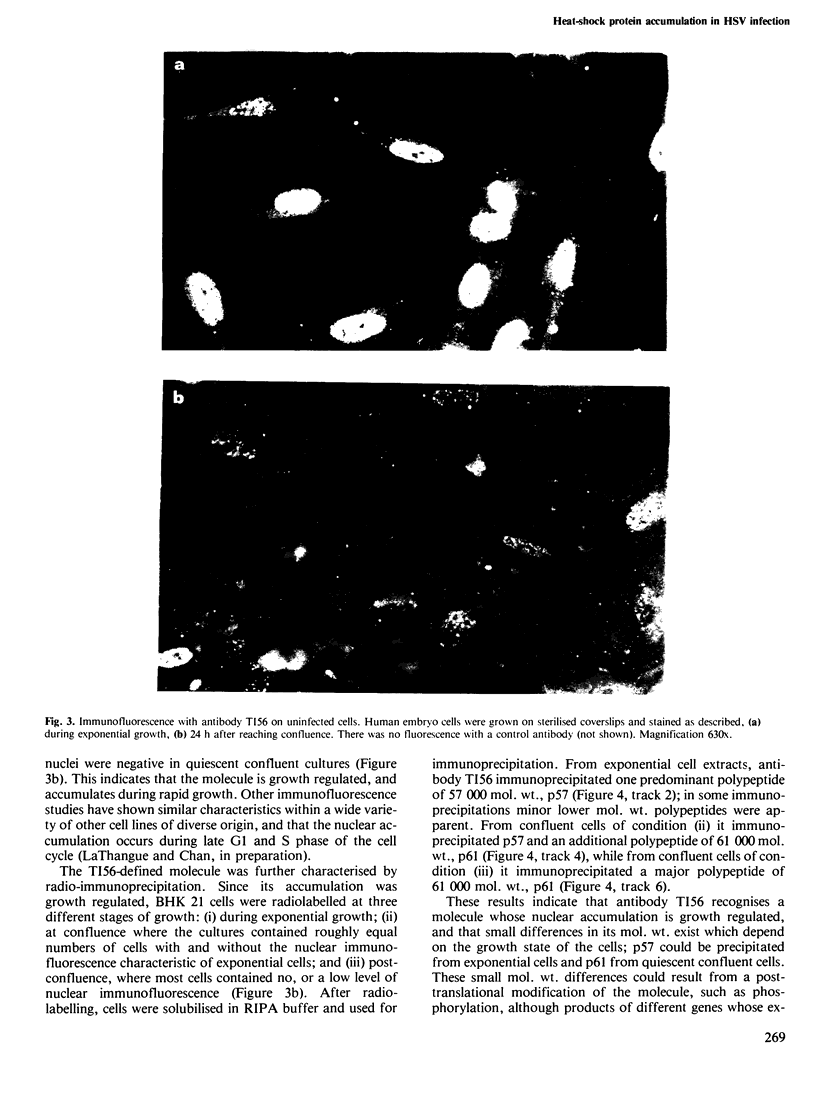



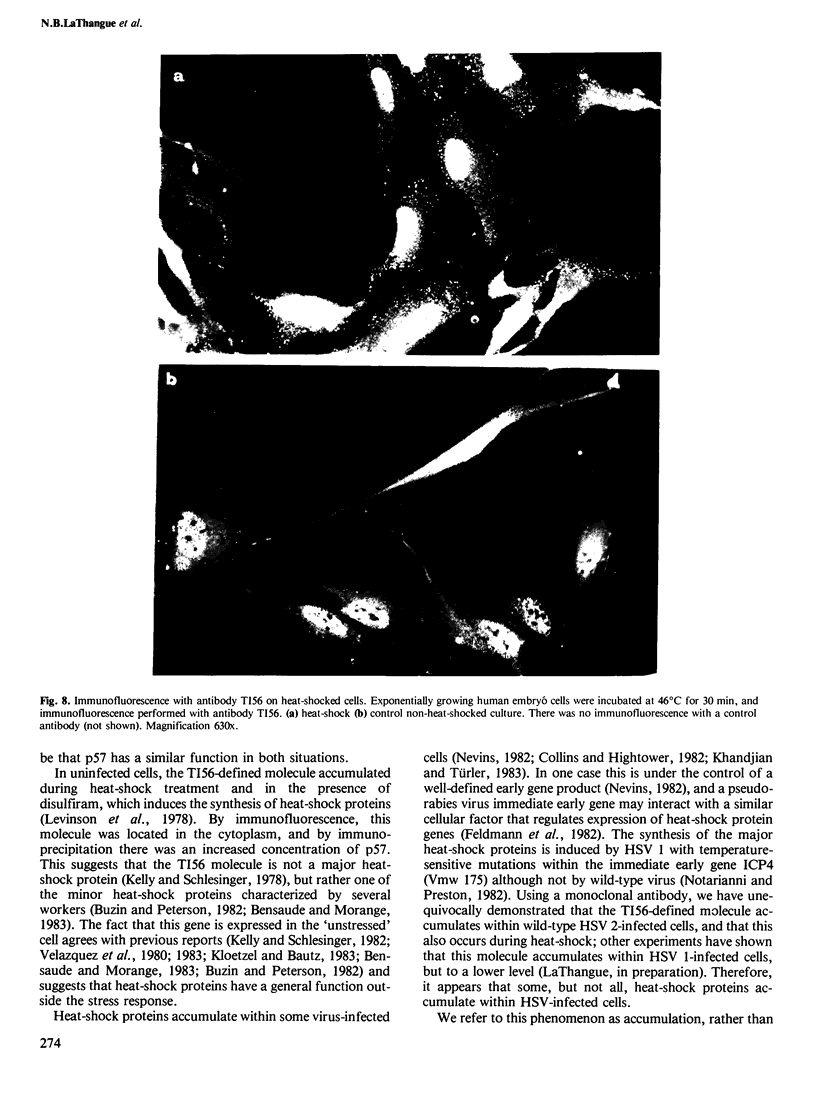


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashburner M., Bonner J. J. The induction of gene activity in drosophilia by heat shock. Cell. 1979 Jun;17(2):241–254. doi: 10.1016/0092-8674(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Baringer J. R. Recovery of herpes simplex virus from human sacral ganglions. N Engl J Med. 1974 Oct 17;291(16):828–830. doi: 10.1056/NEJM197410172911606. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buzin C. H., Petersen N. S. A comparison of the multiple Drosophila heat shock proteins in cell lines and larval salivary glands by two-dimensional gel electrophoresis. J Mol Biol. 1982 Jun 25;158(2):181–201. doi: 10.1016/0022-2836(82)90428-4. [DOI] [PubMed] [Google Scholar]
- Chan W. L. Protective immunization of mice with specific HSV-1 glycoproteins. Immunology. 1983 Jun;49(2):343–352. [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E. Newcastle disease virus stimulates the cellular accumulation of stress (heat shock) mRNAs and proteins. J Virol. 1982 Nov;44(2):703–707. doi: 10.1128/jvi.44.2.703-707.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Imperiale M. J., Nevins J. R. Activation of early adenovirus transcription by the herpesvirus immediate early gene: evidence for a common cellular control factor. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4952–4956. doi: 10.1073/pnas.79.16.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., Corey L., McDougall J. K., Tolentino E., Nowinski R. C. Monoclonal antibodies to herpes simplex viruses: use in antigenic typing and rapid diagnosis. J Infect Dis. 1983 May;147(5):829–837. doi: 10.1093/infdis/147.5.829. [DOI] [PubMed] [Google Scholar]
- Haarr L., Marsden H. S. Two-dimensional gel analysis of HSV type 1-induced polypeptides and glycoprotein processing. J Gen Virol. 1981 Jan;52(Pt 1):77–92. doi: 10.1099/0022-1317-52-1-77. [DOI] [PubMed] [Google Scholar]
- Hayward W. S., Neel B. G., Astrin S. M. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature. 1981 Apr 9;290(5806):475–480. doi: 10.1038/290475a0. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Sinden R. R., Sadler J. R. Herpes simplex virus types 1 and 2 induce shutoff of host protein synthesis by different mechanisms in Friend erythroleukemia cells. J Virol. 1983 Jan;45(1):241–250. doi: 10.1128/jvi.45.1.241-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes E. L., Clark E. A., Smith E., Mitchison N. A. Mouse hybrid cell lines produce antibodies to herpes simplex virus type 1. J Gen Virol. 1979 Jul;44(1):81–87. doi: 10.1099/0022-1317-44-1-81. [DOI] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. Antibodies to two major chicken heat shock proteins cross-react with similar proteins in widely divergent species. Mol Cell Biol. 1982 Mar;2(3):267–274. doi: 10.1128/mcb.2.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Schlesinger M. J. The effect of amino acid analogues and heat shock on gene expression in chicken embryo fibroblasts. Cell. 1978 Dec;15(4):1277–1286. doi: 10.1016/0092-8674(78)90053-3. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W., Türler H. Simian virus 40 and polyoma virus induce synthesis of heat shock proteins in permissive cells. Mol Cell Biol. 1983 Jan;3(1):1–8. doi: 10.1128/mcb.3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloetzel P. M., Bautz E. K. Heat-shock proteins are associated with hnRNA in Drosophila melanogaster tissue culture cells. EMBO J. 1983;2(5):705–710. doi: 10.1002/j.1460-2075.1983.tb01488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Levinson W., Mikelens P., Oppermann H., Jackson J. Effect of antabuse (disulfiram) on Rous sarcoma virus and on eukaryotic cells. Biochim Biophys Acta. 1978 Jun 22;519(1):65–75. doi: 10.1016/0005-2787(78)90062-x. [DOI] [PubMed] [Google Scholar]
- Luka J., Jörnvall H., Klein G. Purification and biochemical characterization of the Epstein-Barr virus-determined nuclear antigen and an associated protein with a 53,000-dalton subunit. J Virol. 1980 Sep;35(3):592–602. doi: 10.1128/jvi.35.3.592-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notarianni E. L., Preston C. M. Activation of cellular stress protein genes by herpes simplex virus temperature-sensitive mutants which overproduce immediate early polypeptides. Virology. 1982 Nov;123(1):113–122. doi: 10.1016/0042-6822(82)90299-9. [DOI] [PubMed] [Google Scholar]
- Petersen N. S., Mitchell H. K. Recovery of protein synthesis after heat shock: prior heat treatment affects the ability of cells to translate mRNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1708–1711. doi: 10.1073/pnas.78.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Seth P., Rawls W. E., Duff R., Rapp F., Adam E., Melnick J. L. Antigenic differences between isolates of herpesvirus type 2. Intervirology. 1974;3(1-2):1–14. doi: 10.1159/000149738. [DOI] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez J. M., DiDomenico B. J., Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell. 1980 Jul;20(3):679–689. doi: 10.1016/0092-8674(80)90314-1. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., Sonoda S., Bugaisky G., Lindquist S. Is the major Drosophila heat shock protein present in cells that have not been heat shocked? J Cell Biol. 1983 Jan;96(1):286–290. doi: 10.1083/jcb.96.1.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. B., Nakane P. K. The covalent coupling of proteins to periodate-oxidized sephadex: a new approach to immunoadsorbent preparation. J Immunol Methods. 1976;12(1-2):171–181. doi: 10.1016/0022-1759(76)90107-1. [DOI] [PubMed] [Google Scholar]