Abstract
Mineral trioxide aggregate (MTA) is a powder containing calcium silicate composed of hydrophilic particles which harden at the presence of moisture. MTA was initially introduced as a root end filling material. Due its practical advantages that include superior biocompatility, effective sealing capability, and the ability to improve regeneration of the pulp and peripheral root tissues, it is used in different clinical applications such as pulp capping, apexification, pulpotomy and perforation. Despite being a promising material in endodontic treatment, MTA is not commonly used. Long setting time is the main clinical disadvantage of MTA. The aim of this review is to provide an overview of the current literature concerning the setting mechanism of MTA, accelerators and devices used to evaluate various steps of the hardening process.
Keywords: Mineral trioxide aggregate, setting, vicat, gillmore
Introduction
MTA is a powder that contains calcium silicate and consists of hydrophilic particles which causes setting under humidity conditions (1). MTA is classified in type-1 Portland cement (PS) category combined with 4:1 ratio of bismuth oxide to provide radiopacity according to the American Standards for Testing Materials (ASTM) (1, 2, 3, 4). The main component of Portland cement material consists of mainly tricalcium silicate (3CaO∙SiO2), dicalcium silicate (2CaO∙SiO2), tricalcium aluminate (3CaO∙Al2O3), tricalcium oxide (Ca2O3), silicate oxide (SiO2), aluminoferrite (4CaO∙Al2O3∙Fe2O3), and gypsum (CaSO4∙4H2O) (2, 5, 6, 7). MTA was initially introduced as a root end filling material and it is used in a variety of clinical applications (such as pulp capping, apexification, pulpotomy perforation and root perforation) due its practical advantages that include superior biocompatility, effective sealing capability, and the ability to improve regeneration of the pulp and peripheral root tissues (8, 9, 10, 11, 12, 13, 14, 15). Even though MTA sets slowly approximately in 3 to 4 hours in mouth and this might also be interpreted in favour of the material to prevent microleakage, long setting time is considered as the most important disadvantage of the material (14, 16, 17). Setting mechanism of MTA should be evaluated in order to eliminate this disadvantage (18).
Setting mechanism of MTA
Setting reaction starts with the contact of MTA powder comprising fine hydrophilic particles and water (18, 19, 20).
Hydration of MTA
Chemical reaction that leads to the setting of hydrophilic cement is called “hydration” (20, 21, 22). Components of MTA cement are partially soluble in water. Soluble components react at different speeds and rates, heat occurs during the reaction, and new products form. The resulting new products cause the setting of MTA cement and provide bonding to each other in the content of the components (19, 20,21, 22).
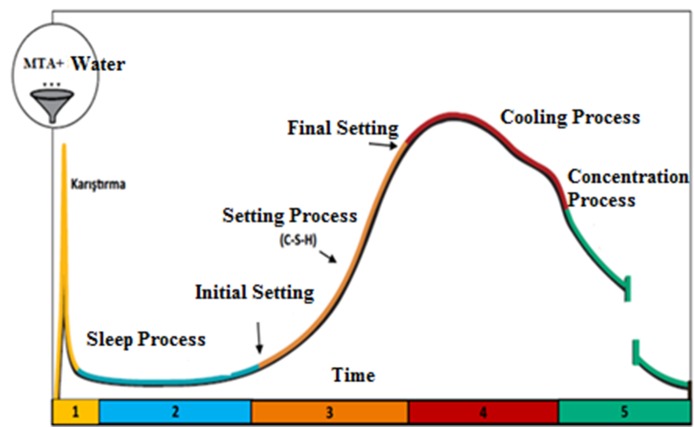
Hydration reaction is divided into different steps including mixing process, sleep process, setting process, cooling process, and condensation process (Figure 1) (20, 21, 22, 23). The characteristics of the components in the process must be known to better understand the hydration reaction. Calcium silicates (C3S,C2S), calcium aluminates (C3A, C4AF) and calcium sulphate (CS2H) in the MTA’s structure directly affects the hydration reaction (20, 21, 22).
Figure 1.
Hydration reaction is divided into different steps including mixing process, sleep process, setting process, cooling process, and condensation process.
Silicates
Silicates consist of combination of silicon dioxide (SiO2) and calcium oxide (CaO) (20, 21, 22, 24).
Tricalcium silicate - Alita (C3S)
It comprises approximately 55% of the volume of MTA cement. It provides rapid hydration and setting of the cement. It is largely held responsible for initial setting and early strength (21).
Dicalcium silicate - Axiom (C2S)
It comprises approximately 20% of the volume of MTA cement. It provides slow hydration and setting of the cement. It is largely held responsible for late strength (21).
Aluminates
Tetracalcium aluminate - Aluminite (C3A)
Alumina is the combination of calcium oxides with tricalcium aluminate (C3A) and combination of iron oxides with tetracalcium aluminoferrite (C4AF). Aluminites comprise approximately 20% of cement. During hydration, C3A reacts too fast and resists to sulphate (CS2H) (21).
Tetracalcium aluminoferrite - Ferrite (C4AF)
It balances and decreases the heat that occurs during the setting of cement. Moreover, due to the iron component, it causes coloration (21).
Calcium sulphate
It controls the effect of C3A which starts the setting reaction quickly and reduces the reaction rate. When there is no sulphate in cement (CS2H), the setting occurs very early. In addition, initial setting of CS2H controls the rise in early strength (21).
Processes encountered during cement setting Mixing Process
In this process, aluminate and gypsum (CaSO4∙4H2O) dissolve in water and react within a few minutes. As a result of rapid dissolution of gypsum added to cement, aluminates dissociated from cement forms a gel-like layer around the powder particles by reacting with water. This gellike layer prevents the quick reaction of aluminates and, therefore, rapid setting of cement (20, 21, 24).
Sleep Process
During this process, cement can be transported, placed or processed. The duration might vary with chemical additives. Rate of heat generation remains almost constant. However, reaction still continues. Cement components dissolve and saturate with water calcium in the cement (Ca2+) and hydroxyl (OH-) ions (20, 21, 24).
Setting Process
When the water of cement is oversaturated with soluble calcium ions, new hydration products begin to form. As a result of this reaction, temperature increases. It is called the beginning of setting. Once the setting starts on the surface of cement, neither the vibrator usage nor applications such as surface finishing, cannot be performed. Such interventions lead to permanent separation (20, 21). The amount of new products formed in the setting period increases constantly. The resulting product is collected around the hydrophilic particles that connect each other and surrounds the particles. Through this process the cement starts to set and solidify (20, 21). Initial setting time is the duration between the mixing of MTA powder with water and the moment when the cement start precipitation by showing physical changes (20, 21). Final setting time is the duration between the mixing of MTA powder with water and the moment when the cement solidifies (20, 21, 24).
Cooling Process
During this process, a reaction called “topochemical” occurs. Cement has become saturated in terms of components. Hydration starts at the surface of cement particles (C3S), and hydration products (C-S-H and CH) are formed at the surface. The process of cement gaining strength also begins in this period (21, 24).
Concentration Process
In this period, the reaction slows down and the heat output is reduced significantly. Hydration products continue to generate and develop slowly. Cement reaches the most rigid and robust structural properties that can be gained (5, 21, 24).
The chemical reactions that occur in the hydration step of MTA cement that we have described above are as follows: As a result of the hydrolysis of calcium silicate, calcium hydroxide in the aqueous cement (CaOH) and some calcium silicate hydrate (3CaO∙SiO2, 2CaO∙SiO2) are formed (5, 7, 24). The reaction of dicalcium silicate and tricalcium silicate is as follows:
3CaO∙SiO2+ H2O → CaO∙2SiO2∙3H2O+Ca(OH)2
2CaO∙SiO2+ H2O → 3CaO∙2SiO2∙3H2O+ Ca(OH)2
As a result of this reaction, crystal structure of hydrate is weak which forms a porous solid. This structure is called “silica gel”. Ca ions in silica gel combine with the OH ion and convert into Ca(OH)2 (24).
Once tricalcium aluminate (3CaAl2O4) is hydrated in the presence of calcium sulphate (CaSO4), it forms etringite (or sulphoaluminat calcium) with high sulphate concentration (6CaO•Al2O3•3SO3•32H2O). Etringite formation continues until all sulphate ions are used. Ettringite is converted to monosulphate once sulphate (SO2) ions are depleted.
3CaO•Al2O3•6 H2O+ H2O+ CaSO4• 2H2O → 6CaO•Al2O3•3SO3•32H2O
The resulting ettringite is disintegrated on the surface of cement particle and silicate hydrate coating is formed. After the destruction of the silicate hydrate coating, hydration can take years (20). It has been reported that MTA sets slowly approximately 3-4 hours in clinical conditions (3, 25). Long setting time of MTA can cause clinical problems. Researchers have added various chemicals to MTA in order to shorten the long curing time of MTA and to strengthen the structure of gritty texture and low density (26, 27, 28, 29).
The accelerators added to MTA
Substances which are used to improve mechanical and chemical properties of cement are called “additives” (1, 30, 31). There are numerous additives with various compositions that can be used selectively (14). Because of the difficulty of indentification of each additives, additives are divided into groups and thus, their common features can be better understood (32). Chemical additives can be summarized in four groups according to their main function (29, 31, 33, 34): setting time accelerators, setting time abbreviators, those that alleviative normal or high the amount of water, air-dragging Accelerator/abbreviator additives are used to reduce the setting time of MTA (18, 26). MTA has similar structure to Portland cement (6, 7, 35, 36). Portland cement was first produced in 1824 to be used in the construction industry. Therefore, adding of Portland cement to make the concrete and the use of additives in this concrete had begun after that date (7, 22, 36). Considering studies with cement, the first used accelerator material is calcium chloride (CaCI2). Patents were assigned in Germany in 1873 and in England in 1885 concerning the CaCI2 cement production (6, 30, 37). Chemicals such as sodium silicate (SiO2•Na2O•H2O), potassium silicate (SiO2•K2O•H2O), sodium carbonate (Na2CO3), potassium carbonate (K2CO3), sodium nitrite (NaNO2), sodium aluminate (Na2Al2O4), oxalate (NaC2O4), hydrated lime, sodium gluconate (C6H11NaO7) or urea (C5H4N4O3) were used as accelerators to reduce the setting time of Portland cement (17, 21, 26). After the construction industry, Portland cement has been introduced in dentistry following a variety of heat treatments (22, 35, 38). Because of its low radiopacity, new products have developed, and finally MTA was produced (2, 4, 39). Setting time of MTA is similar to Portland cement as well as its other properties (7, 40). Therefore, chemicals which are used to shorten the setting time of Portland cement were also used to shorten the setting time of MTA (1, 17, 26, 29, 31, 36). These are calcium chloride, calcium nitrite/ nitrate, calcium lactate gluconate, sodium chloride, disodium hydrogen phosphate (1, 11, 29, 31, 34, 41, 42, 43, 44). Besides, the effects of dental materials such as sodium hypochlorite (NaOCl), saline, lidocaine and chlorhexidine gluconate on the setting time of MTA have been examined (1, 26, 27, 29, 45).
Measurement of setting time of MTA
Because of silica particles in MTA/Portland cement, setting reaction is initiated by contact with water and described as “hydraulic cement” (1, 19, 22, 46). Initial and final setting times of cement are determined by the setting time test (penetration test) using various instruments and according to different standards (29, 31, 47).
Gillmore Apparatus (Gillmore Needle)
Gillmore apparatus is used to test the initial and final setting time of hydraulic cement (35). The apparatus consists of two horizontal arms with different weights and two stainless steel needles with cylindrical flat-end needles (Figure 2). Cylindrical flat-end needles are called “Gillmore needles”. ASTM C266-04 standard is used for test of setting time performed by Gillmore needle (48). One of Gillmore needles, with which the initial setting time is measured, is 2.12 mm in diameter, weighing 113.4 g. The second needle is 1.06 mm in diameter, weighing 453.6 g and it is used to measure final setting time. Gillmore apparatus’ base diameter is 76 mm, top diameter is 50 mm and thickness is 13 mm height (1, 48).
Figure 2.

Gillmore Apparatus.
According to the ASTM C191-04 standard, it has been reported that 650 g cement has to be used during the test. Initial and final setting times are defined as the time points when there are no detectable traces of the needle on the cement surface (1, 48).
Vicat Apparatus (Vicat needle)
Vicat apparatus is one of the tools used to test the initial and final setting time of hydraulic cements (47). ASTM C191-04 standard is used for testing procedures (Figure 3) (49, 50). There are two types Vicat apparatus defined as “standard” and “automatic”. Standard Vicat apparatus is set on a flat plate, and it is a tool that has a cylindrical shaft which is perpendicular to the axis of the plate. There are indicators on the cylinder. The Vicat needle is mounted on the lower end of the shaft, the shaft has a weight of 300g. Vicat needle is a cylinder with 1 mm in diameter (Figure 3) and (Figure 4).
Figure 3.
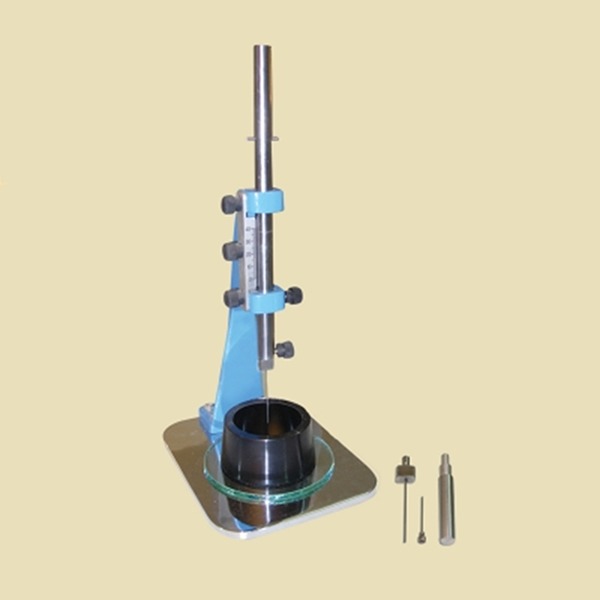
Original Vicat Apparatus.
Figure 4.

Automatic Vicat Apparatus.
Hydraulic cement is placed in a conical mold in Vicat apparatus with 40 mm depth, 70 mm top inner diameter and 80 mm base inner diameter. According to the ASTM C191-04 standard, it has been reported that 500 g cement has to be used during the test. After the mixed cement is inserted in the mold, measurement starts by releasing Vicat needle right after the contact with the cement surface (49).
Initial setting time measured with Vicat apparatus is the time between the contact of powder with water and when penetration is measured as 25 mm. Final setting time is considered as the time between the contact of powder with water to the moment when no circular trace of needle on cement surface is detectable (49, 50).
Automatic Vicat apparatus (Vicatronic) was designed to eliminate user-related errors, to obtain precise measurements data and to easily collect data (Figure 3)(51). Automatic Vicat apparatus has an electronic memory in which and the various standards used in the setting of cements are already stored. In addition, the test data can be archived by users (51). Standard Vicat needle has a free end operated by the user which may cause overflowing of test material beyond the mold and the deterioration of the prepared cement surface.
In automatic Vicat apparatus, a mode called “driven drop”, has been designed to adjust the dropping of Vicat needle on the cement surface to eliminate such disadvantages. After selecting the appropriate mode, the test procedure can be automatized and be resumed if necessary. The measurement values are displayed on the screen and can be printed instantly using the integral printer (51).
Conclusion
Hydration reaction of MTA is categorized into mixing process, sleep process, setting process, cooling process and condensation process. The physical and biochemical characteristics of the components which participate in the setting process should be well understood in order to control the long setting time which is usually measured by using traditional Vicat or Gillmore apparatus. Automatic Vicat device is a viable alternative to minimize the dependency on user skills and data loss.
Footnotes
Source of funding: None declared.
Conflict of interest: None declared.
References
- 1.Darvell BW, Wu RC. “Mta”-an hydraulic silicate cement: Review update and setting reaction. Dent Mater. 2011;27(5):407–422. doi: 10.1016/j.dental.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21(7):349–353. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 3.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review--part i: Chemical, physical, and antibacterial properties. J Endod. 2010;36(1):16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Grazziotin-Soares R, Nekoofar MH, Davies TE, Bafail A, Alhaddar E, Hübler R, Busato AL, Dummer PM. Effect of bismuth oxide on white mineral trioxide aggregate: chemical characterization and physical properties. Int Endod J. 2013 Sep 12;47(6):520–533. doi: 10.1111/iej.12181. [DOI] [PubMed] [Google Scholar]
- 5.Asgary S, Parirokh M, Eghbal MJ, Brink F. A comparative study of white mineral trioxide aggregate and white portland cements using x-ray microanalysis. Aust Endod J. 2004;30(3):89–92. doi: 10.1111/j.1747-4477.2004.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Dammaschke T, Gerth HU, Zuchner H, Schafer E. Chemical and physical surface and bulk material characterization of white proroot mta and two portland cements. Dent Mater. 2005;21(8):731–738. doi: 10.1016/j.dental.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 7.Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005;21(4):297–303. doi: 10.1016/j.dental.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Alhadainy HA. Root perforations. A review of literature. Oral Surg Oral Med Oral Pathol. 1994;78(3):368–374. doi: 10.1016/0030-4220(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 9.Behnia A, Strassler HE, Campbell R. Repairing iatrogenic root perforations. J Am Dent Assoc. 2000;131(2):196–201. doi: 10.14219/jada.archive.2000.0147. [DOI] [PubMed] [Google Scholar]
- 10.Bodem O, Blumenshine S, Zeh D, Koch MJ. Direct pulp capping with mineral trioxide aggregate in a primary molar: A case report. Int J Paediatr Dent. 2004;14(5):376–379. doi: 10.1111/j.1365-263X.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 11.Bortoluzzi EA, Broon NJ, Bramante CM, Consolaro A, Garcia RB, de Moraes IG, Bernadineli N. Mineral trioxide aggregate with or without calcium chloride in pulpotomy. J Endod. 2008;34(2):172–175. doi: 10.1016/j.joen.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Ferris DM, Baumgartner JC. Perforation repair comparing two types of mineral trioxide aggregate. J Endod. 2004;30(6):422–424. doi: 10.1097/00004770-200406000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Friedman S. Retrograde approaches in endodontic therapy. Endod Dent Traumatol. 1991;7(3):97–107. doi: 10.1111/j.1600-9657.1991.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 14.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review--part iii: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36(3):400–413. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Roberts HW, Toth JM, Berzins DW, Charlton DG. Mineral trioxide aggregate material use in endodontic treatment: A review of the literature. Dent Mater. 2008;24(2):149–164. doi: 10.1016/j.dental.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25(3):197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 17.Huang TH, Shie MY, Kao CT, Ding SJ. The effect of setting accelerator on properties of mineral trioxide aggregate. J Endod. 2008;34(5):590–593. doi: 10.1016/j.joen.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 18.Sluyk SR, Moon PC, Hartwell GR. Evaluation of setting properties and retention characteristics of mineral trioxide aggregate when used as a furcation perforation repair material. J Endod. 1998;24(11):768–771. doi: 10.1016/S0099-2399(98)80171-4. [DOI] [PubMed] [Google Scholar]
- 19.Storm B, Eichmiller FC, Tordik PA, Goodell GG. Setting expansion of gray and white mineral trioxide aggregate and portland cement. J Endod. 2008;34(1):80–82. doi: 10.1016/j.joen.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri J. Hydration mechanisms of mineral trioxide aggregate. Int Endod J. 2007 Apr 24;40(6):462–470. doi: 10.1111/j.1365-2591.2007.01248.x. [DOI] [PubMed] [Google Scholar]
- 21.Kosmatka SH, Voigt G, Taylor P. Integrated materials and construction practices for concrete pavement: A state-of-the-practice manual[Internet] UNITED STATES: CTRE, IOWA STATE UNIVERSITY; 2006. Available from: http://www.cptechcenter.org/technical-library/documents/imcp/imcp_manual_october2007.pdf . [Google Scholar]
- 22.Camilleri J. Characterization and chemical activity of portland cement and two experimental cements with potential for use in dentistry. Int Endod J. 2008;41(9):791–799. doi: 10.1111/j.1365-2591.2008.01439.x. [DOI] [PubMed] [Google Scholar]
- 23.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25(5):787–793. doi: 10.1016/S0142-9612(03)00591-X. [DOI] [PubMed] [Google Scholar]
- 24.Camilleri J. Characterization of hydration products of mineral trioxide aggregate. Int Endod J. 2008 Feb 20;41(5):408–417. doi: 10.1111/j.1365-2591.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 25.Torabinejad M, Hong CU, Lee SJ, Monsef M, Pitt Ford TR. Investigation of mineral trioxide aggregate for root-end filling in dogs. J Endod. 1995;21(12):603–608. doi: 10.1016/S0099-2399(06)81112-X. [DOI] [PubMed] [Google Scholar]
- 26.Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of mta. J Endod. 2006;32(6):569–572. doi: 10.1016/j.joen.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and portland cement. J Endod. 2007;33(10):1235–1238. doi: 10.1016/j.joen.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 28.Huang C, Tay FR, Wei SH, Kei LH, Cheung GS, Pashley DH. Tensile strength and ultrastructure of a compomer and a composite in aqueous and non-aqueous storage media. Am J Dent. 2003;16 Spec No:82A–87A. [PubMed] [Google Scholar]
- 29.Hsieh SC, Teng NC, Lin YC, Lee PY, Ji DY, Chen CC, Ke ES, Lee SY, Yang JC. A novel accelerator for improving the handling properties of dental filling materials. J Endod. 2009;35(9):1292–1295. doi: 10.1016/j.joen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 30.Erdogan S, Erdogan T. Kimyasal katkı maddeleri ve tarihi geçmişleri.2.Yapılarda Kimyasal Katkılar Sempozyumu. New York: TMMOB KİMYA MÜHENDİSLERİ ODASI, TMMOB İNŞAAT MÜHENDİSLERİ ODASI; 2007. pp. 21–33. [Google Scholar]
- 31.Ji DY, Wu HD, Hsieh SC, Teng NC, Chen CC, Ke ES, Lin YC, Lee SY, Yang JC. Effects of a novel hydration accelerant on the biological and mechanical properties of white mineral trioxide aggregate. J Endod. 2011;37(6):851–855. doi: 10.1016/j.joen.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Ramachandran VS. Concrete admixtures handbook: Properties, science and technology. Cambridge University Press. 1996;pp:10–103. [Google Scholar]
- 33.Standard terminology relating to concrete and concrete aggregate. Annual Book of ASTM Standards. West Conshohocken, PA: ASTM; 2002. Available from: https://www.astm.org/Standards/C125.htm . [Google Scholar]
- 34.Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and ph of mineral trioxide aggregate and white portland cement with a radiopacifier. J Endod. 2009;35(4):550–554. doi: 10.1016/j.joen.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Islam I, Chng HK, Yap AU. X-ray diffraction analysis of mineral trioxide aggregate and portland cement. Int Endod J. 2006;39(3):220–225. doi: 10.1111/j.1365-2591.2006.01077.x. [DOI] [PubMed] [Google Scholar]
- 36.Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005;38(11):834–842. doi: 10.1111/j.1365-2591.2005.01028.x. [DOI] [PubMed] [Google Scholar]
- 37.de Almeida J, Felippe MC, Bortoluzzi EA, Teixeira CS, Felippe WT. Influence of the exposure of MTA with and without calcium chloride to phosphate-buffered saline on the push-out bond strength to dentine. Int Endod J. 2013 Aug 28;47(5):449–453. doi: 10.1111/iej.12168. [DOI] [PubMed] [Google Scholar]
- 38.Lea F, Hewlett P. pp. London,Arnold: 1998. Chemistry of cement and concrete; pp. 10–58. [Google Scholar]
- 39.Camilleri J. Hydration characteristics of calcium silicate cements with alternative radiopacifiers used as root-end filling materials. J Endod. 2010 Jan 25;36(3):502–508. doi: 10.1016/j.joen.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 40.Monteiro Bramante C, Demarchi AC, de Moraes IG, Bernadineli N, Garcia RB, Spangberg LS, Duarte MA. Presence of arsenic in different types of mta and white and gray portland cement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106(6):909–913. doi: 10.1016/j.tripleo.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 41.Rao A, Rao A, Shenoy R. Mineral trioxide aggregate--a review. J Clin Pediatr Dent. 2009;34(1):1–7. doi: 10.17796/jcpd.34.1.n1t0757815067g83. [DOI] [PubMed] [Google Scholar]
- 42.Hong ST, Bae KS, Baek SH, Kum KY, Lee W. Microleakage of accelerated mineral trioxide aggregate and portland cement in an in vitro apexification model. J Endod. 2008;34(1):56–58. doi: 10.1016/j.joen.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Justnes H, Nygaard EC. Technical calcium nitrate as set accelerator for cement at low-temperatures. Cement and Concrete Research. 1995;25(8):1766–1774. [Google Scholar]
- 44.Ding SJ, Kao CT, Shie MY, Hung C. Huang TH. The physical and cytological properties of white mta mixed with na2hpo4 as an accelerant. J Endod. 2008;34(6):748–751. doi: 10.1016/j.joen.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 45.Stowe TJ, Sedgley CM, Stowe B, Fenno JC. The effects of chlorhexidine gluconate (0.12%) on the antimicrobial properties of tooth-colored proroot mineral trioxide aggregate. J Endod. 2004;30(6):429–431. doi: 10.1097/00004770-200406000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Parirokh M, Asgary S, Eghbal MJ, Kakoei S, Samiee M. A comparative study of using a combination of calcium chloride and mineral trioxide aggregate as the pulp-capping agent on dogs' teeth. J Endod. 2011;37(6):786–788. doi: 10.1016/j.joen.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 47.Ber BS, Hatton JF, Stewart GP. Chemical modification of proroot mta to improve handling characteristics and decrease setting time. J Endod. 2007 Jul 19;33(10):1231–1234. doi: 10.1016/j.joen.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 48.Standard test method for time of setting of hydraulic-cement paste by gillmore needles. West Conshohocken, PA: ASTM; 2004. Available from: https://www.astm.org/DATABASE.CART/HISTORICAL/C266-04.htm . [Google Scholar]
- 49.Standard test methods for time of setting of hydraulic cement by vicat needle. West Conshohocken, PA: ASTM; 2008. p. C191. Available from: https://www.astm.org/Standards/C191.htm . [Google Scholar]
- 50.Grech L, Mallia B, Camilleri J. Investigation of the physical properties of tricalcium silicate cement-based root-end filling materials. Dent Mater. 2012 Nov 27;29(2) doi: 10.1016/j.dental.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 51.Vicatronic [Internet] Scotland, United Kingdom: Impact Test Equipment Ltd; 2011. Available from: http://www.impact-test.co.uk/docs/CE350_HB.pdf . [Google Scholar]