Abstract
Background and Purpose
Psychological stress exacerbates symptoms of urinary bladder dysfunction; however, the underlying brain mechanisms are unclear. We have demonstrated that centrally administered bombesin, a stress‐related neuropeptide, facilitates the rat micturition reflex. Brain bombesin‐like peptides modulate the serotoninergic nervous system activity under stress conditions; therefore, we examined whether brain 5‐HT is involved in the bombesin‐induced increased frequency of urination in urethane‐anaesthetised male Sprague–Dawley rats.
Experimental Approach
Evaluation of intercontraction intervals (ICI) and maximal voiding pressure (MVP) during cystometrograms were started 1 h before i.c.v. administration of bombesin or i.c.v. pretreatment with the 5‐HT receptor antagonists.
Key Results
Bombesin (0.03 nmol per animal, i.c.v.) significantly reduced ICI without affecting MVP. The bombesin‐induced response was significantly suppressed by acute depletion of brain 5‐HT, which was induced by pretreatment with p‐chlorophenylalanine, a 5‐HT synthesis inhibitor. Bombesin at a lower dose (0.01 nmol per animal, i.c.v.) showed no significant effect on ICI, while it significantly reduced ICI in the presence of WAY‐100635 (5‐HT1A receptor antagonist, 0.1 or 0.3 μg per animal, i.c.v.), which can block the negative feedback control of 5‐HT release. Bombesin (0.03 nmol per animal)‐induced ICI reduction was significantly attenuated by SB269970 (5‐HT7 receptor antagonist, 0.1 or 0.3 μg per animal, i.c.v.) but not by ritanserin (5‐HT2 receptor antagonist, 0.3 or 1 μg per animal, i.c.v.).
Conclusions and Implications
The brain serotoninergic nervous system is involved in the facilitation of the rat micturition reflex induced by bombesin‐like peptides at least in part through brain 5‐HT7 receptors.
Abbreviations
- DMF
N,N‐dimethylformamide
- GRP
gastrin‐releasing peptide
- IACUC
Institutional Animal Care and Use Committees
- ICI
intercontraction intervals
- MVP
maximal voiding pressure
- PFC
prefrontal cortex
- Rv
post‐voiding residual urine volume
Introduction
There are previous reports showing a relationship between bladder function and psychological stress not only in experimental animals but also in humans (Lutgendorf et al., 2000; Smith et al., 2011; Merrill et al., 2013; Lai et al., 2015). For example, in rats, psychological stress exposure increases micturition frequency and decreases voiding interval (Smith et al., 2011). In female patients with bladder pain syndrome/interstitial cystitis, exposure to acute mental stress worsens symptoms of bladder pain and urinary urgency, but not in controls (Lutgendorf et al., 2000). These findings suggest that psychological stress plays an important role in the induction of frequent urination and exacerbation of bladder dysfunction including overactive bladder and bladder pain syndrome/interstitial cystitis. Psychological stress‐related information is conveyed to the brain, which recruits neuronal and neuroendocrine systems for adaptation to stressful conditions, thereby inducing physical and behavioural responses to psychological stress (stress responses) (Ulrich‐Lai and Herman, 2009). However, the brain pathophysiological mechanisms underlying psychological stress‐induced effects on bladder function are still unclear.
A brain neuropeptide, bombesin, has been reported to regulate stress responses (Merali et al., 2002; Jensen et al., 2008). Bombesin itself is a frog peptide and is not expressed in mammals, while bombesin‐like peptides such as neuromedin B and gastrin‐releasing peptide (GRP) are expressed in the mammalian brain (Jensen et al., 2008). In fact, in rodent models, acute stress exposure such as immobilization stress increased immunoreactivity and in vivo release of these bombesin‐like peptides in the brain (Kent et al., 1998; Merali et al., 2008). Recently, we reported that centrally administered bombesin, a non‐specific peptide agonist of bombesin receptors, reduced intercontraction intervals (ICI) without affecting maximal voiding pressure (MVP) and reduced single‐voided volume and bladder capacity without affecting post‐voiding residual urine volume (Rv) or voiding efficiency in cystometrogram experiments in rats (Shimizu et al., 2016). These results suggest that brain bombesin‐like peptides might facilitate sensory inputs to the micturition centre, thereby inducing frequent urination because the centrally administered bombesin had no significant effect on cystometrogram parameters of bladder efferent activity such as MVP, Rv or voiding efficiency.
Brain bombesin‐like peptides can modulate activity of the serotoninergic nervous system. Under restraint stress condition, GRP increases serotoninergic neuron activity in the rat hypothalamic paraventricular nucleus (Garrido et al., 2002). In addition, neuromedin B injected into the rat dorsal raphe nucleus, in which 5‐HT‐containing neurons are widely distributed, promoted in vivo release of 5‐HT in the hippocampus (Merali et al., 2006). These findings suggest that the brain serotoninergic nervous system is a downstream pathway of brain bombesin‐like peptides. The serotoninergic nervous system in the CNS has been reported to modulate micturition, both inhibitory and excitatory (Sugaya et al., 1998; Ishizuka et al., 2002; Ramage, 2006; Kadekawa et al., 2009; Chiba et al., 2016a). Based on these findings, we hypothesized that brain bombesin‐like peptides can induce frequent urination via modulation of the central serotoninergic nervous system. Therefore, in this study, we examined the brain mechanisms for the centrally administered bombesin‐induced frequent urination in rats focusing on the brain 5‐HT and 5‐HT receptors. We used p‐chlorophenylalanine (fenclonine, an inhibitor of 5‐HT biosynthesis enzyme L‐tryptophan hydroxylase) that has been utilized to investigate the effects of near‐total depletion of 5‐HT in the CNS, such as the frontal cortex, hypothalamus, brain stem and spinal cord (Steinman et al., 1987; Christianson et al., 2008; Delaville et al., 2012; Yoshimura et al., 2014). In addition, we focused on three 5‐HT receptor subtypes, 5‐HT1A, 5‐HT2 (5‐HT2A, 5‐HT2B and 5‐HT2C) and 5‐HT7, because several groups have reported that these 5‐HT receptor subtypes are involved in the modulation of micturition (Ramage, 2006).
Methods
Animals
All animal experiments were conducted in accordance with the NIH guidelines and approved by the University of Pittsburgh Institutional Animal Care and Use Committees (IACUC) (#15096571). All efforts were made to minimize the suffering of the animals and the number of animals needed to obtain reliable results. A total of 60 male Sprague–Dawley rats weighing 300–350 g (Harlan Laboratories Inc., Indianapolis, IN, USA) were used in this study, and they were housed with two animals per cage (length, 36.2 cm; width, 24.8 cm; height, 17.8 cm) upon the IACUC recommendation for humane animal care, maintained in an air‐conditioned room at 22–24°C under the 12/12 h light–dark cycle with lights on at 0700 h, and given food (LabDiet #5P76, LabDiet, St. Louis, MO, USA) and water ad libitum. These rats were divided into 10 groups randomly and were used for the experiments described below. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Surgery
In urethane‐anaesthetised (0.9–1.0 g·kg−1, i.p.) male Sprague–Dawley rats, after a laparotomy, a catheter (PE‐50; Clay Adams, Parsippany, NJ, USA) was inserted into the bladder from the dome in order to perform continuous cystometrogram. Each rat was then placed in the prone position in a stereotaxic apparatus for the brain (SR‐6R; Narishige, Tokyo, Japan) until the end of continuous cystometric evaluation, as described previously (Shimizu et al., 2016). The skull was drilled for i.c.v. administration of drugs using a stainless‐steel cannula (outer diameter of 0.3 mm). The stereotaxic coordinates of the tip of the cannula were as follows (in mm): AP −1.0, L 1.5, V 4.5 (AP, anterior from the bregma; L, lateral from the midline; V, below the surface of the brain), according to the rat brain atlas (Paxinos and Watson, 2005). Three hours after the surgery, the steel cannula was inserted into the right lateral ventricle and each drug was administered as described below (Figure 1). During the surgery and continuous cystometry, we monitored sufficient levels of anaesthesia by confirming negative reflex responses to toe pinch every 30 min. If the level was insufficient, additional doses of urethane (0.05 g·kg−1 per injection, i.p.) were administered. In some experiments, p‐chlorophenylalanine or vehicle (saline with 1% Tween 80) was administered (200 mg·kg−1, i.p.) in a volume of 10 mL·kg−1 once a day for 2 days (Figure 1A). It has been reported that the administration of p‐chlorophenylalanine at this dose resulted in approximately 80–95% depletion of 5‐HT in the rat brain (Christianson et al., 2008; Yoshimura et al., 2014). At 1 day after the second administration, p‐chlorophenylalanine‐treated rats, which exhibited no obvious behavioural abnormality, underwent the surgery described above (Figure 1A).
Figure 1.
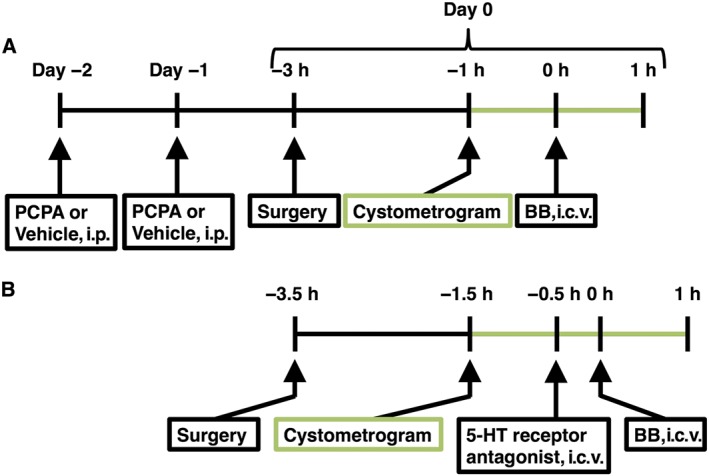
Experiment outline of this study. (A) In some rats, p‐chlorophenylalanine (PCPA) (200 mg·kg−1, i.p.), a 5‐HT synthesis inhibitor, or vehicle (saline containing 1% tween 80, 10 mL·kg−1, i.p.) was administered once a day for 2 days. At 1 day after the second administration, bombesin (BB) was i.c.v. administered 3 h after the surgery for continuous cystometrogram and i.c.v. administration. In vivo continuous cystometrograms were started 1 h before the i.c.v. administration and continued for 1 h after the BB administration. (B) In other rats without PCPA or vehicle treatment, 3 h after the surgery, each 5‐HT receptor antagonist was i.c.v. pretreated 30 min before the BB administration. In vivo continuous cystometrograms were started 1 h before the first i.c.v. administration and continued for 1 h after the BB administration
Drug administration
Bombesin dissolved in sterile saline (0.001 or 0.003 nmol·μL−1) was slowly administered into the right lateral ventricle in a volume of 10 μL using a cannula connected to a 10 μL Hamilton syringe at a rate of 10 μL·min−1, and the cannula was retained until the end of the experiment. For pretreatment with 5‐HT receptor antagonists, WAY‐100635 dissolved in 5 μL of sterile saline, or ritanserin or SB269970 dissolved in 3 μL of N,N‐dimethylformamide (DMF) was i.c.v. administered using a cannula connected to a 10 μL Hamilton syringe at a rate of 10 μL·min−1. Sterile saline in a volume of 5 μL was administered as a vehicle of WAY‐100635, and DMF in a volume of 3 μL was administered as a vehicle of ritanserin and SB269970. The cannula was retained in the ventricle for 15 min to avoid the leakage of each antagonist and then removed from the ventricle. Subsequently, bombesin (0.01 or 0.03 nmol·10 μL−1) was i.c.v. administered 30 min after each pretreatment (Figure 1B). One hour after the bombesin administration, Cresyl Violet solution was injected through the cannula. Thereafter, the rats were decapitated under anaesthesia, and the brains were removed in order to confirm the exact location of the cannula inserted in the brain and to verify whether the solution had spread throughout the entire ventricular space. Due to cannula misplacement, six rats were excluded from 60 rats used; therefore, the data were obtained from 54 rats.
Continuous cystometrogram
Cystometrogram studies were performed according to the methods previously reported (Shimizu et al., 2016). Briefly, after the surgery described above, the bladder catheter was connected to a pressure transducer for measurements of intravesical pressure and to a syringe pump for continuous infusion of sterile saline into the bladder at a rate of 12 mL·h−1. Intravesical pressure was recorded using a PowerLab System (AD Instruments, Bella Vista, Australia). Continuous infusion of saline and measurements of ICI, which was the interval of two voiding bladder contractions, and MVP, which was the maximum pressure during a micturition cycle, were started 1 h before the first i.c.v. administration, and the infusion was continued for 1 h after bombesin administration (Figure 1).
Data analysis and statistics
All values are expressed as means ± SEM. Relative values of ICI and MVP were calculated as the ratio of averaged ICI and MVP measured for each 10 min after bombesin administration to those measured for 10 min prior to the bombesin administration. During each 10 min evaluation period, five to eight micturition cycles were recorded and used to calculate the average value of each cystometric parameter. These data analyses were performed by an investigator (Y.H.) blinded to experimental conditions. The sample size in each experimental group was determined based on the expected difference in a desired endpoint measurement between the test and control groups and the mean of the standard deviations for the two groups reported in our previous report (Shimizu et al., 2016). In experiments in two groups of rats pretreated with p‐chlorophenylalanine or vehicle, five rats per group were used at first to examine the tendency of p‐chlorophenylalanine to produce an inhibitory effect. However, two more rats were added to the p‐chlorophenylalanine‐pretreated group because of a high dispersion of values in this group. In experiments in eight groups of rats treated with 5‐HT receptor antagonists or corresponding vehicle of each antagonist, six rats per group were used. However, in six out of eight groups, one rat per group was excluded due to cannula misplacement as described above. Statistical differences were determined using one‐way ANOVA, followed by post hoc analysis with the Bonferroni method when relative data values were compared at the same time range. When only two means were compared at the same time range, Student's unpaired t‐test was used. When values were compared with those measured for 10 min prior to the bombesin administration, raw data values were compared using one‐way ANOVA, followed by post hoc analysis with the Bonferroni method. P values less than 0.05 were taken to indicate statistical significance. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Materials
The following drugs were used: bombesin (catalogue #, 1149) from R&D Systems Inc. (Minneapolis, MN, USA); p‐chlorophenylalanine (fenclonine) (catalogue #, C6506) from Sigma Aldrich (St. Louis, MO, USA); WAY‐100635 maleate (N‐[2‐[4‐(2‐methoxyphenyl)‐1‐piperazinyl]ethyl]‐N‐2‐pyridinylcyclohexanecarboxamide maleate) (catalogue #, 4380) and ritanserin (6‐[2‐[4‐[bis(4‐fluorophenyl)methylene]‐1‐piperidinyl]ethyl]‐7‐methyl‐5H‐thiazolo[3,2‐a]pyrimidin‐5‐one) (catalogue #, 1955) from Tocris Bioscience (Bristol, UK); SB269970 hydrochloride (3‐[[(2R)‐2‐[2‐(4‐methyl‐1‐piperidinyl)ethyl]‐1‐pyrrolidinyl]sulfonyl]‐phenol, monohydrochloride) (catalogue #, 17 081) from Cayman Chemical (Ann Arbor, MI, USA). All other reagents of the highest grade available were obtained from Sigma Aldrich. The dosage of drugs was determined according to previous studies (Read et al., 2003; Yoshiyama et al., 2003; Christianson et al., 2008; Yoshimura et al., 2014; Shimizu et al., 2016) and our preliminary experiments.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a, 2015b).
Results
Acute depletion of brain 5‐HT by p‐chlorophenylalanine attenuated the centrally administered bombesin‐induced ICI reduction
Results of continuous cystometrograms in rats with or without p‐chlorophenylalanine pretreatment are shown in Figures 2 and 3. The baseline values of ICI (s) and MVP (cmH2O) during the −10 to 0 min period were 98 ± 17 and 44.5 ± 0.9 in the vehicle (saline containing 1% tween 80, 10 mL·kg−1)‐pretreated group (n = 5), and 90 ± 18 and 45.3 ± 2.6 in the p‐chlorophenylalanine (200 mg·kg−1)‐pretreated group (n = 7), respectively, and there were no significant differences in baseline values of ICI or MVP prior to bombesin administration between p‐chlorophenylalanine‐treated and vehicle‐treated groups. In vehicle‐pretreated rats, centrally administered bombesin at a dose of 0.03 nmol per animal (i.c.v.) significantly reduced ICI without affecting MVP compared with the values before the bombesin administration (−10 to 0 min) (Figure 3). These data are in agreement with our previous results (Shimizu et al., 2016). However, in p‐chlorophenylalanine‐pretreated rats, ICI values were not significantly decreased after bombesin administration, indicating that the bombesin‐induced reduction in ICI was significantly suppressed after 5‐HT depletion (Figure 3).
Figure 2.
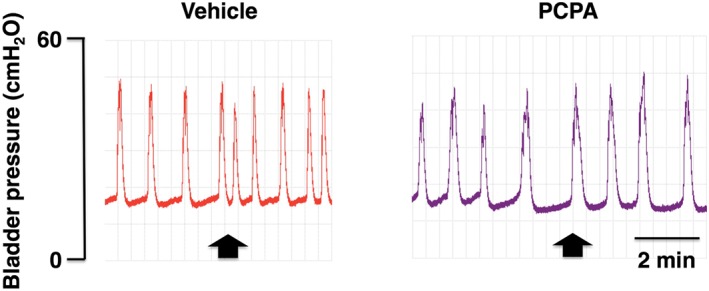
Representative in vivo continuous cystometrogram traces in Sprague–Dawley rats. Para‐chlorophenylalanine (PCPA), a 5‐HT synthesis inhibitor, was administered (200 mg·kg−1, i.p.) once a day for 2 days. At 1 day after the second administration, bombesin was administered (0.03 nmol per animal, i.c.v.). Arrows indicate the timing of bombesin injection. Left and right panels show traces in a vehicle‐ or PCPA‐pretreated rat respectively. Note that acute depletion of brain 5‐HT attenuated the centrally administered bombesin‐induced changes.
Figure 3.
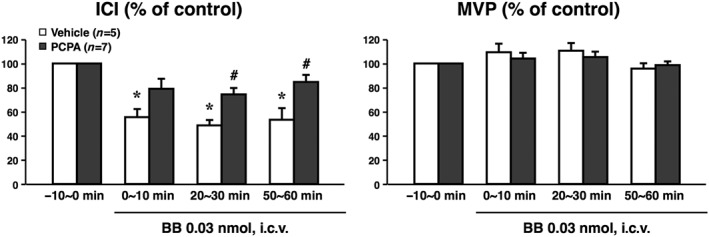
Effects of centrally administered bombesin (BB) on ICI and MVP. Sprague–Dawley rats were pretreated with vehicle (saline containing 1% tween 80, 10 mL·kg−1, i.p.) once a day for 2 days (Vehicle, n = 5) or with p‐chlorophenylalanine (200 mg·kg−1, i.p.) once a day for 2 days (PCPA, n = 7). At 1 day after the second PCPA administration, BB was administered (0.03 nmol per animal, i.c.v.). Data calculated as the ratio to the values during the −10 to 0 min period prior to BB administration (−10 to 0 min) present means ± SEM. *P < 0.05, when raw data values were compared with the Bonferroni method to the values prior to BB (−10 to 0 min). # P < 0.05, when relative data values were compared with Student's t‐test to the Vehicle group. The number of animals per group is indicated in parentheses. Note that acute depletion of brain 5‐HT by PCPA attenuated the centrally administered BB‐induced reduction in ICI.
Centrally administered bombesin‐induced ICI reduction was potentiated by WAY‐100635, a 5‐HT1A receptor antagonist
The baseline values of ICI during the −10 to 0 min period were 92 ± 14 s in the vehicle (5 μL saline per animal, i.c.v.)‐pretreated group (n = 6), 74 ± 7 s in the WAY‐100635 (0.1 μg per animal, i.c.v.)‐pretreated group (n = 5) and 100 ± 20 s in the WAY‐100635 (0.3 μg per animal, i.c.v.)‐pretreated group (n = 5), and there were no significant differences among three groups. Centrally administered bombesin at a lower dose of 0.01 nmol per animal (i.c.v.) showed no significant effect on ICI compared with the values prior to bombesin administration (−10 to 0 min) (Figure 4). Pretreatment with WAY‐100635 at a lower dose of 0.1 μg per animal (i.c.v.) had no significant effect on the bombesin (0.01 nmol per animal, i.c.v.)‐induced response, while the bombesin significantly reduced ICI in the presence of a higher dose of WAY‐100635 (0.3 μg per animal, i.c.v.) (Figure 4). There were no significant effects of the treatment with WAY‐100635 alone (0.1 and 0.3 μg per animal, i.c.v.) on ICI or MVP (data not shown).
Figure 4.
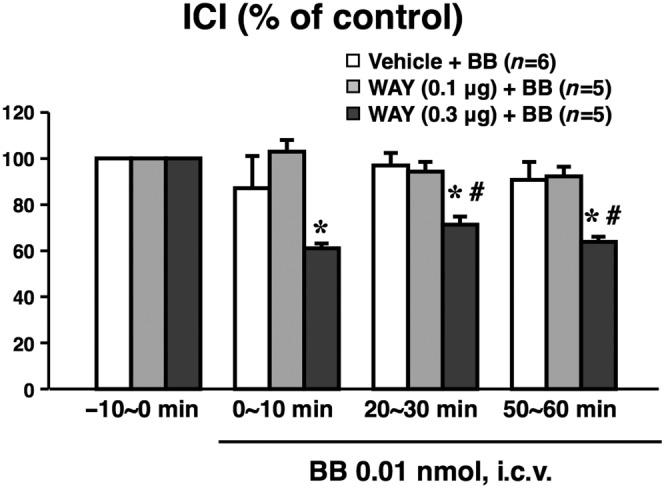
Effect of pretreatment with WAY‐100635 (WAY), a 5‐HT1A receptor antagonist, on the centrally administered bombesin (BB)‐induced reduction in ICI. WAY (0.1 or 0.3 μg per animal, n = 5 each) or vehicle (5 μL saline per animal, n = 6) was i.c.v. administered 30 min before the administration of BB (0.01 nmol per animal, i.c.v.). Data calculated as the ratio to the values during the −10 to 0 min period prior to BB administration (−10 to 0 min) present means ± SEM. *P < 0.05, when raw data values were compared with the Bonferroni method to the values prior to BB (−10 to 0 min). # P < 0.05, when relative data values were compared with the Bonferroni method to the Vehicle + BB group. The number of animals per group is indicated in parentheses. Note that the centrally administered BB‐induced ICI reduction was potentiated by WAY.
Centrally administered bombesin‐induced ICI reduction was suppressed by SB269970, a 5‐HT7 receptor antagonist, but not by ritanserin, a non‐selective 5‐HT2 receptor antagonist
The same vehicle‐treated rats were used for statistical comparison of SB269970 and ritanserin experiments in Figure 5A, B respectively. The baseline values of ICI during the −10 to 0 min period were 135 ± 21 s in the vehicle (3 μL DMF per animal, i.c.v.)‐pretreated group (n = 5), 140 ± 29 s in the ritanserin (0.3 μg per animal, i.c.v.)‐pretreated group (n = 5), 137 ± 3 s in the ritanserin (1 μg per animal, i.c.v.)‐pretreated group (n = 5), 96 ± 18 s in the SB269970 (0.1 μg per animal, i.c.v.)‐pretreated group (n = 5) and 76 ± 9 s in the SB269970 (0.3 μg per animal, i.c.v.)‐pretreated group (n = 6). There were no significant differences in these baseline values among the three groups in Figure 5A or the three groups in Figure 5B. Pretreatment with ritanserin had no significant effect at either dose (0.3 or 1 μg per animal, i.c.v.) on the bombesin (0.03 nmol per animal, i.c.v.)‐induced reduction in ICI (Figure 5A). In contrast, SB269970 at both doses (0.1 and 0.3 μg per animal, i.c.v.) significantly suppressed the bombesin‐induced ICI reduction (Figure 5B). There were no significant effects of the treatment with SB269970 alone (0.1 and 0.3 μg per animal, i.c.v.) on ICI or MVP (data not shown).
Figure 5.
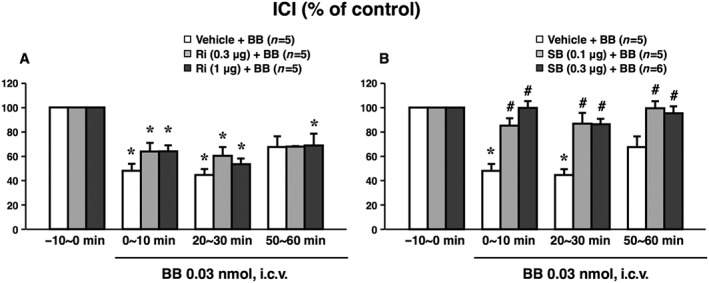
Effects of pretreatment with ritanserin (Ri), a 5‐HT2 receptor antagonist, or SB269970 (SB), a 5‐HT7 receptor antagonist, on the centrally administered bombesin (BB)‐induced reduction in ICI. Ri (0.3 or 1 μg per animal, n = 5 each, A), SB (0.1 or 0.3 μg per animal, n = 5 or 6, respectively, B) or vehicle (3 μL DMF per animal, n = 5) was i.c.v. administered 30 min before the administration of BB (0.03 nmol per animal, i.c.v.). Data calculated as the ratio to the values during the −10 to 0 min period prior to BB administration (−10 to 0 min) present means ± SEM. The same vehicle‐treated rats were used for statistical comparison in (A) and (B) respectively. *P < 0.05, when raw data values were compared with the Bonferroni method to the values prior to BB (−10 to 0 min). # P < 0.05, when relative data values were compared with the Bonferroni method to the Vehicle + BB group. The number of animals per group is indicated in parentheses. Note that the centrally administered BB‐induced ICI reduction was suppressed by SB, but not by Ri.
Discussion and conclusions
In the present study, we demonstrated that i.c.v. administered bombesin‐induced ICI reduction was attenuated in p‐chlorophenylalanine‐pretreated rats, in which brain 5‐HT was depleted. In the presence of WAY‐100635, i.c.v. administered bombesin induced ICI reduction even at a dose that had no effect in the absence of WAY‐100635. Central pretreatment with SB269970, but not ritanserin, significantly inhibited the reduction of ICI induced by i.c.v. administered bombesin. These results suggest that the brain serotoninergic nervous system is involved in bombesin‐induced frequent urination in rats at least through brain 5‐HT7 receptors, whereas WAY‐100635 that can inhibit 5‐HT1A receptors‐mediated negative feedback control of serotoninergic neuron firing and 5‐HT release at serotoninergic nerve terminals (Mundey et al., 1996; Adell and Artigas, 1998) is likely to strengthen the 5‐HT‐mediated enhancement of the micturition reflex induced by centrally administered bombesin.
The serotoninergic pathways in the CNS have been shown to modulate micturition. Electric stimulation of the raphe nucleus, a major 5‐HT‐containing area, causes inhibition of micturition in decerebrated rats (Sugaya et al., 1998), and intrathecally administered 5‐HT inhibits bladder contractions in rats (Kadekawa et al., 2009). These lines of evidence suggest that 5‐HT‐containing pathways play an inhibitory role in the control of micturition through the descending pathway from the raphe nucleus to the spinal cord. In addition, 5‐HT is reported to be involved in suppression of micturition in the rat prefrontal cortex (PFC) (Chiba et al., 2016a), which is known to perform executive functions to suppress voiding until a socially appropriate timing (Funahashi and Andreau, 2013). On the other hand, i.c.v. administered 5‐HT and some agonists for 5‐HT receptor subtypes such as 5‐HT1A, 5‐HT2 and 5‐HT4 enhance the micturition reflex in rats (Ishizuka et al., 2002), indicating that 5‐HT‐containing pathways might be excitatory in the control of micturition through the ascending pathway. Considering that 5‐HT in the CNS can play both inhibitory and excitatory roles in regulation of micturition, the findings that the pretreatment with p‐chlorophenylalanine alone showed no effect on micturition, in current and previous studies (Yoshiyama et al., 1994), are not unexpected. However, in this study, in p‐chlorophenylalanine‐pretreated rats, centrally administered bombesin‐induced frequent urination was attenuated compared with control rats. Even though p‐chlorophenylalanine pretreatment can suppress the inhibitory regulation of 5‐HT‐containing descending pathways in the micturition control, the pretreatment suppressed the bombesin‐induced stimulation of micturition. Therefore, brain bombesin‐like peptides seem to induce frequent urination at least through 5‐HT‐containing ascending pathways, which are excitatory, in the brain.
Subsequently, we used a potent 5‐HT1A receptor antagonist, WAY‐100635, which displays 100‐fold selectivity for 5‐HT1A over other 5‐HT receptor subtypes (Forster et al., 1995). 5‐HT1A receptors are well known as 5‐HT autoreceptors, which can inhibit 5‐HT neuron firing and 5‐HT release from the presynaptic nerve terminals (Mundey et al., 1996; Adell and Artigas, 1998). In this study, in rats pretreated with WAY‐100635, centrally administered bombesin induced frequent urination, even at a dose which alone had no effect on normal micturition. On the other hand, centrally administered WAY‐100635 by itself had no effect on micturition. It has previously been reported that systemically administered WAY‐100635 alone had no effect on in vivo release of 5‐HT in the median raphe nucleus in rats, while the WAY‐100635 inhibited 5‐HT release reduction induced by systemically administered 8‐OH‐DPAT, a selective 5‐HT1A receptor agonist (Adell and Artigas, 1998). These findings suggest that the 5‐HT1A receptors‐mediated negative feedback control of 5‐HT neuron firing and 5‐HT release is put into effect when 5‐HT release is enhanced or 5‐HT1A receptors are stimulated. Therefore, it might be reasonable to assume that WAY‐100635 can inhibit activation of the 5‐HT1A receptors‐mediated negative feedback control after administration of bombesin, which induces enhancement of 5‐HT release, to potentiate the bombesin‐induced frequent urination. Taken together, our results indicate that brain bombesin‐like peptides are involved in facilitation of the rat micturition reflex through the brain serotoninergic nervous system.
We further investigated the effects of ritanserin and SB269970, potent antagonists of 5‐HT2 (5‐HT2A, 5‐HT2B and 5‐HT2C) and 5‐HT7 receptors respectively (Watanabe et al., 1992; Lovell et al., 2000). In this study, central pretreatment with SB269970 almost completely suppressed the centrally administered bombesin‐induced frequent urination in rats. On the other hand, ritanserin had no effects on the bombesin‐induced response. These results indicate that brain bombesin‐like peptides facilitate the micturition reflex through brain 5‐HT7 receptors, but not 5‐HT2 receptors. It has previously been reported that i.c.v. administered SB269970 alone at a higher dose compared with those used in this study abolished the micturition reflex in rats, but not when given intrathecally (Read et al., 2003; Ramage, 2006). These findings suggest that 5‐HT7 receptors play an excitatory role in the control of bladder function at a supraspinal level and can support our present data. On the other hand, in the PFC, 5‐HT7 receptors are reported to mediate the inhibitory control of micturition (Chiba et al., 2016b), suggesting that roles of brain 5‐HT7 receptors on micturition might be either excitatory or inhibitory depending on different brain regions where 5‐HT7 receptors are expressed and activated. We used i.c.v. administration of bombesin and each 5‐HT receptor antagonist; therefore, a limitation of this study is that it is unclear which 5‐HT‐containing pathway in the brain is involved in the bombesin‐induced frequent urination. Further studies are therefore needed to examine the specific regions in the brain that contribute to bombesin and 5‐HT‐mediated frequent urination. In addition, another limitation of this study was to use anaesthetised rats because urethane anaesthesia can affect brain activity. Therefore, future studies under the conscious condition are necessary in order to clarify the physiological mechanisms of stress underlying lower urinary tract dysfunction.
Chronic reductions of monoamines, 5‐HT and noradrenaline in the CNS reportedly lead to frequent urination and bladder overactivity in rats, which were reversed by fluoxetine, a selective 5‐HT reuptake inhibitor (Lee et al., 2003). Imipramine, a tricyclic antidepressant, and duloxetine, a 5‐HT and noradrenaline reuptake inhibitor, ameliorated frequent urination in a mouse model of chemically induced cystitis (Redaelli et al., 2015). In addition, duloxetine improved symptoms of overactive bladder in human patients (Steers et al., 2007). Thus, chronic alterations in central serotoninergic and/or noradrenergic nervous systems can induce bladder dysfunction. The present study has demonstrated that the brain serotoninergic nervous system is involved in frequent urination induced by bombesin, a stress‐related neuropeptide, in rats. Although it might be difficult to directly apply the findings in our study using acute experimental protocols to the chronic disease condition, the central serotoninergic nervous system could be a useful target for alleviation of psychological stress‐induced exacerbation of bladder dysfunction.
In summary, our results suggest that the brain bombesin‐like peptide system is involved in facilitation of the rat micturition reflex through the brain serotoninergic nervous system and that frequent urination is mediated by activation of brain 5‐HT7 receptors. These findings would be useful for understanding the underlying mechanisms of stress‐related exacerbation of lower urinary tract symptoms in overactive bladder and bladder pain syndrome/interstitial cystitis, for which brain 5‐HT7 receptors could be a new therapeutic target.
Author contributions
T.S., S.S., M.S. and N.Y. designed this research. T.S., N.W., S.T, N.S., K.K. and T.M. performed the experiments. T.S. and Y.H. analysed the data. T.S., S.S., M.S. and N.Y. interpreted the results of experiments. T.S. and N.Y. drafted the manuscript. T.S., S.S., N.W., S.T., N.S., Y.H., K.K., T.M., M.S. and N.Y. approved the final version of the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported in part by a Grant‐in‐Aid for Scientific Research (C) (no. JP26460909 and JP17K09303 to T.S.) from Japan Society for the Promotion of Science, a grant from Narishige Neuroscience Research Foundation in Japan, a grant from Smoking Research Foundation in Japan, a grant from The Japan Health Foundation, a grant from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney, USA (R01‐DK088836 to N.Y.) and a Grant from the Department of Defence, USA (W81XWH‐12‐1‐0565 to N.Y.).
Shimizu, T. , Shimizu, S. , Wada, N. , Takai, S. , Shimizu, N. , Higashi, Y. , Kadekawa, K. , Majima, T. , Saito, M. , and Yoshimura, N. (2017) Brain serotoninergic nervous system is involved in bombesin‐induced frequent urination through brain 5‐HT7 receptors in rats. British Journal of Pharmacology, 174: 3072–3080. doi: 10.1111/bph.13941.
References
- Adell A, Artigas F (1998). A microdialysis study of the in vivo release of 5‐HT in the median raphe nucleus of the rat. Br J Pharmacol 125: 1361–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba H, Mitsui T, Kitta T, Ohmura Y, Moriya K, Kanno Y et al. (2016a). The role of serotonergic mechanism in the rat prefrontal cortex for controlling the micturition reflex: an in vivo microdialysis study. NeurourolUrodyn 35: 902–907. [DOI] [PubMed] [Google Scholar]
- Chiba H, Mitsui T, Kitta T, Ohmura Y, Moriya K, Kanno Y et al. (2016b). 5‐HT in the rat prefrontal cortex controls the micturition reflex via 5‐HT2A and 5‐HT7 . J Urol 195: e794–e795. [Google Scholar]
- Christianson JP, Rabbett S, Lyckland J, Drugan RC (2008). The immobility produced by intermittent swim stress is not mediated by serotonin. Pharmacol Biochem Behav 89: 412–423. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaville C, Navailles S, Benazzouz A (2012). Effects of noradrenaline and serotonin depletions on the neuronal activity of globus pallidus and substantia nigra pars reticulata in experimental parkinsonism. Neuroscience 202: 424–433. [DOI] [PubMed] [Google Scholar]
- Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y et al. (1995). A pharmacological profile of the selective silent 5‐HT1A receptor antagonist, WAY‐100635. Eur J Pharmacol 281: 81–88. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Andreau JM (2013). Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris 107: 471–482. [DOI] [PubMed] [Google Scholar]
- Garrido MM, Fuentes JA, Manzanares J (2002). Gastrin‐releasing peptide mediated regulation of 5‐HT neuronal activity in the hypothalamic paraventricular nucleus under basal and restraint stress conditions. Life Sci 70: 2953–2966. [DOI] [PubMed] [Google Scholar]
- Ishizuka O, Gu B, Igawa Y, Nishizawa O, Pehrson R, Andersson KE (2002). Role of supraspinal serotonin receptors for micturition in normal conscious rats. NeurourolUrodyn 21: 225–230. [DOI] [PubMed] [Google Scholar]
- Jensen RT, Battey JF, Spindel ER, Benya RV (2008). International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol Rev 60: 1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadekawa K, Nishijima S, Sugaya K, Miyazato M, Saito S (2009). Mechanisms by which the serotonergic system inhibits micturition in rats. Life Sci 85: 592–596. [DOI] [PubMed] [Google Scholar]
- Kent P, Anisman H, Merali Z (1998). Are bombesin‐like peptides involved in the mediation of stress response? Life Sci 62: 103–114. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai H, Gardner V, Vetter J, Andriole GL (2015). Correlation between psychological stress levels and the severity of overactive bladder symptoms. BMC Urol 15: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KS, Na YG, Dean‐McKinney T, Klausner AP, Tuttle JB, Steers WD (2003). Alterations in voiding frequency and cystometry in the clomipramine induced model of endogenous depression and reversal with fluoxetine. J Urol 170: 2067–2071. [DOI] [PubMed] [Google Scholar]
- Lovell PJ, Bromidge SM, Dabbs S, Duckworth DM, Forbes IT, Jennings AJ et al. (2000). A novel, potent, and selective 5‐HT7 antagonist: (R)‐3‐(2‐(2‐(4‐methylpiperidin‐1‐yl)ethyl)pyrrolidine‐1‐sulfonyl) phenol (SB‐269970). J Med Chem 43: 342–345. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Kreder KJ, Rothrock NE, Ratliff TL, Zimmerman B (2000). Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol 164: 1265–1269. [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Anisman H, James JS, Kent P, Schulkin J (2008). Effects of corticosterone on corticotrophin‐releasing hormone and gastrin‐releasing peptide release in response to an aversive stimulus in two regions of the forebrain (central nucleus of the amygdala and prefrontal cortex). Eur J Neurosci 28: 165–172. [DOI] [PubMed] [Google Scholar]
- Merali Z, Bédard T, Andrews N, Davis B, McKnight AT, Gonzalez MI et al. (2006). Bombesin receptors as a novel anti‐anxiety therapeutic target: BB1 receptor actions on anxiety through alterations of serotonin activity. J Neurosci 26: 10387–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Kent P, Anisman H (2002). Role of bombesin‐related peptides in the mediation or integration of the stress response. Cell Mol. Life Sci 59: 272–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Malley S, Vizzard MA (2013). Repeated variate stress in male rats induces increased voiding frequency, somatic sensitivity, and urinary bladder nerve growth factor expression. Am J Physiol Regul Integr Comp Physiol 305: R147–R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundey MK, Fletcher A, Marsden CA (1996). Effects of 8‐OHDPAT and 5‐HT1A antagonists WAY100135 and WAY100635, on guinea‐pig behaviour and dorsal raphe 5‐HT neurone firing. Br J Pharmacol 117: 750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005). In: Paxinos G, Watson C. (eds). The Rat Brain in Stereotaxic Coordinates. Burlington: Elsevier Academic Press. [Google Scholar]
- Ramage AG (2006). The role of central 5‐hydroxytryptamine (5‐HT, serotonin) receptors in the control of micturition. Br J Pharmacol 147: S120–S131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read KE, Sanger GJ, Ramage AG (2003). Evidence for the involvement of central 5‐HT7 receptors in the micturition reflex in anaesthetized female rats. Br J Pharmacol 140: 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redaelli M, Ricatti MJ, Simonetto M, Claus M, Ballabio M, Caretta A et al. (2015). Serotonin and noradrenaline reuptake inhibitors improve micturition control in mice. PLoS One 10: e0121883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Shimizu S, Higashi Y, Nakamura K, Yoshimura N, Saito M (2016). A stress‐related peptide bombesin centrally induces frequent urination through brain bombesin receptor types 1 and 2 in the rat. J Pharmacol Exp Ther 356: 693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Leung J, Kun S, Zhang R, Karagiannides I, Raz S et al. (2011). The effects of acute and chronic psychological stress on bladder function in a rodent model. Urology 78: 967.e1–967.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steers WD, Herschorn S, Kreder KJ, Moore K, Strohbehn K, Yalcin I et al. (2007). Duloxetine compared with placebo for treating women with symptoms of overactive bladder. BJU Int 100: 337–345. [DOI] [PubMed] [Google Scholar]
- Steinman JL, Carlton SM, Haber B, Willis WD (1987). Differential effects of p‐chlorophenylalanine on indoleamines in brainstem nuclei and spinal cord of rats. I. Biochemical and behavioral analysis. Brain Res 426: 297–309. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Ogawa Y, Hatano T, Koyama Y, Miyazato T, Oda M (1998). Evidence for involvement of the subcoeruleus nucleus and nucleus raphe magnus in urine storage and penile erection in decerebrate rats. J Urol 159: 2172–2176. [DOI] [PubMed] [Google Scholar]
- Ulrich‐Lai YM, Herman JP (2009). Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Usui H, Kobayashi S, Yoshiwara H, Shibano T, Tanaka T et al. (1992). Syntheses and 5‐HT2 antagonist activity of bicyclic 1,2,4‐triazol‐3(2H)‐one and 1,3,5‐triazine‐2,4(3H)‐dione derivatives. J Med Chem 35: 189–194. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Hagimoto M, Matsuura T, Ohkubo J, Ohno M, Maruyama T et al. (2014). Effects of food deprivation on the hypothalamic feeding‐regulating peptides gene expressions in serotonin depleted rats. J Physiol Sci 64: 97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama M, Kakizaki H, de Groat WC (2003). Suppression of the micturition reflex in urethane‐anesthetized rats by intracerebroventricular injection of WAY100635, a 5‐HT1A receptor antagonist. Brain Res 980: 281–287. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Roppolo JR, de Groat WC (1994). Interactions between glutamatergic and monoaminergic systems controlling the micturition reflex in the urethane‐anesthetized rat. Brain Res 639: 300–308. [DOI] [PubMed] [Google Scholar]


