Abstract
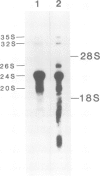
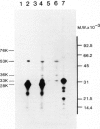
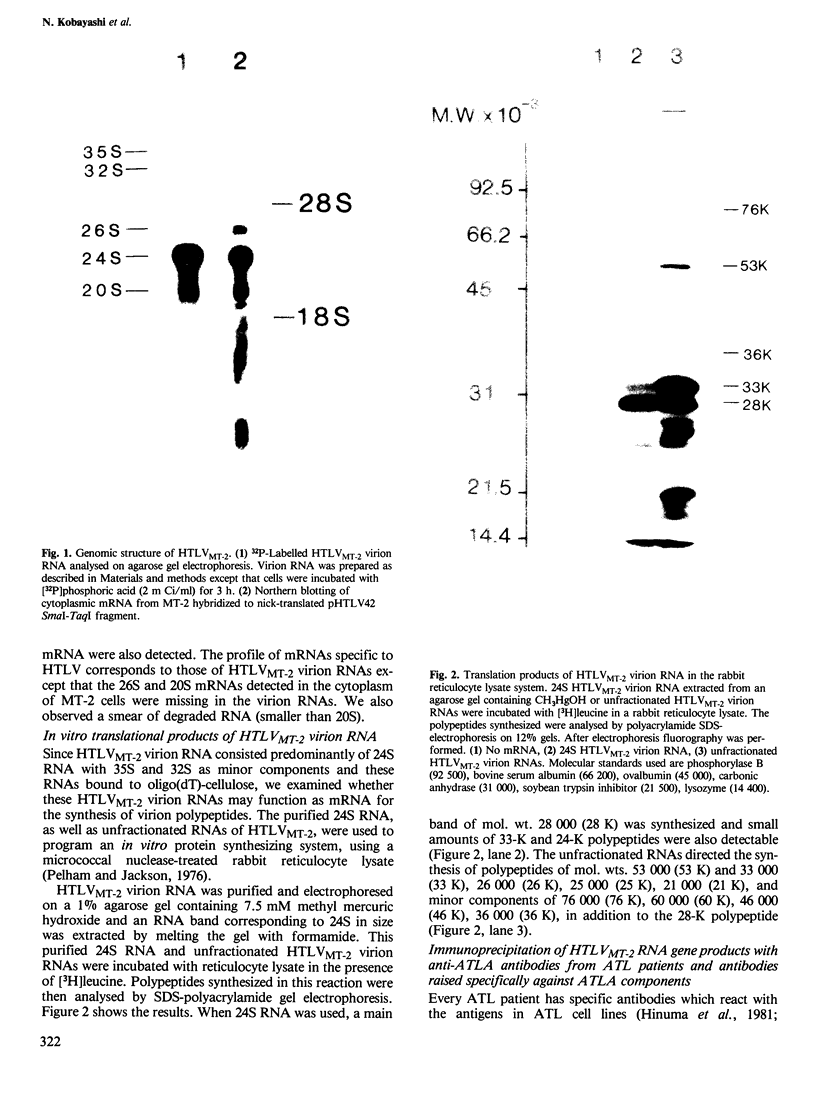
A human type-C retrovirus, designated HTLV (human T-cell leukemia virus), was isolated from the HTLV producer cell line MT-2. Agarose gel electrophoresis analysis 32P-labeled HTLVMT-2 virion RNA revealed that HTLVMT-2 virion RNA consists mainly of 24S and small amounts of 35S and 32S RNAs. The 24S HTLVMT-2 virion RNA and unfractionated HTLVMT-2 virion RNA were translated in a rabbit reticulocyte lysate system in vitro. The predominant polypeptide synthesized from 24S RNA had an apparent mol. wt. of 28 000 (28 K); unfractionated HTLVMT-2 virion RNA directed the synthesis of 53 000 (53 K), 33 000 (33 K) and 28 000 (28 K) polypeptides as main components. Most of the polypeptides synthesised in vitro by translation of HTLVMT-2 virion RNAs possess the same sizes as the proteins formerly designated as ATLA (ATL-associated antigen) in SDS-polyacrylamide gel electrophoresis and immunologically precipitated with sera of ATL patients. Therefore, the antigens termed ATLA, found by the serological study of ATL, are HTLVMT-2 encoded polypeptides.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Stephenson J. R. Endogenous type-C RNA viruses of mammalian cells. Biochim Biophys Acta. 1976 Dec 23;458(4):323–354. doi: 10.1016/0304-419x(76)90006-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Hanafusa T., Wang L. H., Anderson S. M., Karess R. E., Hayward W. S., Hanafusa H. Characterization of the transforming gene of Fujinami sarcoma virus. Proc Natl Acad Sci U S A. 1980 May;77(5):3009–3013. doi: 10.1073/pnas.77.5.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Gotoh Y., Sugamura K., Nagata K., Goto T., Nakai M., Kamada N., Matsumoto T., Kinoshita K. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gan. 1982 Apr;73(2):341–344. [PubMed] [Google Scholar]
- Hinuma Y., Komoda H., Chosa T., Kondo T., Kohakura M., Takenaka T., Kikuchi M., Ichimaru M., Yunoki K., Sato I. Antibodies to adult T-cell leukemia-virus-associated antigen (ATLA) in sera from patients with ATL and controls in Japan: a nation-wide sero-epidemiologic study. Int J Cancer. 1982 Jun 15;29(6):631–635. doi: 10.1002/ijc.2910290606. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Nagata K., Hanaoka M., Nakai M., Matsumoto T., Kinoshita K. I., Shirakawa S., Miyoshi I. Adult T-cell leukemia: antigen in an ATL cell line and detection of antibodies to the antigen in human sera. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6476–6480. doi: 10.1073/pnas.78.10.6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyanaraman V. S., Sarngadharan M. G., Nakao Y., Ito Y., Aoki T., Gallo R. C. Natural antibodies to the structural core protein (p24) of the human T-cell leukemia (lymphoma) retrovirus found in sera of leukemia patients in Japan. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1653–1657. doi: 10.1073/pnas.79.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Pawson A., Robins T., Moscovici C., Duesberg P. H. Fujinami sarcoma virus: an avian RNA tumor virus with a unique transforming gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2018–2022. doi: 10.1073/pnas.77.4.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzari V., Wong-Staal F., Franchini G., Colombini S., Gelmann E. P., Oroszlan S., Staal S., Gallo R. C. Human T-cell leukemia-lymphoma virus (HTLV): cloning of an integrated defective provirus and flanking cellular sequences. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1574–1578. doi: 10.1073/pnas.80.6.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi I., Kubonishi I., Sumida M., Hiraki S., Tsubota T., Kimura I., Miyamoto K., Sato J. A novel T-cell line derived from adult T-cell leukemia. Gan. 1980 Feb;71(1):155–156. [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Mellon P., Duesberg P. H., Martin G. S. env Gene of Rous sarcoma virus: identification of the gene product by cell-free translation. J Virol. 1980 Mar;33(3):993–1003. doi: 10.1128/jvi.33.3.993-1003.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Ruscetti F. W., Gazdar A. F., Bunn P. A., Minna J. D., Gallo R. C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7415–7419. doi: 10.1073/pnas.77.12.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitz M. S., Jr, Poiesz B. J., Ruscetti F. W., Gallo R. C. Characterization and distribution of nucleic acid sequences of a novel type C retrovirus isolated from neoplastic human T lymphocytes. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1887–1891. doi: 10.1073/pnas.78.3.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiki M., Hattori S., Hirayama Y., Yoshida M. Human adult T-cell leukemia virus: complete nucleotide sequence of the provirus genome integrated in leukemia cell DNA. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3618–3622. doi: 10.1073/pnas.80.12.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shealy D. J., Mosser A. G., Rueckert R. R. Novel p19-related protein in Rous-associated virus type 61: implications for avian gag gene order. J Virol. 1980 May;34(2):431–437. doi: 10.1128/jvi.34.2.431-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Koyanagi Y., Chosa T., Yamamoto N., Hinuma Y. Monoclonal antibody reactive with both p28 and p19 of adult T-cell leukemia virus-specific polypeptides. Gan. 1983 Jun;74(3):327–330. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Yodoi J., Sagawa K., Takatsuki K., Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977 Sep;50(3):481–492. [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Hinuma Y. Antigens in an adult T-cell leukemia virus-producer cell line: reactivity with human serum antibodies. Int J Cancer. 1982 Sep 15;30(3):289–293. doi: 10.1002/ijc.2910300306. [DOI] [PubMed] [Google Scholar]
- Yamamoto N., Schneider J., Hinuma Y., Hunsmann G. Adult T-cell leukemia-associated antigen (ATLA): detection of a glycoprotein in cell- and virus-free supernatant. Z Naturforsch C. 1982 Jul-Aug;37(7-8):731–732. doi: 10.1515/znc-1982-7-828. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Miyoshi I., Hinuma Y. Isolation and characterization of retrovirus from cell lines of human adult T-cell leukemia and its implication in the disease. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2031–2035. doi: 10.1073/pnas.79.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]