Abstract
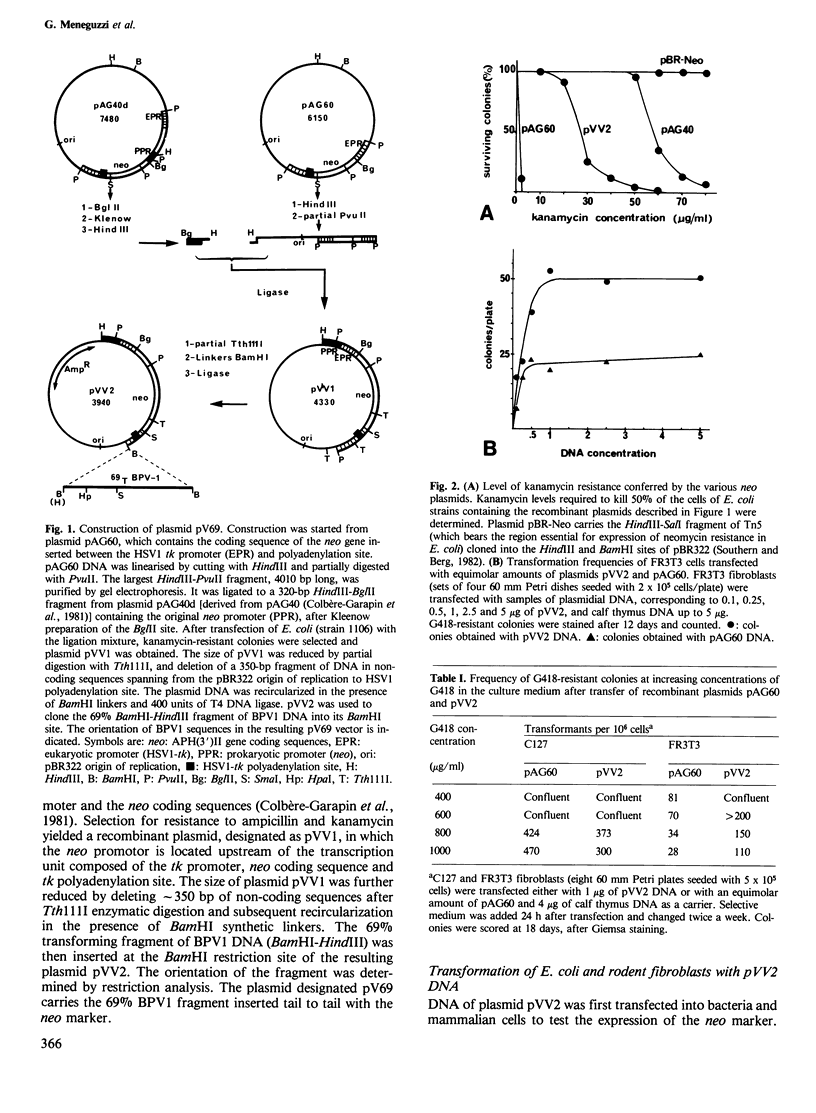
A recombinant plasmid was constructed (pV69) which comprises a subgenomic fragment of bovine papilloma virus type 1 (BPV1) DNA, part of plasmid pBR322 DNA and a drug resistance gene expressed in both mammalian fibroblasts and Escherichia coli. This gene (vv2) is a modified form of the bacterial neomycin resistance gene (neo) linked to the herpes simplex virus thymidine kinase (tk) promoter (plasmid pAG60), to which the original bacterial neo promoter from transposon Tn5 was added back, upstream of the eukaryotic promoter. It induced kanamycin resistance in E. coli, as well as resistance to the drug G418 in rat and mouse fibroblasts. Its expression in FR3T3 rat cells was enhanced as compared with the original tk-neo construction. After transfer of plasmid pV69 into C127 mouse cells or FR3T3 rat cells, the number of resistant colonies selected in medium containing G418 was one to two orders of magnitude higher than that of transformed foci in normal medium. In eight independent cell lines selected by drug resistance, pV69 DNA was found to be maintained in a plasmidial state, without any detectable rearrangement or deletion and could be transferred back in E. coli. In contrast, cell lines selected by focus formation in normal medium maintained deleted forms of the original plasmid DNA, and only part of them were resistant to G418. Most of the drug-resistant clones had kept the morphology and growth control of the normal fibroblasts. However, with further passages in culture, these cells spontaneously produced transformed foci with increasing frequencies.
Full text
PDF
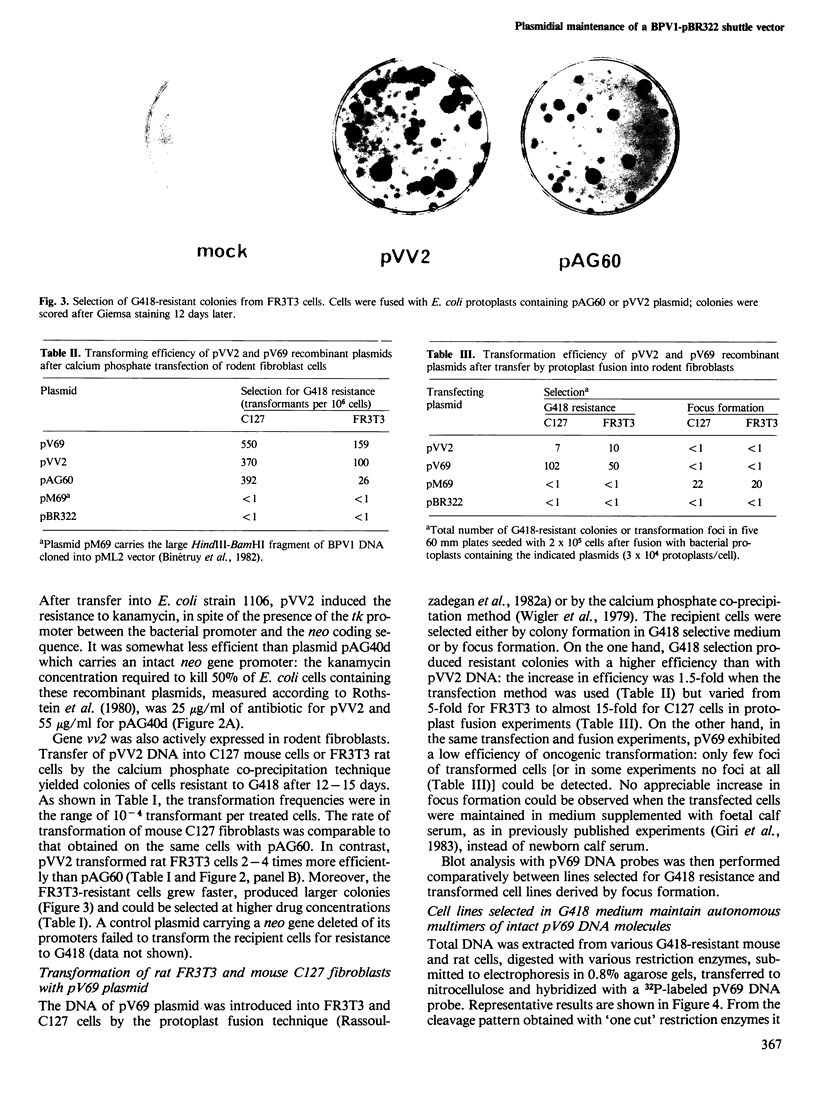
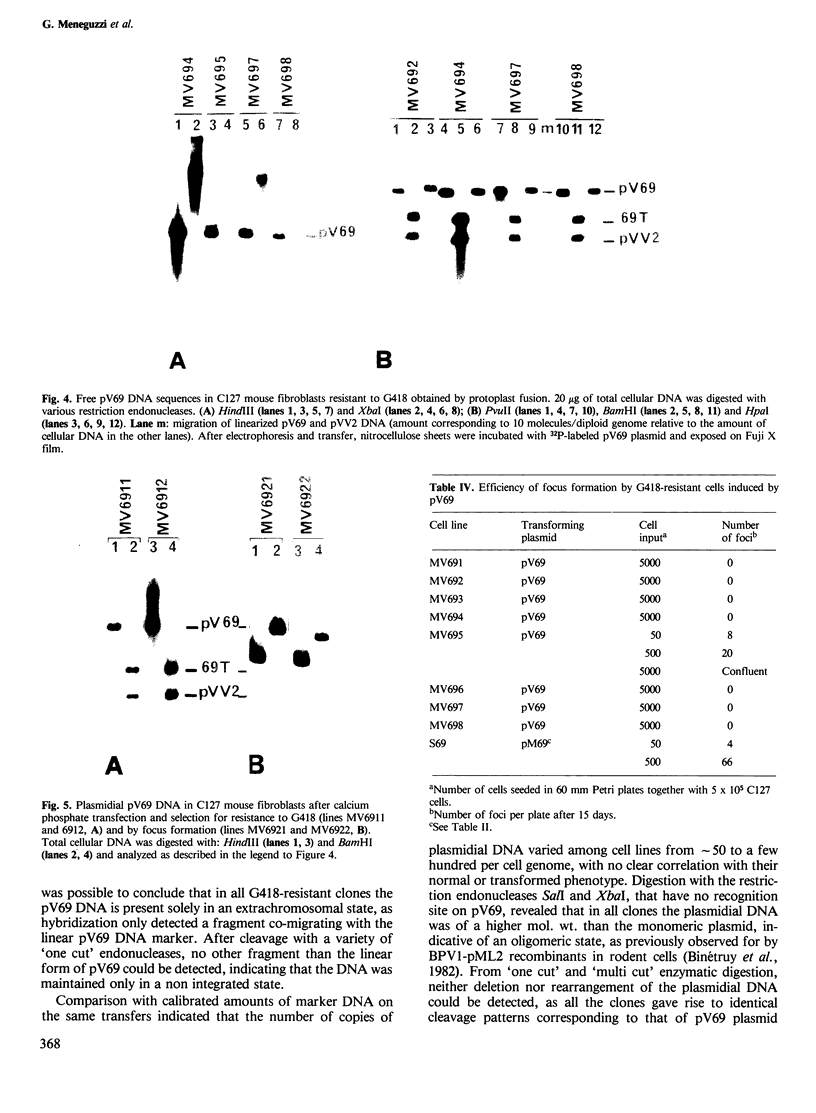



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binétruy B., Meneguzzi G., Breathnach R., Cuzin F. Recombinant DNA molecules comprising bovine papilloma virus type 1 DNA linked to plasmid DNA are maintained in a plasmidial state both in rodent fibroblasts and in bacterial cells. EMBO J. 1982;1(5):621–628. doi: 10.1002/j.1460-2075.1982.tb01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbère-Garapin F., Horodniceanu F., Kourilsky P., Garapin A. C. A new dominant hybrid selective marker for higher eukaryotic cells. J Mol Biol. 1981 Jul 25;150(1):1–14. doi: 10.1016/0022-2836(81)90321-1. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri I., Jouanneau J., Yaniv M. Comparative studies of the expression of linked Escherichia coli gpt gene and BPV-1 DNAs in transfected cells. Virology. 1983 Jun;127(2):385–396. doi: 10.1016/0042-6822(83)90152-6. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Lane D., Lipsich L., Wigler M., Botchan M. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell. 1980 Aug;21(1):127–139. doi: 10.1016/0092-8674(80)90120-8. [DOI] [PubMed] [Google Scholar]
- Heilman C. A., Engel L., Lowy D. R., Howley P. M. Virus-specific transcription in bovine papillomavirus-transformed mouse cells. Virology. 1982 May;119(1):22–34. doi: 10.1016/0042-6822(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Jarrett W. F., McNeil P. E., Grimshaw W. T., Selman I. E., McIntyre W. I. High incidence area of cattle cancer with a possible interaction between an environmental carcinogen and a papilloma virus. Nature. 1978 Jul 20;274(5668):215–217. doi: 10.1038/274215a0. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Levinson B. B., Goodman H. M. A plasmid that replicates in both mouse and E. coli cells. J Mol Appl Genet. 1982;1(6):527–538. [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Scolnick E. M. Helper-independent transformation by unintegrated Harvey sarcoma virus DNA. J Virol. 1978 May;26(2):291–298. doi: 10.1128/jvi.26.2.291-298.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P. D., Bernard H. U., Scott A., Brady G., Hashimoto-Gotoh T., Schütz G. A bovine papilloma virus vector with a dominant resistance marker replicates extrachromosomally in mouse and E. coli cells. EMBO J. 1983;2(9):1487–1492. doi: 10.1002/j.1460-2075.1983.tb01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneguzzi G., Chenciner N., Corallini A., Grossi M. P., Barbanti-Brodano G., Milanesi G. The arrangement of integrated viral DNA is different in BK virus-transformed mouse and hamster cells. Virology. 1981 May;111(1):139–153. doi: 10.1016/0042-6822(81)90660-7. [DOI] [PubMed] [Google Scholar]
- Mitrani-Rosenbaum S., Maroteaux L., Mory Y., Revel M., Howley P. M. Inducible expression of the human interferon beta 1 gene linked to a bovine papilloma virus DNA vector and maintained extrachromosomally in mouse cells. Mol Cell Biol. 1983 Feb;3(2):233–240. doi: 10.1128/mcb.3.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Binetruy B., Cuzin F. High frequency of gene transfer after fusion between bacteria and eukaryotic cells. Nature. 1982 Jan 21;295(5846):257–259. doi: 10.1038/295257a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Sarver N., Byrne J. C., Howley P. M. Transformation and replication in mouse cells of a bovine papillomavirus--pML2 plasmid vector that can be rescued in bacteria. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7147–7151. doi: 10.1073/pnas.79.23.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver N., Gruss P., Law M. F., Khoury G., Howley P. M. Bovine papilloma virus deoxyribonucleic acid: a novel eucaryotic cloning vector. Mol Cell Biol. 1981 Jun;1(6):486–496. doi: 10.1128/mcb.1.6.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- van den Elsen P., de Pater S., Houweling A., van der Veer J., van der Eb A. The relationship between region E1a and E1b of human adenoviruses in cell transformation. Gene. 1982 May;18(2):175–185. doi: 10.1016/0378-1119(82)90115-9. [DOI] [PubMed] [Google Scholar]