Abstract
Background and Purpose
Symptoms of Parkinson's disease are commonly managed using selective dopamine D2/3 receptor agonists, including ropinirole. While D2/3 agonists are useful in early‐stage Parkinson's disease, they tend to lose efficacy in later disease stages and do not appear to modify disease progression. We have recently developed a novel ‘multifunctional’ compound, D‐512: a high‐affinity D2/3 receptor agonist with antioxidant and other neuroprotective properties that may limit Parkinson's disease progression. This study sought to compare the anti‐Parkinsonian properties of the clinically used compound, ropinirole, with those of the novel compound, D‐512.
Experimental Approach
A rat model of Parkinson's disease was created by unilaterally infusing 6‐hydroxydopamine, a dopamine neurotoxin, into the medial forebrain bundle. D‐512 was compared with ropinirole for ability to stimulate spontaneous motor activity and reverse Parkinsonian akinesia. These beneficial effects were compared against each drug's liability to provoke dyskinesia, a common motor side effect.
Key Results
Both compounds increased spontaneous movement, but D‐512 showed a longer duration of action. Only D‐512 was able to significantly reverse forelimb akinesia. Drug‐induced dyskinesia was similar for equivalent doses.
Conclusions and Implications
Compared with ropinirole, D‐512 showed greater peak‐dose efficacy and a longer duration of action, despite a similar side‐effect profile. Our results add to earlier data showing that D‐512 is superior to available D2/3 agonists and could merit clinical investigation.
Abbreviations
- 6‐OHDA
6‐hydroxydopamine
- PD
Parkinson's disease
Introduction
Parkinson's disease (PD) involves the progressive loss of dopaminergic neurons in the substantia nigra, leading to motor symptoms including akinesia, resting tremor, rigidity and postural instability (Jankovic, 2008; Lees, Hardy, and Revesz, 2009). While the dopamine precursor L‐DOPA is the most effective symptomatic treatment for PD, chronic L‐DOPA treatment typically results in the development of drug‐induced dyskinesias (Cenci, Ohlin, and Odin, 2011).
In order to avoid dyskinesias, early to mid‐stage PD is often treated with dopamine D2 and D3 receptor agonists (Stowe et al., 2008). Among these, ropinirole (Figure 1) is frequently used as a monotherapy or adjunctive treatment due to its low dyskinesia liability (Brooks et al., 1998; Rascol et al., 2000). However, the efficacy of D2/3 agonists often wanes in late‐stage PD, requiring these drugs to be combined with other medications and administered more frequently in later‐stage PD patients (Connolly and Lang, 2014).
Figure 1.
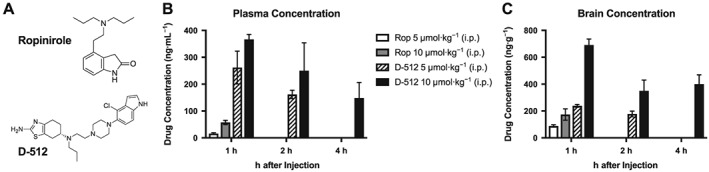
Higher levels of D‐512 in plasma and brain than those of ropinirole (Rop). Concentrations were assessed with LC–MS/MS at 1, 2 and 4 h post‐injection (n = 3 per group). Ropinirole was only analysed at 1 h, while D‐512 (5 μmol·kg−1) was only analysed at 1 and 2 h post‐injection. (A) Molecular structures of D‐512 and ropinirole. (B) Drug concentrations in blood plasma. (C) Drug concentrations in whole brain homogenates. Inferential statistics were not performed due to the limited sample size.
While a number of symptomatic treatments for PD are available, there is limited evidence that any medications are disease‐modifying (Valera and Masliah, 2016). The histopathological hallmark of PD is the presence of Lewy body protein aggregates, which may proliferate in dopaminergic neurons when excessive oxidative stress leads to mitochondrial and lysosomal dysfunction (Wirdefeldt et al., 2011). In support of this theory, retrospective analyses have sometimes found a negative correlation between dietary antioxidant vitamin intake and PD progression, although the effects are not consistent across all studies (Zhang et al., 2002; Knekt et al., 2010; Wirdefeldt et al., 2011). Ongoing clinical trials are examining antioxidants in PD patients, and it was recently reported that administration of the antioxidant coenzyme Q10 was able to reduce disease progression over a 2 year period (Yoritaka et al., 2015).
As an improvement over available pharmacotherapies, we have recently developed a novel series of compounds designed to optimize symptomatic efficacy and provide disease‐modifying properties (Johnson et al., 2012). One of the most promising of these ‘multi‐functional’ compounds, D‐512 (Figure 1A), is a high‐affinity D2/3 agonist (Km < 3 nM) with motor‐stimulating properties in rats that last three times as long as ropinirole when acutely administered i.p. on an equimolar basis (Santra et al., 2013). Additionally, D‐512 provides in vitro and in vivo neuroprotection against the dopaminergic neurotoxins, ostensibly through reducing oxidative stress within dopaminergic neurons (Santra et al., 2013; Shah et al., 2014; Voshavar et al., 2015).
Even though acute administration of D‐512 enhances spontaneous motor activity, the acute and chronic effects of this compound on the motor symptoms of PD have not been examined. Likewise, it is not clear if D‐512 produces significant levels of drug‐induced dyskinesia, a major concern considering that, in general, the most effective PD medications have the greatest dyskinesia liability (Cenci et al., 2011; Connolly and Lang, 2014). In the present study, we used the rat 6‐hydroxydopamine (6‐OHDA) model of PD to examine the potential superiority of D‐512 to ropinirole by directly comparing the profile of each agent for motor stimulation and reversal of PD symptoms.
Methods
Animal care
All animal care and experimental protocols adhered to the most‐current National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Wayne State University and Binghamton University. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Rats were pair‐housed in plastic cages and given free access to water and standard laboratory rat food, except during experimentation.
Experiment 1: pharmacokinetic comparison of ropinirole and D‐512
Pharmacokinetic analyses were performed at Wayne State University, using male Sprague Dawley rats (Harlan, Indianapolis, USA) that were 8 weeks old (initial n = 21; final n = 21; n = 3 per group). Following administration of 5 or 10 μmol·kg−1 of D‐512 or ropinirole (1.1 or 2.2 mL·kg−1 in distilled H2O, respectively, i.p.), blood and brain samples were collected. D‐512 concentrations were examined at three time points post‐injection (1, 2 and 4 h), while ropinirole concentrations were analysed only at 1 h after injection. These time points were chosen because previous research showed that locomotor activation by ropinirole wanes after 1 h, while D‐512 continuously stimulated movement for 6 h (Johnson et al., 2012). Samples were stored at −80°C prior to processing.
On the day of analysis, plasma and brain samples were thawed to room temperature for approximately 20 min, and then the brain samples were homogenized in PBS (4 parts PBS to 1 part brain [v/w]). Concentrations of D‐512 in plasma and brain were quantitated using a set of calibration standards prepared either in blank plasma matrix or in blank brain homogenate matrix on the day of analysis. D‐440 (a structural analogue of D‐512; Santra et al., 2013) was used as an internal standard in the experiment. The standard samples of D‐512 (5, 10, 25, 100, 500, 1000, 1500 and 2000 ng·mL−1) for the calibration curve were prepared by adding 5 μL of appropriate working dilution of D‐512 and 5 μL of D‐440 as an internal standard (100 ng·mL−1 in acetonitrile) to 45 μL of blank rat plasma or blank rat brain homogenate. The bioanalytical sample preparation for brain and plasma analysis followed by quantification of the test compound entailed the addition of 245 μL acetonitrile to every 55 μL of standard and analyte samples containing both D‐512 and the internal standard to precipitate tissue plasma proteins and tissue macromolecules. These mixtures were then vortexed for 15 min at 1400 r.p.m. at 4°C. The suspensions were next clarified by centrifugation (18 000× g, 10 min at 4°C), and each 150 μL of the resulting supernatant was mixed well with 50 μL acetonitrile, and the mixtures were then vortexed for 15 min at 1400 r.p.m. at 4°C. The mixtures were again centrifuged (18 000× g, 10 min at 4°C) before 100 μL of clear supernatant was transferred to autosampler vials for LC–MS/MS analysis.
The sample preparation for brain and plasma analysis of ropinirole was carried out exactly the same way as described for D‐512 except that quinpirole, a close analogue of ropinirole, was used as an internal standard at a fixed concentration of 100 ng·mL−1.
Chromatographic analysis
The analysis of plasma and brain samples was performed using Waters (Milford, CT, USA) Acquity UPLC instrument with a triple quadrupole MS analyser. The LC–MS/MS detection was performed using a positive multiple reaction monitoring method by monitoring the ion transitions of D‐512 and D‐440 from m/z 473.56 ➔ 153.10 and 467.28 ➔ 113.07 respectively. On the other hand, the ion transitions of ropinirole and quinpirole were from m/z 261.22 ➔ 160.08 and m/z 220.20 ➔ 161.18 respectively. The MS/MS conditions for all the analytes are shown in Supporting Information Table S1. The MS control and data acquisition were collected using the Waters MassLynx software v4.1. Chromatographic conditions were achieved using a reverse‐phase C‐18 ethylene‐bridged hybrid column (BEH C18; 2.16100 mm, 1.7 mM). For analysing the plasma and brain uptake of D‐512, 5 μL of sample solutions was injected and samples were eluted using water (solvent A) and acetonitrile (solvent B) mixture with a flow rate of 0.2 mL·min−1. Isocratic elution of the mobile phase was 5% A and 95% B from 1 to 4 min. Similarly, 5 μL sample solutions of ropinirole were injected, and samples were eluted using 10 mM ammonium formate buffer (pH 9) as solvent A and methanol as solvent B with a flow rate of 0.7 mL·min−1. Gradient elution of the mobile phase was 95% A and 5% B from 0 to 2 min, 2% A and 98% B from 2 to 2.6 min and 95% A and 5% B from 2.6 to 4 min.
Experiment 2: comparison of the behavioural effects of ropinirole and D‐512
Behavioural analysis of D‐512 was performed at Binghamton University. Male Sprague Dawley rats (Harlan) were 9 weeks old at the start of the experiment (initial n = 62; final n = 60; n = 10 per group). The colony room was maintained at 22–23°C on a 12 h light/dark cycle (lights on 0700–1900) with experiments performed during the light cycle.
Surgical procedures
For analgesic purposes, rats were given buprenorphine (0.03 mg·kg−1) immediately prior to surgery and 24 h after surgery. Animals were anaesthetized with isoflurane (2–3% for 30–40 min) mixed with oxygen (1.0 L·min−1). 6‐OHDA was suspended in saline with 0.1% ascorbic acid as an antioxidant. Sham or active lesions were created by infusing vehicle or 6‐OHDA, respectively, into the left medial forebrain bundle. Coordinates were based on the rat brain atlas of Paxinos and Watson (1998): from bregma, posterior 1.8 mm; lateral 2.0 mm; ventral 8.6 mm. A syringe with 26 gauge needle was lowered into the target site, and 6‐OHDA (12 μg in 4 μL) or vehicle was injected at a constant flow rate of 2 μL·min−1 for 2 min. The needle was withdrawn 5 min later. Among the 62 animals that underwent surgery, one rat died post‐operatively.
Chronic drug treatments
Drug treatments began 3 weeks after surgery and continued for 22 d, with behavioural testing ongoing throughout treatment. The volume of liquid administered was 1 mL·kg−1. On days 1–7, rats received one of the following drugs daily (i.p.): D‐512 (1 or 3 μmol·kg−1; equivalent to 1 or 3 mg·kg−1), ropinirole (0.7 or 1.7 μmol·kg−1; equivalent to 0.2 or 0.5 mg·kg−1) or their common vehicle, saline. Doses were chosen based on those required to elicit anti‐Parkinsonian benefit in rats (Ravenscroft et al., 2004; Santra et al., 2013). Additionally, a pilot study (conducted on animals that were exposed to chronic L‐DOPA) indicated that these doses of D‐512 or ropinirole caused significant behavioural changes when given acutely. Animals used in the present study were drug‐naïve, and when few drug‐induced changes were observed on days 1–7, the doses were increased on days 8–22: D‐512 (3 or 9 μmol·kg−1; equivalent to 3 or 9 mg·kg−1) and ropinirole (1.7 or 5.1 μmol·kg−1; equivalent to 0.5 or 1.5 mg·kg−1).
Spontaneous locomotion
Motion chambers allow assessment of drug‐induced changes in spontaneous motor activity. Activity was assessed by infrared photocell arrays in acrylic chambers measuring 41 × 41 × 30.5 cm (Accuscan Instruments, Columbus, OH, USA). The software analysed patterns of photobeam breaks to measure total distance travelled (in m) and the number of discrete movements separated by at least 1 s. In the week prior to drug treatment, rats were habituated to the chambers on two occasions for 4 h each. Spontaneous locomotion was analysed using 1 h time bins over 4 h post‐injection on days 3, 10 and 17 of chronic drug treatment (Figure 2).
Figure 2.
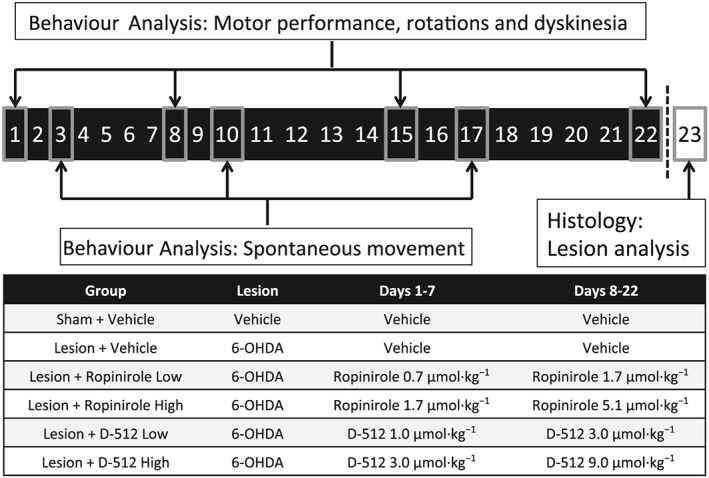
Timeline of treatments and behavioural analysis in experiment 2. Under anaesthesia, all rats received infusions to the left medial forebrain bundle, consisting of 6‐OHDA or vehicle. After a 3 week recovery, rats were treated daily for 22 days with ropinirole, D‐512 or vehicle (all doses were increased on day 8 as shown). On days 1, 8, 15 and 22, rats were assessed for motor performance (with the forepaw adjusting steps test), contralateral rotations (using a visual count) and drug‐induced dyskinesia (with the abnormal involuntary movement scale). On days 3, 10 and 17, spontaneous movement was assessed with motion chambers. No drugs were administered on day 23; instead, rats were transcardially perfused for subsequent immunochemical analysis of dopaminergic lesions in the striatum.
Motor performance
The forepaw adjusting steps test is a measure of akinesia, a cardinal symptom of PD (Jankovic, 2008). Rats with >80% unilateral DA depletion perform poorly on the test with the lesioned side of the body (Chang et al., 1999). To perform the test, an experimenter blind to treatment condition held the rat's hindlimbs and one forelimb such that the free forelimb was forced to bear the rat's body weight. Rats were moved 90 cm laterally over 10 s, in the direction toward the rat's midline. Experimenters ensured that the animal's forelimb was on the table at the start of each trial; during the trial, if the animal manifested forelimb dyskinesia causing the forelimb to be removed from the table for any period, the trial was not stopped or redone. This test was performed three times with each forelimb, and the sum of the three trials is reported as total steps for each time point. With the ‘lesioned forelimb’ (contralateral to brain 6‐OHDA lesion), fewer steps indicate a greater Parkinsonian impairment. The test was also performed with the ‘intact forelimb’ (ipsilateral to brain 6‐OHDA lesion) to determine if D‐512 or ropinirole was affecting motor performance in a healthy motor system. During the chronic drug treatment phase, the forepaw adjusting steps test was performed on days 1, 8, 15 and 22, at 60 and 240 min post‐treatment, immediately after drug‐induced dyskinesia was scored for each time point (Figure 2).
The test was performed twice in the week prior to drug treatment: first to habituate the rats to the test and a second time to assign rats to equivalently impaired treatment groups. In order to be considered sufficiently Parkinsonian, the total steps with the lesioned forelimb needed to be <60% of the steps taken with the intact forelimb (Lindenbach et al., 2015, 2016). Among the 61 rats examined with the stepping test, 51 had received 6‐OHDA infusions and 50 of 51 met this behavioural criterion (yielding n = 60 usable rats in experiment 2, or n = 10 per group). 6‐OHDA‐lesioned rats were assigned to one of five treatment groups such that each group had equivalent mean scores on the stepping test.
Asymmetrical rotations
In a unilaterally Parkinsonian rat, anti‐Parkinsonian medications including D2/3 agonists cause rats to preferentially turn contralateral to brain lesion (Lundblad et al., 2002). Accordingly, the number of contralateral rotations is often used as a positive indicator of anti‐Parkinsonian efficacy (Lane, Cheetham, and Jenner, 2006; Smith et al., 2012). Rotational behaviour was assessed for 1 min every 10 min for 240. Positive numbers indicate a preponderance of contralateral rotations, while negative scores indicate net ipsilateral rotations. To reduce the total number of comparisons, two consecutive time points were collapsed into one score. Rotations were analysed on days 1, 8, 15 and 22 of chronic treatment (Figure 2).
Drug‐induced dyskinesia
The abnormal involuntary movements test is a metric of dyskinesia, a common side effect of many anti‐Parkinsonian medications. Rats were monitored for dyskinesia using the abnormal involuntary movement scale (Cenci and Lundblad, 2007). Rats were observed in clear‐plastic cylinders and were rated by a trained observer (≥95% reliability) for 1 min every 10 min over 240 min concomitant with scoring of rotations. During each rating period, individual dyskinesia severity scores ranging from 0 (not present) to 4 (severe and not interruptible) were given for axial, limb and orolingual dyskinesias. The three subtype scores were summed to create a single score for each time point. Similar to rotation analyses, two sequential time points were collapsed into one score and dyskinesia was scored on days 1, 8, 15 and 22 of chronic treatment (Figure 2).
Histology
In order for dopaminergic lesions to be confirmed, immunohistochemistry was used on half the animals that received a 6‐OHDA lesion followed by chronic systemic saline (n = 5). One day after the final treatment, rats were transcardially perfused, first with PBS and then with 4% paraformaldehyde in PBS. Brains were immersed in 30% sucrose and cut into 40 μm coronal sections using a sliding microtome.
As a marker of dopaminergic neuron viability, sections were analysed for immunoreactivity of tyrosine hydroxylase (TH), the rate‐limiting enzyme in dopamine synthesis. Three coronal slices covering the anterior–posterior axis of the dorsal striatum were selected (at +1.60, +0.20 and −0.80 mm from bregma), stained and quantified using a previously published method (Lindenbach et al., 2015). Briefly, photomicrographs were taken of the striata, converted into 8 bit grayscale, and optical density was analysed using Image J software (National Institutes of Health, Bethesda, MD, USA). Non‐specific background staining was cancelled out by subtracting the optical density of the striatum from the adjacent cingulate cortex on the same coronal section.
Data and statistical analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Statistical analysis was performed using SPSS (v. 20; IBM, Armonk, USA) with α set at 0.05 unless noted. Inferential statistics were not performed on experiment 1 due to the limited sample size (n = 3 per group). In experiment 2, locomotor data and drug‐induced rotations were analysed using a three‐way mixed model ANOVA: Drug * Day * Time (within a day). A similar ANOVA was used for motor performance in experiment 2, except that Limb (lesion or intact) was added as a factor, yielding a four‐way mixed model ANOVA. When merited by omnibus comparison, Tukey's HSD was used in order to compare all six treatment groups to each other.
Dyskinesia data were analysed using non‐parametric statistics because the abnormal involuntary movements scale is ordinal. We used the between‐subjects Kruskal–Wallis omnibus test, followed by Mann–Whitney contrasts, if appropriate. In order to adjust for multiple comparisons with the Mann–Whitney test, we used the same threshold for statistical significance as would be produced by applying Tukey's HSD (for k = 6 groups, P = 0.0082).
When analysing rotations and dyskinesia, if the omnibus comparison indicated a significant difference between a treatment group and vehicle for a given test day, inferential statistics were performed on the time series. In order to reduce type I probability for these analyses, we only compared a given treatment to vehicle and reduced α to 0.01. In practice, this meant that rotations time series were analysed with independent samples t‐tests and dyskinesia time series were analysed with Mann–Whitney contrasts.
Materials
D‐512 was synthesized at Wayne State University by our co‐authors (it was developed by them and is not commercially available). Ropinirole and 6‐OHDA came from Sigma‐Aldrich (St. Louis, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a, 2015b, 2015c).
Results
Experiment 1: pharmacokinetic comparison of ropinirole and D‐512
Plasma levels and brain uptake of ropinirole and D‐512
Blood plasma levels of D‐512 were higher than that of ropinirole when the drugs were given at two equimolar doses (5 and 10 μmol·kg−1) at 1, 2 or 4h after adminsitration (Figure 1B). Brain uptake of D‐512 was also greater than that of ropinirole. As shown in Figure 1C, D‐512 showed a higher brain concentration than ropinirole 1 h after injection of either 5 μmol·kg−1 or 10 μmol·kg−1.
Experiment 2: behavioural comparison of ropinirole and D‐512
Distance travelled
We assessed the ability of ropinirole and D‐512 to enhance movement and exploratory behaviour in an open field. Rats were habituated to the motion chambers twice before testing, with baseline movement recorded on the second exposure. We confirmed that average motor activity was not changing over the course of the experiment in saline‐treated animals.
Drug‐induced changes in total distance travelled were analysed with a three‐way mixed‐model ANOVA: Drug (six groups) * Day (3, 10 or 17) * Time (4× over 4 h). All four interactions were statistically significant, including the three‐way interaction (F30,324 = 6.65, P < 0.001).
Drugs compared with vehicle
After dose escalation, on day 17, ropinirole 5.1 μmol·kg−1 enhanced distance travelled more than vehicle during the 1st h (Figure 3). D‐512 3 μmol·kg−1 increased locomotion relative to vehicle from 1 to 4 h on day 3 and from 1 to 3 h on days 10 and 17. D‐512 9 μmol·kg−1 enhanced movement relative to vehicle from 1 to 4 h on days 10 and 17.
Figure 3.
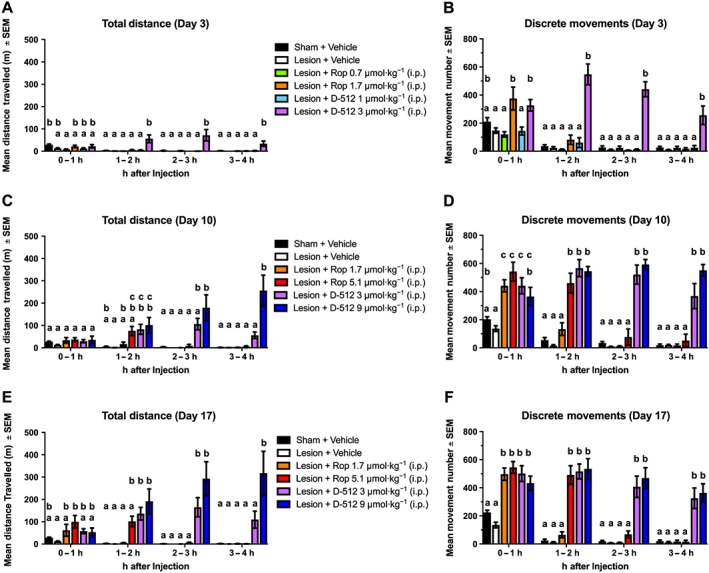
Chronic ropinirole (Rop) and D‐512 increased spontaneous movement in motion chambers (n = 10 per group). Rats were treated for 22 days with ropinirole, D‐512 or vehicle (all doses were increased on day 8). Movement data were analysed for total distance travelled (in mm) and the number of discrete movements (defined as the number of start–stop motions separated by at least 1 s) on (A, B) day 3 (C, D) day 10 and (E, F) day 17. Tukey's HSD was used for between‐group comparisons at each time point: bars that share the same letter are considered statistically comparable, while bars with different letters are considered statistically different.
Ropinirole compared with D‐512
On day 3, D‐512 3 μmol·kg−1 increased movement more than both doses of ropinirole from 1 to 4 h after drug administration. D‐512 9 μmol·kg−1 induced more locomotor activity than ropinirole 1.7 μmol·kg−1 (on day 10, from 2 to 4 h; on day 17, from 1 to 4 h) and ropinirole 5.1 μmol·kg−1 (on days 10 and 17, from 2 to 4 h).
Movement number
The effects of ropinirole and D‐512 on the number of discrete movements (starts/stops separated by at least 1 s) were analysed with a three‐way mixed model ANOVA. All main effects and interactions were statistically significant, including the important three‐way interaction (F30,324 = 6.53, P < 0.001).
Drugs compared with vehicle
Ropinirole 1.7 μmol·kg−1 increased movement number from 0 to 1 h post‐injection on days 3, 10 and 17 (Figure 3). A higher dose of ropinirole (5.1 μmol·kg−1) increased movement from 0 to 2 h on days 10 and 17. D‐512 at 3 or 9 μmol·kg−1 increased discrete movements for all 4 h on all test days.
Ropinirole compared with D‐512
On day 3, ropinirole 1.7 μmol·kg−1 increased discrete movements relative to D‐512 1 μmol·kg−1 from 0 to 1 h on day 3. Also on day 3, D‐512 3 μmol·kg−1 evoked more movements than ropinirole 0.7 μmol·kg−1 for all 4 h. Similarly, D‐512 3 μmol·kg−1 increased the number of movements more than ropinirole 1.7 μmol·kg−1 from 1 to 4 h on all test days. On days 10 and 17, D‐512 9 μmol·kg−1 induced more discrete movements than ropinirole 1.7 μmol·kg−1 (from 1 to 4 h) and ropinirole 5.1 μmol·kg−1 (from 2 to 4 h).
Motor performance
The ability of ropinirole and D‐512 to reverse Parkinsonian motor deficits was analysed with a four‐way ANOVA: Drug (six groups) * Day (1, 8, 15 or 22) * Time (60 or 240 min) * Limb (Lesion or Intact). Two of the three‐way interactions were significant: Drug * Day * Limb (F15,162 = 2.62, P = 0.001) and Drug * Day * Time (F15,162 = 2.83, P = 0.001). As an internal validation, we statistically verified that 6‐OHDA caused a Parkinsonian phenotype: with the affected forelimb, Sham + Vehicle rats took more steps than Lesion + Vehicle rats at all time points and on all test days (Figure 4).
Figure 4.
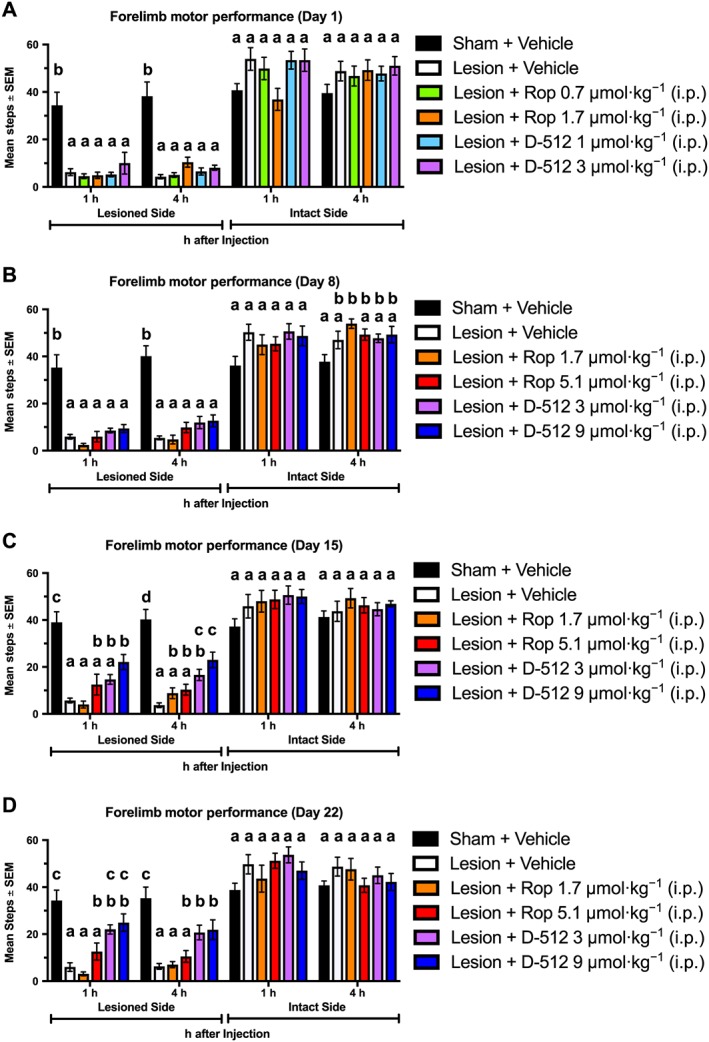
Chronic D‐512, but not ropinirole (Rop), significantly improved motor performance using the forepaw adjusting steps test (n = 10 per group). Rats were treated for 22 days with Rop, D‐512 or vehicle (all doses were increased on day 8). Forelimb ability was assessed at 1 and 4 h post‐injection on (A) day 1, (B) day 8, (C) day 15 and (D) day 22. Tukey's HSD was used for between‐group comparisons at each time point: bars that share the same letter are considered statistically comparable, while bars with different letters are considered statistically different.
Drugs compared with vehicle
With the lesioned forelimb, no dose of ropinirole increased total steps taken more tha those after vehicle at any time point. By contrast, chronic, but not acute, administration of D‐512 increased stepping. D‐512 3 μmol·kg−1 improved motor performance more than vehicle on day 15 (at 4 h) and on day 22 (at 1 and 4 h). The higher dose of D‐512 (9 μmol·kg−1) increased steps more than vehicle at 1 and 4 h on days 15 and 22.
Ropinirole compared with D‐512
D‐512 3 μmol·kg−1 increased stepping relative to ropinirole 1.7 μmol·kg−1 on day 15 (at 1 h) and on day 22 (at 1 and 4 h). D‐512 9 μmol·kg−1 increased stepping more than ropinirole 1.7 μmol·kg−1 at all time points on days 15 and 22. Additionally, D‐512 9 μmol·kg−1 improved forelimb ability more than ropinirole 5.1 μmol·kg−1 on day 15 at 4 h post‐injection.
Contralateral rotations
The ability of anti‐Parkinsonian medications to promote rotations contralateral to a unilateral dopaminergic lesion is often used as a measurement of anti‐Parkinsonian efficacy (Lane et al., 2006; Smith et al., 2012). Rotations were analysed with a three‐way ANOVA: Drug (six groups) * Day (1, 8, 15 or 22) * Time (12× over 240 min). All possible effects were statistically significant, including the three‐way interaction (F165,1782 = 2.31, P < 0.001).
Drugs compared with vehicle
Planned contrasts using Tukey's HSD revealed that ropinirole did not significantly increased rotations relative to vehicle on any test day (Figure 5). By contrast, D‐512 3 μmol·kg−1 increased rotations compared with vehicle on day 8 (at 120, 160 and 200 min), day 15 (from 20 to 220 min) and day 22 (at 20–60 and 100–240 min). D‐512 9 μmol·kg−1 increased rotations compared with vehicle on day 8 (at 20–40, 180 and 220–240 min), day 15 (at 40 and 100–240 min) and day 22 (from 60 to 240 min).
Figure 5.
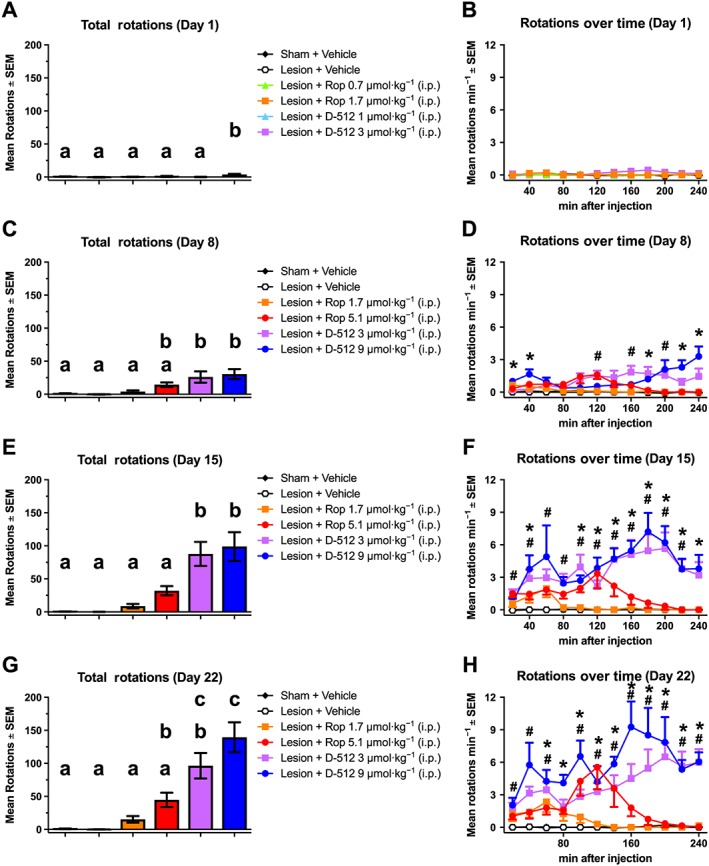
Chronic treatment with D‐512, but not with ropinirole (Rop), significantly induces rotational behaviour (n = 10 per group). Rats were treated for 22 days with ropinirole, D‐512 or vehicle (all doses were increased on day 8). Contralateral rotations were counted for 1 min every 10 min for 240 min post‐injection on (A, B) day 1, (C, D) day 8 and (E, F) day 15 and (G, H) day 22. Tukey's HSD was used for between‐group comparisons on each test day: bars that share the same letter are considered statistically comparable, while bars with different letters are considered statistically different. For the time series within each day: #P < 0.05, D‐512 (3 μmol·kg−1) significantly different from vehicle; *P < 0.05, D‐512 (9 μmol·kg−1) significantly different from vehicle.
Ropinirole compared with D‐512
D‐512 3 μmol·kg−1 produced more rotations than ropinirole 0.7 or 1.7 μmol·kg−1 on all days. D‐512 3 μmol·kg−1 also increased rotations compared with ropinirole 5.1 μmol·kg−1 on day 15. Similarly, D‐512 9 μmol·kg−1 increased rotations compared with ropinirole 1.7 μmol·kg−1 (days 8, 15 and 22) and ropinirole 5.1 μmol·kg−1 (days 15 and 22).
Drug‐induced dyskinesia
Next, we examined the potential of each drug to provoke involuntary hyperkinetic movements. Dyskinesia scores were rated with the abnormal involuntary movement scale and analysed with the Kruskal–Wallis test. Both ropinirole and D‐512 significantly induced dyskinesia (relative to vehicle) on day 8 (χ2 = 34.17, P < 0.001), day 15 (χ2 = 42.12, P < 0.001) and day 22 (χ2 = 42.13, P < 0.001; Figure 6).
Figure 6.
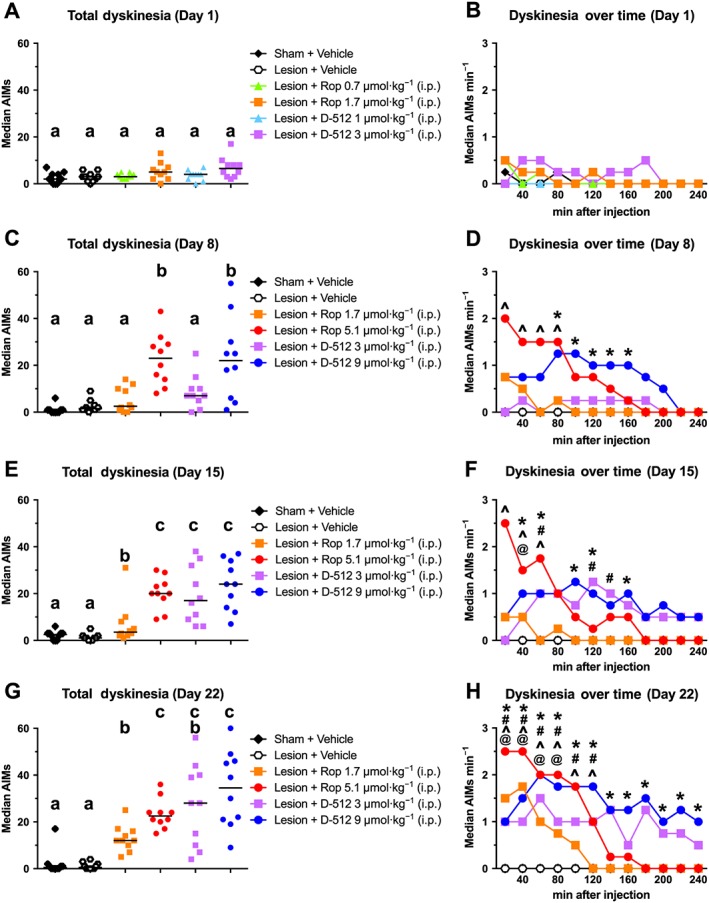
Chronic ropinirole (Rop) and D‐512 caused drug‐induced dyskinesia (n = 10 per group). Rats were treated for 22 days with ropinirole, D‐512 or vehicle (all doses were increased on day 8). Dyskinesia was scored using the abnormal involuntary movements (AIMs) scale over 1 min every 10 min for 240 min post‐injection on (A, B) day 1 (C, D) day 8, (E, F) day 15 and (G, H) day 22. Tukey's HSD was used for between‐group comparisons at each time point: bars that share the same letter are considered statistically comparable, while bars with different letters are considered statistically different. For the time series within each day: @P < 0.05, Rop (1.7 μmol·kg−1) significantly different from vehicle; ^P < 0.05, Rop (5.1 μmol·kg−1) significantly different from vehicle; #P < 0.05, D‐512 (3 μmol·kg−1) significantly different from vehicle; * P < 0.05, D‐512 (9 μmol·kg−1) significantly different from vehicle.
Drugs compared with vehicle
Ropinirole 1.7 μmol·kg−1 caused statistically significant dyskinesia (relative to vehicle) on day 15 (at 40 min) and day 22 (from 20 to 80 min). Ropinirole 5.1 μmol·kg−1 caused dyskinesia on day 8 (from 20 to 80 min), day 15 (from 20 to 60 min) and day 22 (from 20 to 120 min). D‐512 3 μmol·kg−1 caused dyskinesia on day 15 (at 60 and 120–140 min) and day 22 (at 20–80, 120–140 and 180 min). Likewise, D‐512 9 μmol·kg−1 caused dyskinesia on day 8 (from 80 to 160 min), day 15 (at 40–60, 100–120 and 160 min) and day 22 (at 20–120, 160–200 and 240 min).
Ropinirole compared with D‐512
Administration of ropinirole 5.1 μmol·kg−1 caused more total dyskinesia than that of D‐512 3 μmol·kg−1 on day 8, but the two groups were otherwise equivalent. D‐512 3 μmol·kg−1 provoked more dyskinesia than ropinirole 1.7 μmol·kg−1 on day 15. D‐512 9 μmol·kg−1 increased dyskinesia relative to ropinirole 1.7 μmol·kg−1 on days 8, 15 and 22.
Efficacy to side‐effect ratio
With most anti‐Parkinsonian medications, compounds with greater efficacy typically cause greater side effects, principally dyskinesia (Stowe et al., 2008; Cenci et al., 2011). In an attempt to quantitatively derive a cost–benefit ratio, we divided the average number of contralateral rotations (the benefit) by the average amount of dyskinesia (the cost). The resulting ratios were analysed with a Kruskal–Wallis test for each day. While the ratios for each dose of D‐512 were numerically greater than those for each dose of ropinirole using this scale on days 8, 15 and 22, this difference was not statistically significant for any day (Figure 7).
Figure 7.
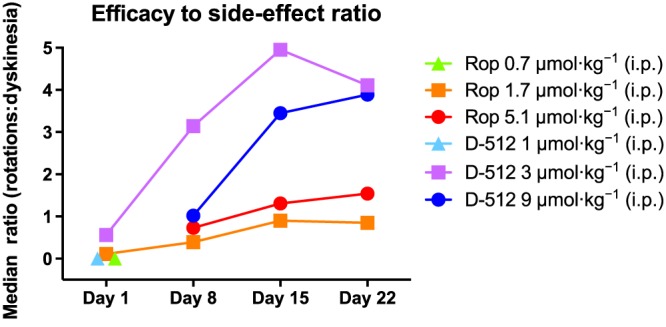
Comparison of anti‐Parkinsonian benefit and dyskinesia liability for doses of ropinirole (Rop) and D‐512 (n = 10 per group). Rats were treated for 22 days with ropinirole or D‐512 (all doses were increased on day 8). For an efficacy to side‐effect ratio to be derived, the total number of contralateral rotations was divided by the total dyskinesia score. The median score for the group is plotted for each day.
Striatal TH expression
The density of striatal TH was used to confirm the lesion caused by 6‐OHDA. These effects were examined with a two‐way ANOVA: Hemisphere (Lesion or Intact) * Striatal Region (Anterior, Middle or Posterior). Striatal TH immunoreactivity was reduced by 89% on the lesioned side relative to the intact side (F1,4 = 175.80, P < 0.001), but depletion was equivalent across striatal regions (Supporting Information Figure S1).
Discussion
The novel multifunctional D2/3 agonist, D‐512, was compared with the clinically used D2/3 agonist, ropinirole, for the treatment of PD symptoms in rats. Higher plasma levels and brain uptake were observed for D‐512 over equimolar doses of ropinirole (Figure 1). Both compounds increased spontaneous movements by a similar magnitude immediately after injection, but the duration of motor activation was longer for D‐512 (Figure 3). Only D‐512 was able to significantly reverse symptoms of forelimb akinesia, a cardinal feature of PD (Figure 4). Rotational behaviour, a marker of anti‐PD efficacy, was greater for D‐512 than for ropinirole even though both drugs produced dyskinesia of similar severity (Figures 5 and 6).
Pharmacokinetic profile
The development of long‐lasting anti‐Parkinsonian agents is critical as drugs with short half‐lives are less convenient for patients and may be more prone to elicit dyskinesia (Jankovic, 2005; Olanow, Obeso, and Stocchi, 2006). Rapidly metabolized compounds lead to frequent ‘wearing off’ episodes, creating such a significant clinical problem that many patients use specialized medical devices offering continuous drug delivery (Nyholm et al., 2005). As shown in Figure 1, D‐512 was present in the brain and blood plasma at more than twice the concentration of ropinirole 1 h after injection of equimolar amounts of each drug. Indeed, the brain concentration of D‐512 was greater at 4 h post‐injection than was observed for ropinirole at 1 h post‐injection, suggesting that D‐512 remains active in the CNS longer than ropinirole.
Spontaneous motor activation
Research in early‐stage PD patients and in animal models of PD has shown that ropinirole produces an anti‐Parkinsonian response of similar magnitude and duration to L‐DOPA (Brooks et al., 1998; Pearce et al., 1998; Rascol et al., 2000; Ravenscroft et al., 2004). The present data suggest that the initial magnitude of the motor response is comparable between D‐512 and ropinirole. Both drugs increased discrete movements during the first hour after drug administration (2–3× above untreated Parkinsonian animals on days 3, 10 and 17; Figure 3B, D, F). There was some suggestion that ropinirole (5.1 μmol·kg−1) may have a more rapid onset since it alone induced significant increases in distance travelled during the first hour of testing on day 17 (Figure 3E). However, D‐512 clearly exhibited longer‐lasting locomotor activation than ropinirole: D‐512 enhanced both distance travelled and discrete movements for all 4 h on all test days, while ropinirole had no significant effect after 2 h on any test day (Figure 3).
Unilaterally Parkinsonian rats exhibit a mild tendency to preferentially turn ipsilateral to lesion, whereas in the same animals, anti‐Parkinsonian compounds strongly and dose‐dependently induce rotations contralateral to lesion (Ungerstedt, 1971). This has led to widespread use of contralateral rotations as a measure of anti‐Parkinsonian efficacy (Lundblad et al., 2002; Smith et al., 2012; Breger, Dunnett, and Lane, 2013). Both ropinirole and D‐512 caused some contralateral turning, but statistically significant increases relative to vehicle were only observed with D‐512 (Figure 5). Indeed, chronic D‐512 at 9 μmol·kg−1 continued to significantly induce rotations at the 240 min time point, when measurements ceased (Figure 5D, F, H). These effects are in agreement with previous work showing that D‐512 caused longer‐lasting rotations than ropinirole when the drugs were administered acutely (Santra et al., 2013).
Even though it is common to measure contralateral rotations as a proxy for anti‐Parkinsonian efficacy, the interpretation of these behavioural effects is complicated by the fact that rotations appear to reflect a combination of anti‐Parkinsonian efficacy and dyskinesia liability (Lane et al., 2006). For example, rotations are dose‐dependently increased by L‐DOPA even when the dose is increased above the therapeutic maximum (Smith et al., 2012; Breger et al., 2013). For this reason, we chose to also examine motor ability with the forepaw adjusting steps test, an assessment that more closely measures Parkinsonian symptoms (Olsson et al., 1995; Chang et al., 1999).
Reversal of forelimb akinesia
The forepaw adjusting steps test assesses the ability of a rat to rapidly initiate and terminate movement and may be the best indicator of anti‐PD efficacy in a rat model of PD because performance is strongly diminished after dopaminergic lesion and restored by dopamine replacement therapy (Olsson et al., 1995). In the present investigation, untreated 6‐OHDA‐lesioned rats averaged 85% fewer steps with their lesioned forelimb than observed in sham‐lesioned animals (Figure 4). Ropinirole did not significantly alter the number of steps relative to vehicle. This result is similar to a previous report showing that quinpirole, a close analogue of ropinirole, did not increase performance on the forepaw adjusting steps test (Olsson et al., 1995). Even though acute treatment with D‐512 did not affect motor performance, chronic administration at 3 and 9 μmol·kg−1 was able to increase stepping relative to vehicle on days 15 and 22 (Figure 4C, D).
Dyskinesia liability
Treatment of PD is complicated by the fact that there is a consistent positive correlation between the magnitude of anti‐PD effects provided by a drug and the severity of dyskinesia it elicits (Stowe et al., 2008; Cenci et al., 2011). In the present study, dyskinesia was similar between ropinirole and D‐512. Examining total dyskinesia on each test day, the low and high doses of ropinirole produced equivalent dyskinesia to the low and high doses of D‐512 respectively (the sole exception being that the low dose of D‐512 caused more dyskinesia than the low dose of ropinirole on day 15; Figure 6A, C, E, G). However, the time series for each test day shows that the high dose of ropinirole produced a magnitude of dyskinesia that was equal to or greater than that of D‐512 in the period immediately after injection (Figure 6B, D, F, H). By contrast, the duration of dyskinesia caused by D‐512 was greater than that of ropinirole on days 8, 15 and 22. Thus, it may be that ropinirole causes greater peak dyskinesia than D‐512, but D‐512 extends the time during which dyskinesia is present. In this sense, the dyskinesia data are consistent with other behavioural assays in suggesting that the anti‐Parkinsonian effects of ropinirole extinguish within 2 h, while the anti‐Parkinsonian effects of D‐512 last for at least 4 h (Figures 3, 4, 5).
Although dopaminergic agonists that target D2/3 receptors are considered to have low dyskinesia liability, our data are consistent with previous research in demonstrating that the risk is present. Studies in Parkinsonian primates have shown that ropinirole causes dyskinesia, but the severity is reduced relative to L‐DOPA (Pearce et al., 1998; Maratos et al., 2001). Similarly, early‐stage PD patients who are otherwise drug‐naïve can experience dyskinesia when given ropinirole as monotherapy, but the odds are much lower than if the patient was started on L‐DOPA monotherapy (Rascol et al., 2001). Although we did not compare ropinirole or D‐512 to L‐DOPA in the present study, our laboratory has consistently reported that a therapeutic dose of L‐DOPA in rats (6 mg·kg−1) induces median peak dyskinesia scores of 5–6 (on the abnormal involuntary movements scale) that are stable for approximately 1–2 h (Bishop et al., 2012; Ostock et al., 2015; Lindenbach et al., 2016). Considering that, in the present study, neither drug caused dyskinesia greater than a median score of 2.5, it seems reasonable to conclude that both ropinirole and D‐512 are less dyskinesiogenic than L‐DOPA.
It is unclear why D2/3 agonists produce dyskinesia, but the reason may relate to the interactions between D1 and D3 receptors. While D1 receptor knockout is sufficient to almost completely abolish dyskinesia, D3 receptor knockout reduces dyskinesia severity by less than half (Darmopil et al., 2009; Solis et al., 2015). D1 and D3 receptors form tetrameric protein complexes. Within these complexes, D3 activation attenuates the effect of D1 receptor agonists on canonical G‐protein‐dependent cAMP formation but potentiates the effect of D1 agonists on G‐protein‐independent MAPK activity (Ferre et al., 2014; Guitart et al., 2014). This finding is relevant for dyskinesia since induction of MAPK activity appears to promote the development and expression of dyskinesia (Pavon et al., 2006; Santini et al., 2007). In support of this notion, D3 receptor knockout in vivo reduces L‐DOPA‐induced dyskinesia, at the same time reducing the ability of L‐DOPA to stimulate the MAPK pathway (Solis et al., 2015). Thus, monotherapy with a D2/3 receptor agonist may elicit some degree of dyskinesia, albeit less than L‐DOPA, by enhancing constitutive activity of canonically D1 receptor‐mediated signalling pathways.
Clinical implications
While D2/3 agonists are often used to treat motor symptoms in early‐stage PD, accumulating evidence suggests that these compounds may also benefit non‐motor symptoms. PD patients often have clinically significant anxiety, depression and/or apathy, with prevalence estimates for at least one symptom ranging from 20 to 60% (Gallagher and Schrag, 2012). D2/3 agonists such as ropinirole or pramipexole reduce non‐motor PD symptoms, even though traditional antidepressants that target the 5‐HT or noradrenaline transporter are often ineffective (Pahwa et al., 2007; Chaudhuri and Schapira, 2009). These clinical findings are bolstered by animal work showing that dopamine depletion with 6‐OHDA causes increased phenotypic expression of anxiety, depression and apathy, which can be reversed by treatment with dopaminergic agonists, especially those targeting the D3 receptor (Bonito‐Oliva, Masini, and Fisone, 2014; Carnicella et al., 2014; Favier et al., 2014). Further studies are needed to assess if D‐512 is more effective than ropinirole or pramipexole, in terms of relieving non‐motor PD symptoms.
D‐512 in vitro, but not ropinirole, attenuated dopaminergic cell loss after 6‐OHDA if the two drugs were administered concomitantly (Santra et al., 2013; Shah et al., 2014). Similar neuroprotection was found in vivo when D‐512 was co‐administered with the monoamine toxin MPTP (Shah et al., 2014). These effects might be due to antioxidant properties and other neuroprotective properties of the indole and aminothiazole moieties in the D‐512 molecule (Johnson et al., 2012; Santra et al., 2013; Shah et al., 2014; Voshavar et al., 2015).
In the present study, the most obvious benefit of D‐512 over ropinirole is an enhancement of peak‐dose efficacy and an extension of the duration of action. Stimulation of spontaneous movement by ropinirole was complete after 2 h, while the effects of ropinirole on motor performance and rotations did not reach statistical significance. By contrast, D‐512 significantly enhanced spontaneous locomotion, motor performance and rotations throughout the 4 h of testing. Dyskinesia was observed with both drugs. However, D‐512 showed a consistently greater ratio of drug‐stimulated rotations relative to dyskinesia, suggesting that the efficacy to side‐effect ratio of D‐512 was greater than that of ropinirole (Figure 7). This is consistent with the theory that increasing the half‐life of dopaminergic agonists makes them less prone to cause dyskinesia due to more consistent stimulation of dopamine receptors across time (Olanow et al., 2006).
There is growing evidence that D‐512 has a better profile to other dopamine receptor agonists in terms of pharmacokinetics (Figure 1), symptomatic efficacy (Figures 3, 4, 5, 6, 7) and neuroprotection (Santra et al., 2013; Shah et al., 2014; Voshavar et al., 2015). Considering that D2/3 receptor agonists remain front‐line treatments for early‐stage PD, our results suggest that further investigation of D‐512 could prove useful in improving the treatment of PD.
Author contributions
D.L., B.D., A.K.D. and C.B. designed research. D.L., B.D., M.M.C. and S.M. performed the research. D.L. and B.D. analysed the data. D.L., B.D., A.K.D. and C.B. wrote the manuscript. D.L., B.D., A.K.D. and C.B. contributed funding.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Mass spectrometry conditions for the samples.
Figure S1 6‐hydroxydopamine lesion reduced TH expression in the striatum (n = 5). Under anaesthesia, rats received 6‐hydroxydopamine infusions in the left medial forebrain bundle. Three weeks later, rats were treated daily for 22 d with saline. On day 23, rats were transcardially perfused and striatal sections were examined for TH expression using optical density analysis. Representative coronal sections (1.60 mm anterior to bregma) are shown for (A) the intact striata and (B) the lesioned striata. (C) Densitometric analysis of lesioned vs. intact striata, in arbitrary units (a.u.). Paired‐samples t‐tests were used to compare between regions and hemispheres: bars that share the same letter are considered statistically comparable, while bars with different letters are considered statistically different.
Acknowledgements
The authors wish to thank Yarden Avnor, Emily Nuss, Mitchell Melikhov‐Sosin, Libby Gross, Nissah Vilceus, Nadia Schuman and Crystal Tasber for assistance with behavioural testing, drug administration and animal care. This work was supported by a grant from the Michael J. Fox Foundation for Parkinson's Research (to D.L., B.D., A.K.D. and C.B.), the Center for Development and Behavioural Neuroscience at Binghamton University (to C.B.) and the National Institutes of Neurological Disorders and Stroke (NS047198 to A.K.D).
Lindenbach, D. , Das, B. , Conti, M. M. , Meadows, S. M. , Dutta, A. K. , and Bishop, C. (2017) D‐512, a novel dopamine D2/3 receptor agonist, demonstrates greater anti‐Parkinsonian efficacy than ropinirole in Parkinsonian rats. British Journal of Pharmacology, 174: 3058–3071. doi: 10.1111/bph.13937.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop C, George J, Buchta W, Goldenberg A, Mohamed M, Dickinson S et al. (2012). Serotonin transporter inhibition attenuates l‐DOPA‐induced dyskinesia without compromising l‐DOPA efficacy in hemi‐parkinsonian rats. Eur J Neurosci 36: 2839–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonito‐Oliva A, Masini D, Fisone G (2014). A mouse model of non‐motor symptoms in Parkinson's disease: focus on pharmacological interventions targeting affective dysfunctions. Front Behav Neurosci 8: 290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breger L, Dunnett S, Lane E (2013). Comparison of rating scales used to evaluate l‐DOPA‐induced dyskinesia in the 6‐OHDA lesioned rat. Neurobiol Dis 50: 142–150. [DOI] [PubMed] [Google Scholar]
- Brooks DJ, Abbott RJ, Lees AJ, Martignoni E, Philcox DV, Rascol O et al. (1998). A placebo‐controlled evaluation of ropinirole, a novel D2 agonist, as sole dopaminergic therapy in Parkinson's disease. Clin Neuropharmacol 21: 101–107. [PubMed] [Google Scholar]
- Carnicella S, Drui G, Boulet S, Carcenac C, Favier M, Duran T et al. (2014). Implication of dopamine D3 receptor activation in the reversion of Parkinson's disease‐related motivational deficits. Transl Psychiatry 4: e401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Lundblad M (2007). Ratings of L‐DOPA‐induced dyskinesia in the unilateral 6‐OHDA lesion model of Parkinson's disease in rats and mice. Curr Protoc Neurosci 9 9.25. [DOI] [PubMed] [Google Scholar]
- Cenci MA, Ohlin KE, Odin P (2011). Current options and future possibilities for the treatment of dyskinesia and motor fluctuations in Parkinson's disease. CNS Neurol Disord Drug Targets 10: 670–684. [DOI] [PubMed] [Google Scholar]
- Chang JW, Wachtel SR, Young D, Kang UJ (1999). Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience 88: 617–628. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AH (2009). Non‐motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 8: 464–474. [DOI] [PubMed] [Google Scholar]
- Connolly B, Lang A (2014). Pharmacological treatment of Parkinson disease: a review. JAMA 311: 1670–1683. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmopil S, Martín AB, De Diego IR, Ares S, Moratalla R (2009). Genetic inactivation of dopamine D1 but not D2 receptors inhibits L‐DOPA–induced dyskinesia and histone activation. Biol Psychiatry 66: 603–613. [DOI] [PubMed] [Google Scholar]
- Favier M, Duran T, Carcenac C, Drui G, Savasta M, Carnicella S (2014). Pramipexole reverses Parkinson's disease‐related motivational deficits in rats. Mov Disord 29: 912–920. [DOI] [PubMed] [Google Scholar]
- Gallagher DA, Schrag A (2012). Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis 46: 581–589. [DOI] [PubMed] [Google Scholar]
- Guitart X, Navarro G, Moreno E, Yano H, Cai NS, Sánchez‐Soto M et al. (2014). Functional selectivity of allosteric interactions within G protein–coupled receptor oligomers: the dopamine D1‐D3 receptor heterotetramer. Mol Pharmacol 86: 417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J (2005). Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord 20: S11–S16. [DOI] [PubMed] [Google Scholar]
- Jankovic J (2008). Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79: 368–376. [DOI] [PubMed] [Google Scholar]
- Johnson M, Antonio T, Reith M, Dutta A (2012). Structure–activity relationship study of N6‐(2‐(4‐(1H‐Indol‐5‐yl)piperazin‐1‐yl)ethyl)‐N6‐propyl‐4,5,6,7‐tetrahydrobenzo[d]thiazole‐2,6‐diamine analogues: development of highly selective D3 dopamine receptor ggonists along with a highly potent D2/D3 agonist and their pharmacological characterization. J Med Chem 55: 5826–5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knekt P, Kilkkinen A, Rissanen H, Marniemi J, Sääksjärvi K, Heliövaara M (2010). Serum vitamin D and the risk of Parkinson disease. Arch Neurol 67: 808–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane EL, Cheetham SC, Jenner P (2006). Does contraversive circling in the 6‐OHDA‐lesioned rat indicate an ability to induce motor complications as well as therapeutic effects in Parkinson's disease? Exp Neurol 197: 284–290. [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T (2009). Parkinson's disease. Lancet 373: 2055–2266. [DOI] [PubMed] [Google Scholar]
- Lindenbach D, Conti MM, Ostock CY, George JA, Goldenberg AA, Melikhov‐Sosin M et al. (2016). The role of primary motor cortex (M1) glutamate and GABA signaling in L‐DOPA‐induced dyskinesia in parkinsonian rats. J Neurosci 36: 9873–9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenbach D, Conti MM, Ostock CY, Dupre KB, Bishop C (2015). Alterations in primary motor cortex neurotransmission and gene expression in hemi‐parkinsonian rats with drug‐induced dyskinesia. Neuroscience 310: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002). Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson's disease. Eur J Neurosci 15: 120–132. [DOI] [PubMed] [Google Scholar]
- Maratos E, Jackson M, Pearce R, Jenner P (2001). Antiparkinsonian activity and dyskinesia risk of ropinirole and L‐DOPA combination therapy in drug naive MPTP‐lesioned common marmosets (Callithrix jacchus). Mov Disord 16: 631–641. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm D, Nilsson Remahl AIM, Dizdar N, Constantinescu R, Holmberg B, Jansson R et al. (2005). Duodenal levodopa infusion monotherapy vs oral polypharmacy in advanced Parkinson disease. Neurology 64: 216–223. [DOI] [PubMed] [Google Scholar]
- Olanow C, Obeso J, Stocchi F (2006). Continuous dopamine‐receptor treatment of Parkinson's disease: scientific rationale and clinical implications. Lancet Neurol 5: 677–687. [DOI] [PubMed] [Google Scholar]
- Olsson M, Nikkhah G, Bentlage C, Björklund A (1995). Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci 15: 3863–3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostock CY, Hallmark J, Palumbo N, Bhide N, Conti MM, George JA et al. (2015). Modulation of l‐DOPA's antiparkinsonian and dyskinetic effects by α2‐noradrenergic receptors within the locus coeruleus. Neuropharmacology 95: 215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahwa R, Stacy MA, Factor SA, Lyons KE, Stocchi F, Hersh BP et al. (2007). Ropinirole 24‐hour prolonged release randomized, controlled study in advanced Parkinson disease. Neurology 68: 1108–1115. [DOI] [PubMed] [Google Scholar]
- Pavon N, Martín AB, Mendialdua A, Moratalla R (2006). ERK phosphorylation and fosB expression are associated with L‐DOPA‐induced dyskinesia in hemiparkinsonian mice. Biol Psychiatry 59: 64–74. [DOI] [PubMed] [Google Scholar]
- Pearce R, Banerji T, Jenner P, Marsden D (1998). De novo administration of ropinirole and bromocriptine induces less dyskinesia than L‐dopa in the MPTP‐treated marmoset. Mov Disord 13: 234–241. [DOI] [PubMed] [Google Scholar]
- Rascol O, Brooks D, Korczyn A, Deyn P, Clarke C, Lang A (2000). A five‐year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. N Engl J Med 342: 1484–1491. [DOI] [PubMed] [Google Scholar]
- Ravenscroft P, Chalon S, Brotchie J, Crossman A (2004). Ropinirole versus l‐DOPA effects on striatal opioid peptide precursors in a rodent model of Parkinson's disease: implications for dyskinesia. Exp Neurol 185: 36–46. [DOI] [PubMed] [Google Scholar]
- Santini E, Valjent E, Usiello A, Carta M, Borgkvist A, Girault JA et al. (2007). Critical involvement of cAMP/DARPP‐32 and extracellular signal‐regulated protein kinase signaling in L‐DOPA‐induced dyskinesia. J Neurosci 27: 6995–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra S, Xu L, Shah M, Johnson M, Dutta A (2013). D‐512 and D‐440 as novel multifunctional dopamine agonists: characterization of neuroprotection properties and evaluation of in vivo efficacy in a Parkinson's disease animal model. ACS Chem Nerosci 4: 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Rajagopalan S, Xu L, Voshavar C, Shurubor Y, Beal F et al. (2014). The high‐affinity D2/D3 agonist D512 protects PC12 cells from 6‐OHDA‐induced apoptotic cell death and rescues dopaminergic neurons in the MPTP mouse model of Parkinson's disease. J Neurochem 131: 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis O, Garcia‐Montes JR, Gonzalez‐Granillo A, Xu M, Moratalla R (2015). Dopamine D3 receptor modulates l‐DOPA‐induced dyskinesia by targeting D1 receptor‐mediated striatal signaling. Cereb Cortex 27: 435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G, Heuer A, Dunnett S, Lane E (2012). Unilateral nigrostriatal 6‐hydroxydopamine lesions in mice II: predicting l‐DOPA‐induced dyskinesia. Behav Brain Res 226: 281–292. [DOI] [PubMed] [Google Scholar]
- Stowe RL, Ives NJ, Clarke C, van Hilten J, Ferreira J, Hawker RJ et al. (2008). Dopamine agonist therapy in early Parkinson's disease. Cochrane Database Syst Rev : CD006564. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U (1971). Postsynaptic supersensitivity after 6‐hydroxy‐dopamine induced degeneration of the nigro‐striatal dopamine system. Acta Physiologica Scand Suppl 367: 69–93. [DOI] [PubMed] [Google Scholar]
- Valera E, Masliah E (2016). Therapeutic approaches in Parkinson's disease and related disorders. J Neurochem 139 (S1): 346–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voshavar C, Shah M, Xu L, Dutta A (2015). Assessment of protective role of multifunctional dopamine agonist D‐512 against oxidative stress produced by depletion of glutathione in PC12 Cells: implication in neuroprotective therapy for Parkinson's disease. Neurotox Res 28: 302–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J (2011). Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol 26 (S1): 1–58. [DOI] [PubMed] [Google Scholar]
- Yoritaka A, Kawajiri S, Yamamoto Y, Nakahara T, Ando M, Hashimoto K et al. (2015). Randomized, double‐blind, placebo‐controlled pilot trial of reduced coenzyme Q10 for Parkinson's disease. Parkinsonism Relat Disord 21: 911–916. [DOI] [PubMed] [Google Scholar]
- Zhang S, Hernán M, Chen H, Spiegelman D, Willett W, Ascherio A (2002). Intakes of vitamins E and C, carotenoids, vitamin supplements, and PD risk. Neurology 59: 1161–1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Mass spectrometry conditions for the samples.
Figure S1 6‐hydroxydopamine lesion reduced TH expression in the striatum (n = 5). Under anaesthesia, rats received 6‐hydroxydopamine infusions in the left medial forebrain bundle. Three weeks later, rats were treated daily for 22 d with saline. On day 23, rats were transcardially perfused and striatal sections were examined for TH expression using optical density analysis. Representative coronal sections (1.60 mm anterior to bregma) are shown for (A) the intact striata and (B) the lesioned striata. (C) Densitometric analysis of lesioned vs. intact striata, in arbitrary units (a.u.). Paired‐samples t‐tests were used to compare between regions and hemispheres: bars that share the same letter are considered statistically comparable, while bars with different letters are considered statistically different.


