Abstract
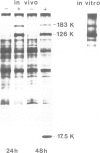
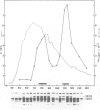
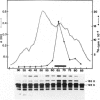
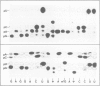
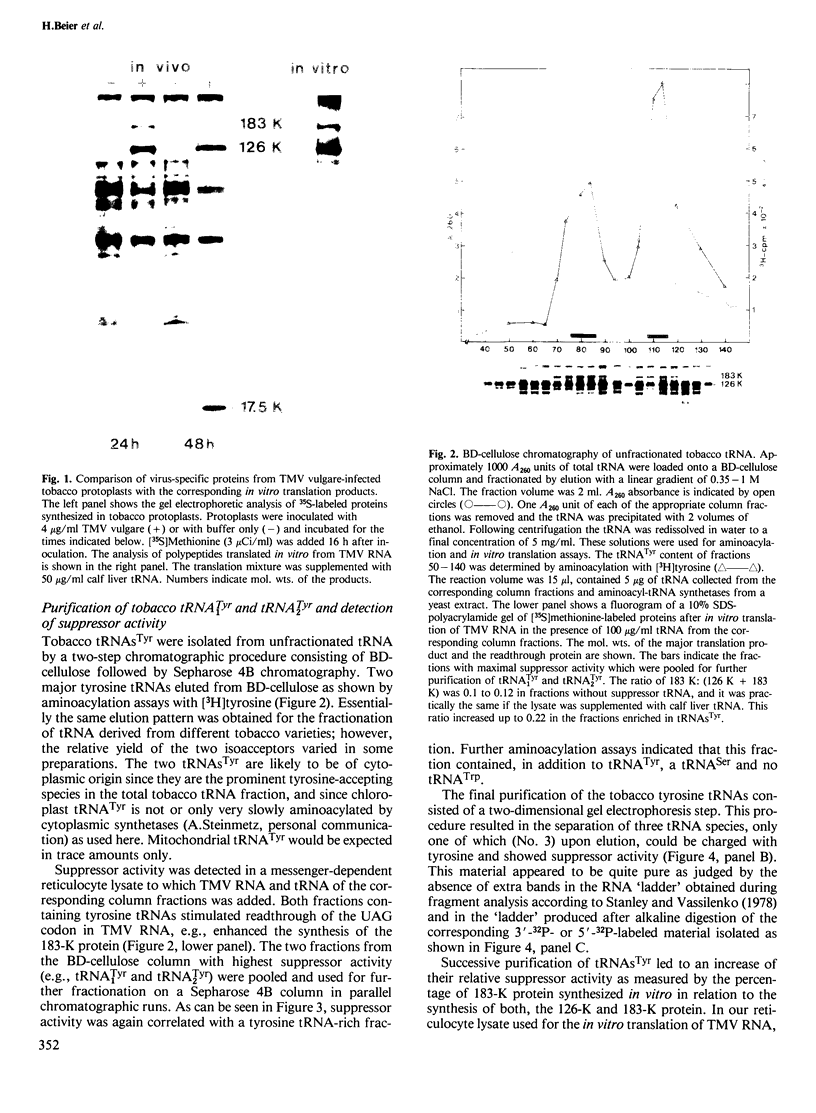
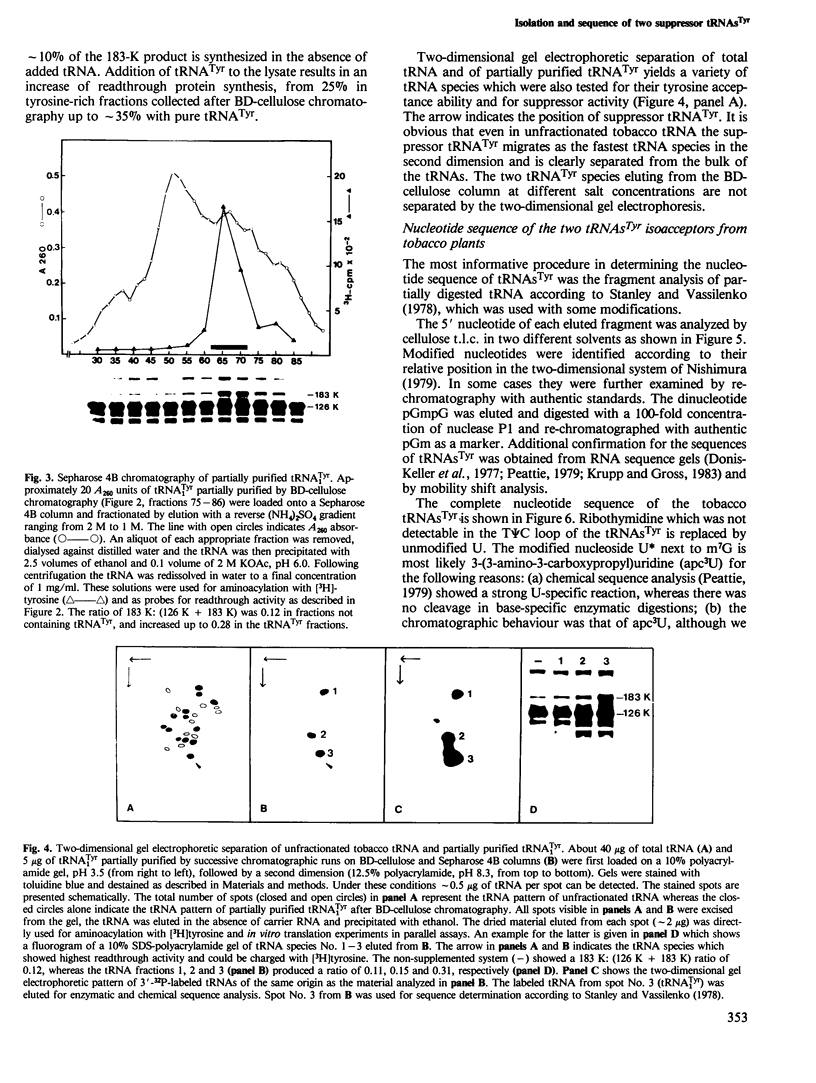
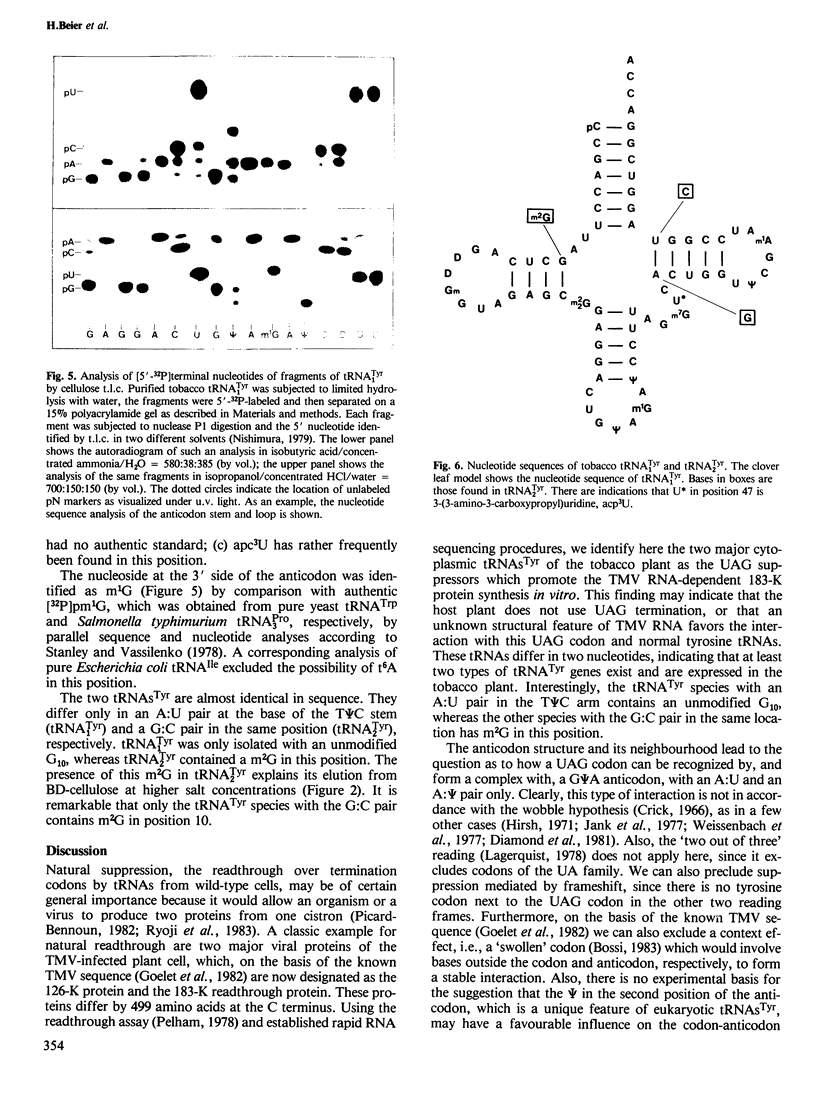
The hypothetical replicase or replicase subunit cistron in the 5'-proximal part of tobacco mosaic virus (TMV) RNA yields a major 126-K protein and a minor 183-K `readthrough' protein in vivo and in vitro. Two natural suppressor tRNAs were purified from uninfected tobacco plants on the basis of their ability to promote readthrough over the corresponding UAG termination codon in vitro. In a reticulocyte lysate the yield of 183-K readthrough protein increases from ˜10% in the absence of added tobacco plant tRNA up to ˜35% in the case of pure tRNATyr added. Their amino acid acceptance and anticodon sequence (GψA) identifies the two natural suppressor tRNAs as the two normal major cytoplasmic tyrosine-specific tRNAs. tRNATyr1 has an A:U pair at the base of the TψC stem and an unmodified G10, whereas tRNATyr2 contains a G:C pair in the corresponding location and m2G in position 10. This is the first case that, in a higher eukaryote, the complete structure is known of both the natural suppressor tRNAs and the corresponding viral RNA on which they exert their function. The corresponding codon-anticodon interaction, which is not in accordance with the wobble hypothesis, and the possible biological significance of the readthrough phenomenon is discussed.
Keywords: readthrough, tobacco mosaic virus (TMV), tobacco plant tRNAsTyr, translation, UAG suppression
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Zaitlin M. Characterization and in vitro translation of the RNAs from less-than-full-length, virus-related, nucleoprotein rods present in tobacco mosaic virus preparations. Virology. 1977 Aug;81(1):160–169. doi: 10.1016/0042-6822(77)90068-x. [DOI] [PubMed] [Google Scholar]
- Beier H., Mundry K. W., Issinger O. G. In vivo and in vitro translation of the RNAs of four tobamoviruses. Intervirology. 1980;14(5-6):292–299. doi: 10.1159/000149199. [DOI] [PubMed] [Google Scholar]
- Bossi L. Context effects: translation of UAG codon by suppressor tRNA is affected by the sequence following UAG in the message. J Mol Biol. 1983 Feb 15;164(1):73–87. doi: 10.1016/0022-2836(83)90088-8. [DOI] [PubMed] [Google Scholar]
- Bruening G., Beachy R. N., Scalla R., Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976 Jun;71(2):498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Diamond A., Dudock B., Hatfield D. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell. 1981 Aug;25(2):497–506. doi: 10.1016/0092-8674(81)90068-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Krupp G., Domdey H., Raba M., Jank P., Lossow C., Alberty H., Ramm K., Sänger H. L. Nucleotide sequence and secondary structure of citrus exocortis and chrysanthemum stunt viroid. Eur J Biochem. 1982 Jan;121(2):249–257. doi: 10.1111/j.1432-1033.1982.tb05779.x. [DOI] [PubMed] [Google Scholar]
- Gupta R. C., Randerath K. Use of specific endonuclease cleavage in RNA sequencing-an enzymic method for distinguishing between cytidine and uridine residues. Nucleic Acids Res. 1977 Oct;4(10):3441–3454. doi: 10.1093/nar/4.10.3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield D., Diamond A., Dudock B. Opal suppressor serine tRNAs from bovine liver form phosphoseryl-tRNA. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6215–6219. doi: 10.1073/pnas.79.20.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D. Tryptophan transfer RNA as the UGA suppressor. J Mol Biol. 1971 Jun 14;58(2):439–458. doi: 10.1016/0022-2836(71)90362-7. [DOI] [PubMed] [Google Scholar]
- Holmes W. M., Hurd R. E., Reid B. R., Rimerman R. A., Hatfield G. W. Separation of transfer ribonucleic acid by sepharose chromatography using reverse salt gradients. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1068–1071. doi: 10.1073/pnas.72.3.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jank P., Shindo-Okada N., Nishimura S., Gross H. J. Rabbit liver tRNA1Val:I. Primary structure and unusual codon recognition. Nucleic Acids Res. 1977 Jun;4(6):1999–2008. doi: 10.1093/nar/4.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Bambara R., Padmanabhan R., Wu R. DNA sequence analysis: a general, simple and rapid method for sequencing large oligodeoxyribonucleotide fragments by mapping. Nucleic Acids Res. 1974 Mar;1(3):331–353. doi: 10.1093/nar/1.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lagerkvist U. "Two out of three": an alternative method for codon reading. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1759–1762. doi: 10.1073/pnas.75.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Alzner-Deweerd B., Heckman J. E., MacGee J., Tabor M. W., RajBhandary U. L. Sequence analysis of 5'[32P] labeled mRNA and tRNA using polyacrylamide gel electrophoresis. Nucleic Acids Res. 1978 Jan;5(1):37–56. doi: 10.1093/nar/5.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E. C., Jr, Wills N., Arlinghaus R. B. Suppression of murine retrovirus polypeptide termination: effect of amber suppressor tRNA on the cell-free translation of Rauscher murine leukemia virus, Moloney murine leukemia virus, and Moloney murine sarcoma virus 124 RNA. J Virol. 1980 May;34(2):464–473. doi: 10.1128/jvi.34.2.464-473.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R., Knight C. A. Protein synthesis in tobacco protoplasts infected with tobacco mosaic virus. Virology. 1975 Mar;64(1):10–22. doi: 10.1016/0042-6822(75)90074-4. [DOI] [PubMed] [Google Scholar]
- Peattie D. A. Direct chemical method for sequencing RNA. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1760–1764. doi: 10.1073/pnas.76.4.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R. Leaky UAG termination codon in tobacco mosaic virus RNA. Nature. 1978 Mar 30;272(5652):469–471. doi: 10.1038/272469a0. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Picard-Bennoun M. Does translational ambiguity increase during cell differentiation? FEBS Lett. 1982 Nov 29;149(2):167–170. doi: 10.1016/0014-5793(82)81094-6. [DOI] [PubMed] [Google Scholar]
- Sakai F., Takebe I. Protein synthesis in tobacco mesophyll protoplasts induced by tobacco mosaic virus infection. Virology. 1974 Dec;62(2):426–433. doi: 10.1016/0042-6822(74)90404-8. [DOI] [PubMed] [Google Scholar]
- Siegel A., Hari V., Kolacz K. The effect of tobacco mosaic virus infection on host and virus-specific protein synthesis in protoplasts. Virology. 1978 Apr;85(2):494–503. doi: 10.1016/0042-6822(78)90456-7. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Tyc K., Kikuchi Y., Konarska M., Filipowicz W., Gross H. J. Ligation of endogenous tRNA 3' half molecules to their corresponding 5' halves via 2'-phosphomonoester,3',5'-phosphodiester bonds in extracts of Chlamydomonas. EMBO J. 1983;2(4):605–610. doi: 10.1002/j.1460-2075.1983.tb01470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. The nucleotide sequence of formylmethionine tRNA from Mycoplasma mycoides sp. capri. Nucleic Acids Res. 1978 Jan;5(1):57–70. doi: 10.1093/nar/5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Dirheimer G., Falcoff R., Sanceau J., Falcoff E. Yeast tRNALeu (anticodon U--A--G) translates all six leucine codons in extracts from interferon treated cells. FEBS Lett. 1977 Oct 1;82(1):71–76. doi: 10.1016/0014-5793(77)80888-0. [DOI] [PubMed] [Google Scholar]
- Zaitlin M., Duda C. T., Petti M. A. Replication of tobacco mosaic virus. V. Properties of the bound and solubilized replicase. Virology. 1973 Jun;53(2):300–311. doi: 10.1016/0042-6822(73)90207-9. [DOI] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. A gel electrophoretic analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology. 1972 Feb;47(2):296–305. doi: 10.1016/0042-6822(72)90265-6. [DOI] [PubMed] [Google Scholar]