Abstract
Purpose:
The discoloration of acrylic resin denture bases may lead to significant esthetic problems. The aim of this in vitro study was to investigate the effects of frequently consumed drinks on the color changes of fresh and aged, heat-polymerized, conventional acrylic resin.
Materials and Methods:
Eighty-four, heat-polymerized acrylic resin specimens (4 mm x 5 mm x 30 mm) were fabricated. Half of the specimens were aged by thermal cycling (between 5°C and 55°C, 60-second dwell time, 3000 cycles). The specimens were stored at 37°C in different drinks as non-aged and aged subgroups including water (control group), black tea, green tea, sour cherry juice, coke and coffee (n=7). The discoloration of each specimen after 1 and 7 days storage in the drinks were measured by a colorimeter based on CIE Lab system. The data of colour differences (ΔE) were analyzed by ANOVA and Dunnet’s tests.
Results:
Thermal cycling and storage in water induced a slight color change. The highest ΔE values were observed in the aged groups, which was also noticeable for black tea and sour cherry juice after 7 days of storage (ΔE>1.5) (p<0.05). The ΔE values of all test groups were detected within the acceptable clinical limits (ΔE<3.5).
Conclusion:
These results suggest that the color stability of denture base acrylic resins is influenced by ageing. Black tea, sour cherry juice and coke can cause significant discolorations on acrylic resin denture bases.
Keywords: Acrylic resin, denture base, discoloration, drinks, thermocycling
Introduction
Acrylic resin denture bases should be color-stable, matching the natural appearance of the intraoral soft tissues. However, acrylic resins are known to undergo color changes because of water sorption over time, which can be affected by a number of factors such as the polymerization process or the surface roughness of the denture base as well as oral hygiene or the consumption of colorant foods and beverages (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12). Considering the widespread use of heat-polymerized acrylic resins; including partial or total removable dentures, teeth- or implantsupported overdentures and implant-supported hybrid prosthesis, the discoloration caused by drinks can be highly important in terms of esthetics (5). In severe cases, the discoloration of acrylic resin may not be removed by only polishing or soaking in denture cleaners (6, 7). Thereby, the replacement of existing denture may even be required. In the current literature, there are few studies showing that drinks can cause significant discolorations on acrylic resin denture bases. However, the type and intensity of the drink, which determine the quantity of colorant and the level of discoloration, and the personal habits can alter this discoloration process. The combined effects of colorant drinks, foods, smoking, as well as the ageing of denture base, can result in severe esthetic problems (2, 5, 6, 9, 10, 12). Patients should be informed about the color changes of acrylic resin denture bases due to colorant foods and drinks to prevent esthetic problems in the long-term.
The aim of this study is therefore to detect the combined effects of ageing and staining drinks that are frequently consumed daily on the discoloration of conventional, heat-polymerized acrylic resin denture bases.
Materials and Methods
Specimen preparation
Eighty-four, rectangular blocks (4 mm x 5 mm x 30 mm) were fabricated using a heat-polymerized acrylic resin (Meliodent, Heraeus Kulzer, Senden, Germany). The conventional lost-wax and flasking technique was used for the preparation of the specimens. Briefly, polymethyl methacrylate dough (35 g powder: 14 ml liquid) was mixed according to the manufacturer’s instructions, and packed in the prepared stone molds in a flask for further processing. Then, the flask was placed in boiling water, and the heat source was switched off. After being kept in hot water for 15 minutes, the flask was boiled for 20 minutes, and left in water bath for cooling slowly to the room temperature. The specimens were then finished and polished by the same operator using acrylic burs, abrasive disks, SiC abrasive papers and a slurry of medium-coarse pumice (Kerr Corp, Orange, CA, USA) with a wet-cloth wheel, respectively. Half of the specimens were thermal cycled for 3000 cycles between 5°C and 55°C with 60-second dwell time. Then, the specimens were divided into 6 main groups together with their non-aged and aged subgroups according to the drink solution used as water (control), black tea, green tea, sour cherry juice, coke and coffee.
Black tea (Yellow Label Tea; Lipton, Rize, Turkey) and green tea (Green Tea, Lipton, Rize, Turkey) solutions were prepared by immersing 2 prefabricated doses (2 x 2 g) of tea into 300 mL of boiling distilled water for 10 minutes. Cola (Coca- Cola; Coca-Cola Co, Istanbul, Turkey) and sour cherry juice (Cappy Special; Coca-Cola Co, Istanbul, Turkey ) were stored at room temperature. Coffee with cream and sugar (Nescafe 3-in-1 coffee; Nestle, Vevey, Switzerland) was prepared according to the manufacturer’s suggested concentration. One package of the prefabricated coffee mixture (total 20 g) was dissolved in 300 mL of boiling distilled water.
Following baseline color measurements, each subgroup was immersed in the same respective storage solution. The specimens were marked from the bottom surfaces, and placed together into the containers with plastic lids by paying attention to avoid specimen-tospecimen contact. The containers were kept at 37 oC in dark until the color measurements were repeated.
Measurement of color values (L*, a*, b*)
The color values (L*, a*, b*) were measured using a colorimeter (CS-100; Minolta, Tokyo, Japan) after 1 and 7 days of storage. Each day of storing samples in test solutions is accepted to simulate 1 month consumption of respective beverages, assuming 3.2 cups of each drink is consumed every day and a cup is considered to be drunk in an average of 15 minutes. Before each measurement session, all specimens were removed from the storage solutions and rinsed in distilled water. Excess water on the surfaces was removed with tissue paper and the specimens were allowed to dry. The colorimeter was calibrated according to the manufacturer’s recommendations by using the supplied white calibration standard. The measurements were repeated 3 times for each specimen, and the mean values of the L*, a*, and b* data were calculated. The color differences (ΔE) in the 3-dimensional L*, a*, b* color space were calculated between the baseline and 1 day storage as well as the baseline and 7 day storage with the following formula; ΔE = [(ΔL*)2 + (Δa*)2 + (Δb*)2] ½.
A ΔE of ≤3.3 was considered clinically acceptable. Additionally, the ΔE values have been quantified by using the National Bureau of Standards (NBS) as expressed by the following formula11; NBS unit = ΔE x 0.92 (Table 1).
Table 1.
Color change quantification according to the National Bureau of Standard (NBS).
| Critical Marks of Color Difference | NBS Units |
|---|---|
| Trace | 0.0-0.5 |
| Slight | 0.5-1.5 |
| Noticeable | 1.5-3.0 |
| Appreciable | 3.0-6.0 |
| Much | 6.0-12.0 |
| Very much | >12.0 |
Statistical analysis
The data of ΔE and NBS values obtained after 1 day and 7 days storage in drink solutions were tested with Shapiro-Wilk tests for normality and equalvariance assumptions. Since these parameters were satisfied, the standard descriptive statistics including the mean and standard deviation were performed to describe the data quantitatively. One-way analysis of variance (ANOVA) and Tukey HSD tests were applied to compare the differences among groups. All statistical tests were performed with SPSS 22.0 (SPSS for Windows, SPSS Inc., Chicago, IL, USA) at p = 0.05.
Results
The mean ± standard deviation (SD) values of ΔE are shown in Figure 1. Color changes expressed in NBS units are shown in Table 2. Storage in water resulted in a slight discoloration, which was more intense for the aged subgroups compared to the non-aged ones. Slight discolorations were observed in the all groups following 1 day storage in drinks (p>0.05). In the aged subgroups, higher ΔE values were detected in general compared to the non-aged, respective subgroups; especially in the groups of black tea, sour cherry juice and coke after 7 days storage.
Figure 1.
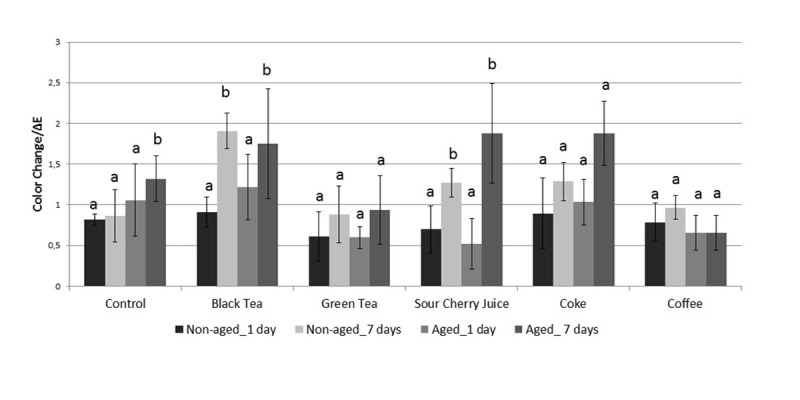
Bar graphic representation of the mean color difference values (ΔE) with standard deviations (SD). The same lower letter indicates no significant difference among the subgroups stored in the same drink solution (p>0.05).
Table 2.
NBS values of the heat-polymerized acrylic resin stored in drinks. The groups showing significant difference were marked with an asterisk (*) (p<0.05).
| Drinks | Non-aged Groups | Aged Groups | ||
|---|---|---|---|---|
| 1 day | 7 days | 1 day | 7 days | |
| Water | 0.75 | 0.79 | 0.97 | 1.21 |
| Black tea | 0.83 | 1.75* | 1.12 | 1.61* |
| Green tea | 0.56 | 0.81 | 0.54 | 0.86 |
| Sour cherry juice | 0.64 | 1.17 | 0.47 | 1.72* |
| Coke | 0.82 | 1.18 | 0.95 | 1.72* |
| Coffee | 0.72 | 0.88 | 0.60 | 0.60 |
There was no significant discoloration in the subgroups of coffee solution (p>0.05). Black tea and sour cherry juice showed noticeable color shifts after 7 days of storage (ΔE=1.5-3), even though the discoloration rates were clinically acceptable (ΔE<3.5). However, the ΔE values were significantly higher than the baseline in the both non-aged and aged subgroups of these drinks after 7 days storage (p<0.05).
Discussion
Eclairage (CIE Lab) system is recommended by the American Dental Association (ADA) as an accurate and repeatable method for the quantitative differentiation of color evaluation. In this system, L* refers to the lightness coordinate, ranging from 0 (black) to 100 (white), and the* and b* are chromaticity coordinates in the red-green axis and the yellow-blue axis, respectively. In the assessments, ΔE=0 indicates no color change in principle, which is regarded as a completely color stable material. A ΔE value of 3.5 or less is considered to be visually imperceptible as well as clinically acceptable (12, 13). This study analyzed the discoloration effects of several drinks, which are frequently consumed on daily basis, on the non-aged and aged, heat-polymerized acrylic resin surfaces by using colorimetry. In addition to the ΔE analysis, the findings were also presented via NBS Units to objectively differentiate the rate of discoloration.
Storage in water caused slight discoloration on both non-aged and aged acrylic surfaces and the intensity of the discoloration was higher for the aged groups. The whitening in the color of acrylic resins due to water sorption has been previously reported by Chandu et al. (3) and Devlin and Kaushik (14). As expected, the aged groups in general demonstrated higher discoloration compared to the non-aged ones, which can also be interpreted as an additional effects of water sorption. Even though it was not on the noticeable level to human eyes, the present study confirmed that water sorption could be considered as a factor affecting the final color of polymerized acrylic resins. In addition, the increase in the L*a*b* values might also be related with the loss of surface gloss (8).
Consistent with the study of Keyf and Etikan (8), black tea caused a noticeable discoloration of on both its non-aged and aged subgroups. Moreover, only black tea showed a significant discoloration compared to the baseline color after 1 day storage. The color change was also noticeable for the aged specimens. In addition, sour cherry juice and coke resulted in a similar color change for the aged subgroups after 7 days of storage, which were in line with the similar studies conducted on acrylic resin denture teeth and denture bases. In accordance with Sepúlveda-Navarro et al. (12), sour cherry juice and coke induced a discoloration as much as black tea in the aged subgroup.
Coffee is known as an intense colorant for dental restoratives (5, 6, 8, 9). Coffee mixture used in the study was the prefabricated packages containing coffee, cream and sugar. In a previous study, sugar has been shown to generate further discoloration most probably by enhancing the adhesion of the colorant molecules to the restoration surface (15). However, in this study, coffee with cream and sugar showed the least discoloration especially in the aged subgroups after 7 days of storage. This might be due to the whitener feature of the creamer. A different coffee solution without creamer could have led to a significant discoloration considering the previously reported discoloration effect of instant coffee drinks with or without sugar (9, 10). Therefore, instant coffee cannot be excluded as a denture colorant based on the findings of this study. In addition, simple measures such as drinking water or rinsing dentures following a cup of staining drink may also lessen these discolorations. Further studies can provide more information about the color changes of acrylic resins due to the various coffee consumption habits.
Porosity caused by improper mixing, monomer contraction or vaporization during polymerization, and the high level of residual monomer can decrease the discoloration resistance of acrylic resins by increasing water sorption together with the reduced mechanical properties (16). It has been reported that excessive heat applied in the beginning of a polymerization cycle could lead to external porosity while internal porosity could be seen if too much heat was applied at the end of polymerization reaction (17). Monomer conversion can ideally occur at about 70 oC. The temperature of the acrylic resin dough can reach up to 100oC since the polymerization itself is an exothermic reaction. The higher temperatures may also cause gaseous porosity by boiling the monomer. The instructed polymerization cycle for the selected acrylic resin material used in this study was quite different than the other polymerization strategies known as short and long curing cycles. However, considering the ideal temperature range required during the polymerization process, it can be asserted that the influence of polymerization-related factors on the discoloration resistance was kept at a minimum level in the present study. Biomechanical and esthetic properties of acrylic resins have been well-proven, even though several aspects still need to be improved such as the polymerization characteristics, dimensional changes due to water sorption and antibacterial properties (18, 19). This study investigated the discoloration effects of several staining drinks on a heat-activated polymethylmethacrylate denture base polymerized with conventional flasking and water-bath method. Different fabrication techniques such as microwave polymerization with compression- or injectionmolding and different polymers such as acetal resin, thermoplastic nylon and thermoplastic acrylic resins could have shown different discoloration trends. Moreover, these staining drinks could affect the hardness and surface roughness of the denture bases, which was not within the scope of this study. More studies are also needed to clarify the effects of colorant drinks on the surface characteristics of acrylic resin-based denture bases.
Conclusion
Within the limitations imposed on this study, all tested drinks induced discoloration on the heatpolymerized acrylic resin surface. Water sorption and ageing also affected the color stability of acrylic resin denture base.
Footnotes
Source of funding: None declared.
Conflict of interest: None declared.
This study has been presented as a poster at the 5th Annual Congress of European Prosthodontics Association, Abstract No: 59, Sep 29-Oct 01, 2011, Bern, Switzerland.
References
- 1.Assuncao WG, Barao VA, Pita MS, Goiato MC. Effect of polymerization methods and thermal cycling on color stability of acrylic resin denture teeth. J Prosthet Dent. 2009;102(6):385–392. doi: 10.1016/S0022-3913(09)60200-6. [DOI] [PubMed] [Google Scholar]
- 2.Ayaz EA, Altintas SH, Turgut S. Effects of cigarette smoke and denture cleaners on the surface roughness and color stability of different denture teeth. J Prosthet Dent. 2014;112(2):241–248. doi: 10.1016/j.prosdent.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Chandu GS, Asnani P, Gupta S, Faisal Khan M. Comparative evaluation of effect of water sorption on the surface properties of heat cure acrylic: An in vitro study. J Int Oral Health. 2015;7(4):63–68. [PMC free article] [PubMed] [Google Scholar]
- 4.Goiato MC, Dos Santos DM, Baptista GT, Moreno A, Andreotti AM, Bannwart LC, Dekon SF. Effect of thermal cycling and disinfection on colour stability of denture base acrylic resin. Gerodontology. 2012;30(4):276–282. doi: 10.1111/j.1741-2358.2012.00676.x. [DOI] [PubMed] [Google Scholar]
- 5.Gregorius WC, Kattadiyil MT, Goodacre CJ, Roggenkamp CL, Powers JM, Paravina RD. Effects of ageing and staining on color of acrylic resin denture teeth. J Dent. 2012;40 Suppl 2 doi: 10.1016/j.jdent.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Hollis S, Eisenbeisz E, Versluis A. Color stability of denture resins after staining and exposure to cleansing agents. J Prosthet Dent. 2015;114(5):709–714. doi: 10.1016/j.prosdent.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Hong G, Murata H, Li Y, Sadamori S, Hamada T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J Prosthet Dent. 2009;101(3):205–213. doi: 10.1016/S0022-3913(09)60032-9. [DOI] [PubMed] [Google Scholar]
- 8.Keyf F, Etikan I. Evaluation of gloss changes of two denture acrylic resin materials in four different beverages. Dent Mater. 2004;20(3):244–251. doi: 10.1016/S0109-5641(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 9.Koksal T, Dikbas I. Color stability of different denture teeth materials against various staining agents. Dent Mater J. 2008;27(1):139–144. doi: 10.4012/dmj.27.139. [DOI] [PubMed] [Google Scholar]
- 10.Oguz S, Mutluay MM, Dogan OM, Bek B. Color change evaluation of denture soft lining materials in coffee and tea. Dent Mater J. 2007;26(2):209–216. doi: 10.4012/dmj.26.209. [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Lin H, Huang Q, Zheng G. Determining color difference thresholds in denture base acrylic resin. J Prosthet Dent. 2015;114(5):702–708. doi: 10.1016/j.prosdent.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Sepulveda-Navarro WF, Arana-Correa BE, Borges CP, Jorge JH, Urban VM, Campanha NH. Color stability of resins and nylon as denture base material in beverages. J Prosthodont. 2011;20(8):632–638. doi: 10.1111/j.1532-849X.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- 13.Moon A, Powers JM, Kiat-Amnuay S. Color stability of denture teeth and acrylic base resin subjected daily to various consumer cleansers. J Esthet Restor Dent. 2014;26(4):247–255. doi: 10.1111/jerd.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devlin H, Kaushik P. The effect of water sorption on acrylic surface properties. J Prosthodont. 2005;14(4):233–238. doi: 10.1111/j.1532-849X.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 15.Guler AU, Yilmaz F, Kulunk T, Guler E, Kurt S. Effects of different drinks on stainability of resin composite provisional restorative materials. J Prosthet Dent. 2005;94(2):118–124. doi: 10.1016/j.prosdent.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Compagnoni MA, Barbosa DB, de Souza Raphael F, Pero AC. The effect of polymerization cycles on porosity of microwave-processed denture base resin. J Prosthet Dent. 2004;91(3):281–285. doi: 10.1016/S0022391304000083. [DOI] [PubMed] [Google Scholar]
- 17.Barbosa DB, de Souza RF, Pero AC, Marra J, Compagnoni MA. Flexural strength of acrylic resins polymerized by different cycles. J Appl Oral Sci. 2007;15(5):424–428. doi: 10.1590/S1678-77572007000500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Haddad A, Vahid Roudsari R, Satterthwaite JD. Fracture toughness of heat cured denture base acrylic resin modified with chlorhexidine and fluconazole as bioactive compounds. J Dent. 2014;42(2):180–184. doi: 10.1016/j.jdent.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 19.de Castro DT, Valente ML, Agnelli JA, Lovato da Silva CH, Watanabe E, Siqueira RL, Alves OL, Holtz RD, dos Reis AC. In vitro study of the antibacterial properties and impact strength of dental acrylic resins modified with a nanomaterial. J Prosthet Dent. 2015;115(2):238–246. doi: 10.1016/j.prosdent.2015.09.003. [DOI] [PubMed] [Google Scholar]


