Abstract
Stress is a precipitating factor for anxiety-related disorders, which are among the leading forms of psychiatric illness and impairment in the modern world. Rodent-based behavioral tests and models are widely used to understand the mechanisms by which stress triggers anxiety-related behaviors and to identify new treatments for anxiety-related disorders. Although substantial progress has been made and many of the key neural circuits and molecular pathways mediating stress responsiveness have been characterized, these advances have thus far failed to translate into fundamentally new treatments that are safer and more efficacious in humans. The purpose of this article is to describe methods that have been historically used for this type of research and to highlight new approaches that align with recent conceptualizations of disease symptomatology and that may ultimately prove to be more fruitful in facilitating the development of improved therapeutics.
Keywords: rodent model, anxiety, human, translational behavioral test
Abstract
El estrés es un factor precipitante para los trastornos relacionados con la ansiedad, los cuales están entre las principales formas de enfermedad psiquiátrica y discapacidad en el mundo moderno. Los modelos y las pruebas conductuales basadas en roedores son ampliamente empleadas para la comprensión de los mecanismos mediante los cuales el estrés gatilla conductas relacionadas con la ansiedad y para identificar nuevos tratamientos para los trastornos relacionados con la ansiedad. Aunque ha habido un significativo progreso y se han caracterizado muchos de los circuitos neurales y vías moleculares clave que median la respuesta de estrés, estos avances han sido insuficientes para traducirse en tratamientos fundamentalmente nuevos que sean más seguros y más eficaces en humanos. El propósito de este artículo es describir los métodos que se han empleado históricamente en este tipo de investigación y destacar los nuevos enfoques que sean concordantes con las conceptualizaciones recientes de la sintomatología de la enfermedad, susceptibles de ser finalmente los más fructíferos para facilitar el desarrollo de los mejores tratamientos.
Abstract
Le stress est un facteur précipitant des troubles liés à l'anxiété qui représentent la majorité des maladies et du handicap psychiatriques du monde moderne. Des modèles et des tests comportementaux sont largement utilisés chez les rongeurs pour comprendre les mécanismes par lesquels le stress déclenche des comportements anxieux et pour identifier de nouveaux traitements des troubles liés à l'anxiété. Des progrès importants ont été réalisés et de nombreux circuits neuronaux et voies moléculaires clés véhiculant la réactivité au stress ont été caractérisés, mais ces avancées n'ont pas, jusqu'à présent, réussi à se traduire en nouveaux traitements réellement plus sûrs et plus efficaces chez l'homme. Cet article a pour but de décrire les méthodes historiques utilisées pour ce type de recherche et de souligner les nouvelles approches concordant avec les conceptions récentes de la symptomatologie de la maladie susceptibles d'être finalement plus fructueuses pour faciliter le développement de meilleurs traitements.
Introduction
The use of animals such as rats and mice for research on the causes of, and treatments for, psychiatric illness is challenging: signs and symptoms of such conditions often reflect motivations, emotions, and thought processes not realistically attributable to these species. Laboratory studies of anxiety using rodents generally focus on ethologically relevant behavioral paradigms, particularly for assessing the efficacy of drugs used to treat anxiety in humans. Often described as “models,” many of these approaches are better conceptualized as “assays” designed to identify new chemicals or manipulations that produce effects resembling those of standard antianxiety (anxiolytic) medications (eg, benzodiazepines). Indeed, behavioral assays that identify effective therapeutic treatments in rodents tend to have few characteristics directly applicable to human populations or to require anthropomorphic projection of complex human traits. This can lead to data overinterpretation, eg, describing a mouse that spends less time on the open arm of a maze as being “anxious” rather than (more appropriately) as expressing an “anxiety-like” behavior. Frequently, treatments that produce effects opposite to those of standard anxiolytics are considered “anxiogenic.” For such reasons, the broad utility of animal models in psychiatric drug discovery efforts has been questioned.1 With the advent of Research Domain Criteria (RDoC) principles, which focus on domains (signs and symptoms) that span multiple disorders,2 more and more preclinical studies of the neurobiology of anxiety focus on behavioral and physiological measures that can also be studied in humans.
Here, we briefly summarize classical anxiety assays and putative preclinical models of anxiety-related disorders. We then describe new investigational directions in the search for more translational behavioral tests that can facilitate studies that better predict clinical outcomes in humans, improve the success of clinical trials, and hasten the development of novel therapeutics.
Classical tests of anxiety-like behavior
Anxiety and fear produce similar behavioral responses, including increased vigilance, freezing and/or hypoactivity, elevated heart rate, and suppressed food consumption.3 Many comprehensive review articles describe the brain regions that mediate fear- versus anxiety-like behavioral responses.4,5 “Anxiety-like” behaviors—so-called because they resemble anxiety behaviors in humans, though in rodents they might actually represent something else entirely—are defined as those elicited by aversive stimuli that are diffuse, unpredictable, distal, or of long duration.3,6,7 Extensive examination of brain areas that mediate fear versus anxiety suggest that the amygdala mediates fear-like behaviors to short, discrete, and proximal aversive cues, whereas the bed nucleus of the stria terminalis (BNST) mediates anxiety-like or worry-like behaviors.7,8 These two brain areas regulate one another to coordinate activation of brain regions that mediate similar behavioral and physiological output. We provide a brief overview of various anxiety behavioral assays used in rodents; however, more comprehensive reviews are available.9
Approach-avoidant behaviors
Classical models of anxiety-like behavior in rodents focus on ethologically relevant assays to assess approach versus avoidance behavior, baseline vigilance, or defensive behaviors. Approach-avoidant paradigms exploit scenarios in which environmental stimuli may be perceived as threatening; latency to approach or time spent with a novel object (a potential threat) is measured and used as a putative indicator of anxiety. Rodents tend to prefer dark and enclosed spaces, which reduces risk of predation.10 The light-dark box assay consists of a box divided into two sections: a minimally lit side with black walls (dark side) and a brightly lit side with white walls (light side).10-12 Shorter latencies to enter and/or greater amounts of time spent in the light side of the box are interpreted as indicating less anxiety-like behavior or an anxiolytic-like effect. Similarly, the elevated plus maze (EPM) combines natural preferences for dark spaces and aversions to illuminated, open, and/or elevated areas. The EPM consists of two opposing enclosed arms and two nonenclosed (open) arms, shaped as a “plus sign” and elevated several feet above the ground13,14; latency to enter, time spent within, and number of entries into each arm type are used as proxies of anxiety levels, with greater amounts of time spent in the open arms interpreted as lower anxiety levels. The social interaction test can also assess approach-avoidant behavior.15,16 In this test, the “target” subject is first placed into an arena where no social partner is present, and the baseline amount of time that it spends in a zone that will later contain a social interaction partner is measured. Once a social partner is present, the latency and time spent with the partner is measured, and the ratio of the time spent in the zone with and without the social partner is used as an indicator of anxiety. Although social interaction tests are thought to assess traits other than anxiety (eg, rewarding effects of social interaction), anxiety-related states influence the amount of time a rodent interacts with a partner, as indicated by the prosocial effects of standard anxiolytics.17 The open field test is often used to assess both anxiety and locomotor activity; it involves an open box with anxiety levels inferred by the latency to enter and time spent in the center of the arena (where the subject would be hypothetically most vulnerable to predators). Finally, the novelty suppressed feeding (NSF)—also known as the hyponeophagia test—involves an open field and the latency to approach and consume a food item (eg, graham cracker). This test exploits the natural apprehension of rodents to consume novel foods. Mice or rats are placed in a novel environment (eg, a beaker, an open field) with a highly palatable food spread throughout. Different foods can be used, allowing repeated testing.18 A close variant, the novelty-induced hypophagia (NIH) test, involves exposing mice to sweetened condensed milk for several days and then assessing latency to consume it in a novel environment.19 In each assay, the test conditions can be altered to be more sensitive to manipulations that produce either anxiolytic- or anxiogenic-like effects, including lighting levels, habituation to the environment before testing, and background noise.16,20
Defensive behaviors
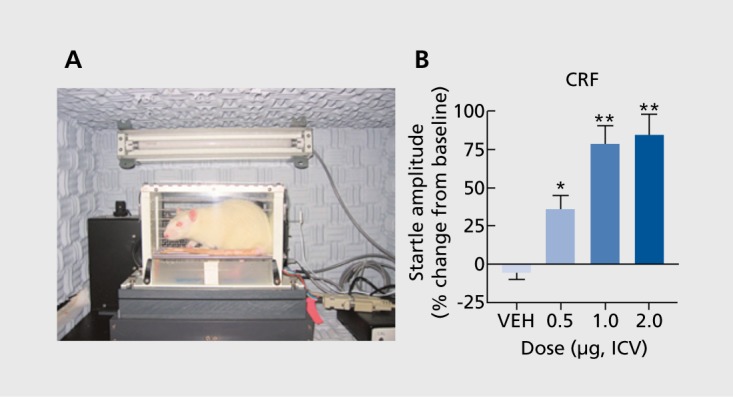
Other classical assays of anxiety quantify the ability of stimuli to elicit defensive behaviors. Rodents avoid—or show greater amounts of defensive behaviors in—environments containing cues that indicate the presence of a predator. Behaviors observed when the predator is present are thought to reflect fear; behaviors observed when only stimuli associated with the predator (eg, odor) are present are thought to reflect anxiety.21-24 Similarly, Pavlovian conditioning to a light/tone paired with a shock causes fear evoked by the light/tone. However, if the duration of light/tone cue presented before the shock is long, the behavior is thought to reflect anxiety.8,25 In general, the metrics used for these tests are acoustic startle (an active ”flinch-like“ response to a white noise burst, measurable in units of force26) or freezing behavior (a passive response where movement is minimized27). Acoustic startle has appeal because it is sensitive to treatments (eg, administration of the stress peptide corticotropin-releasing factor [CRF]) that also cause stress responses in humans (Figure 1) and because the same fundamental response can be studied across species. These behavioral paradigms are used extensively and provide insight into the neurobiological underpinnings of anxiety and fear.
Figure 1. Assessment of the acoustic startle reflex in rodents. (A) Sprague-Dawley rat in an apparatus that quantifies startle responses. Rats are placed within a nonconfining holder to minimize restraint stress. Startle response is induced by a burst of white noise delivered via a speaker located behind the rat. The force displaced is quantified by an accelerometer beneath the holder. (B) Acute effects of the stress-peptide corticotropin-releasing factor (CRF; administered intracerebroventricularly [ICV]) or vehicle only (VEH) on acoustic startle in 150-minute test sessions. Data are expressed as (%) change from pretreatment tests. CRF produces a dosedependent increase in startle. *P<0.05;**P<0.01 ; Tukey's t-tests.
Rodent models of anxiety-related disorders
Environmental manipulations across development can alter anxiety levels in rodents. Many such manipulations include exposing rodents to a form of stress and later measuring anxiety levels. In humans, stress can precipitate or exacerbate anxiety-related disorders; thus, stress has been extensively studied in rodents to understand the neurobiology underlying this effect. Unfortunately, few rodent stress paradigms used are immediately applicable to humans, although some are designed to mimic the variability, duration, and severity of stress encountered by humans. These paradigms involve various types of stressors, including footshock, hypothermia, forced swim, restraint, elevated pedestal, or oscillation stress, or combinations thereof. Stress duration varies between paradigms. Further, some designs may involve exposure to multiple stressors each day across multiple days, whereas others involve a single severe stressor (eg, 100 tailshocks over a 2-hour period). Here, we describe a few of the most commonly used stress paradigms.
Chronic mild stress
In the chronic mild stress (CMS) model, rodents are subjected to a series of unpredictable small stressors over a period of weeks. There are many variations of this model, involving different stressors and durations; it may be referred to by different names, including chronic unpredictable stress (CUS) and chronic variable stress (CVS), despite minimal procedural differences.28,29 In one variant, rodents are subjected to a combination of mild stressors, including overnight illumination, food deprivation, cage tilt, or change of cage mate over a period of weeks.28 Another variation involves footshock, oscillation stress, elevated pedestal stress, forced swim, and restraint stress.30 These approaches produce an array of behavioral changes, including the development of anxiety-like behaviors, and have been used as a model of depression because they can induce anhedonia (reduced sensitivity to reward) and respond to antidepressant treatment.28,29 This model's main limitation is that it can be difficult to replicate from lab to lab, or even within labs over time, for reasons that are not understood.31
Single prolonged stress
The single prolonged stress (SPS) paradigm was developed to model posttraumatic stress disorder (PTSD).32 In SPS, rodents are subjected to a sequence of severe stressors: 2 hours of restraint stress, followed by 20 minutes of forced swim, followed by exposure to ether until loss of consciousness. Typically, the animals are then left undisturbed for 1 week to allow the full development of symptoms. This procedure produces many neurochemical and behavioral characteristics resembling PTSD. For example, SPS-exposed rats have enhanced glucocorticoid negative feedback, increased contextual fear conditioning, and impaired fear extinction.33-35
Chronic social defeat stress
Chronic social defeat stress (CSDS) produces behavioral and physiological consequences relevant to psychiatric illnesses, including anxiety and depression. CSDS is suitable for rats36 or mice37; a typical regimen for mice consists of 10 days of exposure of an “intruder” to an aggressive “resident” for 10 minutes.38 The mice are then housed together separated by a Plexiglas barrier, allowing sensory, but not physical, contact. This procedure causes profound alterations in behavior in the intruder, including decreased exploratory behavior in the EPM and open field, increased latency to enter the light side of a light-dark box, reduced social interaction, and anhedonia.37,39,41 Some of these alterations persist after cessation of the stress regimen; others quickly recover.41 The primary advantages of this procedure are that it is an ethologically relevant stressor that takes advantage of a type of stress that a rodent may encounter in a natural environment and that the resulting behavioral phenotypes are sensitive to chronic but not acute administration of standard antidepressants.37 Despite these advantages, there are many limitations: most important, female rodents tend not to engage in the type of territorial aggression needed to produce the phenotype, suitability is limited to procedures that require tethering (eg, microdialysis, optogenetics), and there is risk of physical injury to animals in which heavy investments have been made.
Developmental models
Postnatal stress
In humans, childhood trauma is associated with anxiety disorders, depression, and other psychiatric disorders in adulthood. In rodents, early prenatal and postnatal experience can also cause lifelong alterations in responsiveness to stress.42-43 In the maternal separation model, pups are withheld from their mothers during the early postnatal period for various periods (from minutes to weeks.44). Maternal separation results in heightened anxiety-like behaviors in adulthood and hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis.44,45 However, divergent outcomes may result, dependent on the timing of the stress: maternal separation during postnatal days 3-4 (PND 3-4) causes a hyperresponsive HPA axis; separation during PND 11-12 causes the opposite effect (hyporesponsiveness).46 The quality of maternal care, such as maternal licking, grooming, and nursing, plays a protective role, dampening the stress responses during this early postnatal period. A naturalistic model of chronic early-life stress has been developed in which the mother and pups are placed in an impoverished cage environment with minimal bedding material and a single paper towel for nesting material from PND 2-9.47 This causes fragmented and erratic maternal care, resulting in offspring with heightened anxiety-like behavior and memory impairments in adulthood.48-50
Prenatal stress
Stress during maternal gestation can also have long-term behavioral consequences in the offspring. Prospective studies suggest that higher levels of maternal stress are associated with infants that are more irritable, greater levels of sleeping and feeding problems, and higher levels of emotional problems in childhood and adolescence.51,52 Rodent models of prenatal stress have been performed with a variety of parameters, but typically involve a stressor such as restraint or footshock applied one to three times a day, most often performed during the last postnatal week of pregnancy.53 Although results can be variable, reliable alterations include HPA-axis dysregulation and behavioral abnormalities, including heightened anxiety-like behaviors and anhedonia.53 Interestingly, a maternal immune activation model in which pregnant rodents are administered a viral and/or bacterial mimetic to induce an innate immune response can also produce heightened anxiety-like behaviors in the offspring.54,55 Considering the highly interconnected nature of the stress and the immune responses, similar mechanisms may underlie the full spectrum of effects seen in these models. Indeed, pairing prenatal immune activation with a second peripubertal stress markedly enhanced the effects.56
Considerations
Use of a chronic stress model requires important considerations. The first priority is to select a model that is robust and reproducible and has high construct validity (ie, based on the causative factors of the disease). Construct validity is often the most difficult to satisfy, but choosing a model where the stressor matches what is known about the etiology of the disorder (eg, postnatal stress to model a predisposition for anxiety-disorders in those with childhood trauma) is a desirable approach. The influence of sex as it relates to both the susceptibility to and outcomes of anxiety is emerging as an important area of study likely to intensify with recent guidelines by government funding agencies requiring incorporation of sex as a biological variable. Many anxiety-related disorders show differences in prevalence by sex (the biological term) or gender (the social term), with PTSD being almost twice as common in women as in men.57 Further, genetic variants have been discovered that cause a predisposition to PTSD only in women.58,59 It is important to consider that stress models may have different effects, depending on sex; this is especially true for CSDS, which is most effective in males, although variants have been described in female rats60 and mice.61 Additionally, besides a difference in the degree of stress response, behavioral adaptations in females may have different manifestations. For example, a novel active fear response called darting occurs almost exclusively in female rats.62 Historical factors, including the perception of higher variability due to estrous cycle, have hampered inclusion of female subjects, despite evidence to the contrary.63 Also, there can be substantial strain differences in stress responsiveness, eg, the difference between the C57BL/6 strain, which tends to be stress resilient, and the BALB/c strain, which tends to be stress susceptible.29,64 Strain differences, as well as genetic drift, have been found in stress-related behavioral responses, autonomic responses, HPA-axis responses, and physiological end points, such as the microstructure of sleep.64-67
New directions in behavioral analysis
The past decade has seen a rapid evolution of techniques useful for dissecting the neural circuitry of anxiety-related behavior. At the forefront has been the development of genetic tools, such as light-activated channel rhodopsins (for optogenetics) and chemicalactivated receptors (ie, designer receptors exclusively activated by designer drugs, or DREAADs) that allow control of neuronal activity with unprecedented specificity and precision. The development of mouse lines with promoter-driven expression in defined neuronal subsets will aid transformational advances in understanding of neuroanatomy at the level of genetically defined neural populations and circuits. Coinciding with this enhanced ability to manipulate the central nervous system (CNS) has been the development of new techniques that measure circuit function, including Ca2* imaging techniques that capture activity of large neuronal populations simultaneously.
Technical and conceptual innovations in behavioral and physiological end points have not advanced at a similar pace. Rather, there has been an increasing reliance on simplifying behavioral end points, which may be more amendable to dissection of the underlying neural circuits. The lack of advancement in modeling disease-relevant end points for psychiatric disorders may be the single greatest obstacle in translating new insights on CNS function into treatment of human disease. There are two major challenges facing researchers who use classical behavioral tests. First, they typically rely upon anthropomorphism—inferring how situations should affect the emotional state of the animal. This results in an inherent subjectivity that can make studies difficult to replicate, even among researchers working within the same lab. Second, behavioral tests performed in rodents do not often have direct correlates in humans, potentially limiting translational value. However, emerging approaches reflect attempts to overcome these limitations.
Comprehensive behavioral approaches
The recent advent of genomic, transcriptomic, and other “-omic” approaches has led to interest in new insights that large data sets may hold. A data-driven approach can be used, where new discoveries can emerge from data sets themselves; such approach is less reliant on theory-driven a priori hypotheses. Further, large data sets are amenable to modern analytic methods, such as machine learning, that offer discovery of new patterns and associations between variables. However, insights on how to apply these approaches to behavioral neuroscience are still nascent. The main obstacle is the sheer quantity of data. Whereas many classical behavioral tests offer limited amounts of data during a brief snapshot of time, new approaches can provide constant (24/7) data streams over long periods. To gain more data there are two main options: increasing the quantity of behavioral tests used (ie, multiple high-throughput behavioral assays run in parallel) or using new behavioral tests that individually produce vast quantities of data.
Several groups now use continuous and advanced behavioral tracking to extract more comprehensive behavioral phenotypes. One example is the development of proprietary, large-scale behavior-based systems (eg, SmartCube)68 that can screen large quantities of drug candidates in rodents by using custom hardware and continuous tracking that measures locomotion, trajectory complexity, and simple behavioral sequences, collecting upwards of 500 000 data points for each subject. The system then uses supervised machine-learning algorithms to generate specific behavioral signatures of different classes and subclasses of drugs, such as those with known anxiolytic or anxiogenic effects in humans. Other versions incorporate alternate components such as paw contact, body position, and limb movement (outcome parameters more relevant to many chronic pain paradigms), or social behavior, circadian, and cognitive behavior (more relevant to neuropsychiatric disease).68 Another example is the use of three-dimensional depth tracking techniques to fully characterize the activity of freely behaving mice.69 A machine-learning-based analysis is used to parcel out individual behavioral modules (eg, low rear, pause, walk) and their associated transition probabilities. A repertoire of behavioral motifs is generated that is akin to a language of body movements. As proof-of-principle, mutant (knockout) mice were compared with wild-type mice via this technique, and a new alteration in behavior (a combination of brief pauses and head bobs) was discovered.69 These systems may be able to uncover behavioral patterns that are impossible to detect solely by human observation but that are fundamental elements of rodent behavior.
A similar approach was recently used to examine behavioral patterns in mice after an acute stressor,70 a period that may include broad patterns of behaviors that facilitate the return to a baseline state. Mice were recorded for 15-minute periods in either naive or stress conditions, and the activity was broken down into eight distinct behavioral categories by a blinded observer. After a brief footshock, a distinct temporally organized behavioral pattern gradually emerged, consisting of increases in grooming, rearing, and walking behavior. This behavioral profile was highly sensitive to context, as different environments produced different sets of responses. However, optogenetic excitation of hypothalamic CRF neurons impaired regulation of behavior by these environmental cues. These data suggest that after stress, mice de-escalate their behaviors in a highly specific pattern that is influenced both by environment and the activity of hypothalamic CRF neurons.70 This type of approach has several advantages for studies of anxiety. Foremost, continuously capturing a wide array of behaviors and their temporal patterns is more likely to generate an ethologically relevant behavioral response to stress that does not rely on anthropomorphism. Additionally, video recordings in a home cage or similar environment are not inherently stressful, and thus do not introduce the potentially confounding influence of having the observer present or the stress evoked from the anxiety test itself (known as “observer effects”). These approaches may help to uncover previously elusive anxiety-related behaviors in rodents, including those relevant to anxiety that are spontaneous or in response to internal cues, which are characteristic of panic disorder and other anxiety disorders.71
Translational behavioral approaches
Many classical assays used to study anxiety rely upon ethologically relevant rodent behaviors and cannot be directly applied to humans, questioning the translational validity of such models. Consequently, there is increasing emphasis on end points that can be measured both in humans and laboratory animals. This approach will ultimately allow experiments to be performed across species and enhance use of preclinical data to predict clinical outcomes. Three such measures are described here: acoustic startle, decision-making, and sleep.
A noise burst elicits a startle response both in humans and laboratory animals.72 Startle is hypothesized to reflect vigilance, with higher startle magnitudes indicative of increased anxiety. It is well established that hypervigilance, reflected by elevations in the acoustic startle response, is a core feature of anxiety disorders, including PTSD.73,74 In rodents, startle is typically reflected by the force of a whole-body “flinch” response measured by an accelerometer located beneath a holding cage; in humans, it is typically reflected by an eyeblink response measured by an orbicularis oculi electromyogram (EMG). Manipulations including varying the intensity of lighting,75-79 exposure to stress or potential for aversive events before startle,30,78,80 or treatment with pharmacological agents77,81 can alter the magnitude of the startle reflex in humans and rodents.
Examining features of other conditions comorbid with anxiety disorders may offer new translational end points. For example, cognitive or decision-making tasks represent end points that may be aberrant in anxiety-related conditions. Cognitive impairment is a core feature of depressive disorders frequently comorbid with anxiety disorder.73 In fact, clinical depression has been associated with particularly poor performance after errors,82 which may reflect negative affect or a “catastrophic response to perceived failure.”83,84 The 5-choice serial reaction time task (5-CSRTT) quantifies attention in rodents and is analogous to the continuous performance task used to measure attention in humans.85 In this task, rats are placed in an apparatus and trained to detect the location of a stimulus light that is randomly presented in one of five apertures. Administration of stress peptides can decrease performance on this task; eg, intracerebroventricular (ICV) infusion of CRF produced dose-dependent decreases in correct responses, increases in omissions, and increases in correct response latencies.86 Similar detriments in performance were found after infusion of pituitary adenylate cyclase-activating polypeptide (PACAP) or a K-opioid agonist,87,88 both of which produce stress-like effects in rodents. The types of error-processing deficits seen in humans with depressive illness have many similarities with those seen in rodents treated with CRF.89
Sleep is also highly sensitive to chronic stress. Accumulating evidence indicates that sleep quality is altered across various types of psychiatric illness, especially those associated with anxiety. Most anxiety disorders are associated with increased sleep latency and sleep disruption.90 As a prominent example, PTSD is highly associated with sleep disturbances, including increased nightmares and insomnia, as well as changes in sleep architecture, including increased time spent in rapid eye movement (REM) and decreased time spent in slow-wave sleep (SWS) stages.91 Depression is associated with similar patterns of sleep disruption characterized by increased time in REM, decreased latency to first REM, and decreased SWS.92 It is currently unknown if sleep dysregulation precedes (or is a risk factor for) the development of anxiety disorders and PTSD, but it clearly exacerbates preexisting symptoms of these illnesses. As examples, immobilization stress, CMS, and footshock all affect sleep architecture.93-97
The fact that many types of stress produce alterations in sleep that are similar to those found in anxiety disorders98 suggests that examining sleep architecture represents a currently underappreciated approach to identifying physiological biomarkers with translational relevance.
In rodents, analysis of electroencephalogram (EEG)/EMG data can provide deeper insight into sleep states than simply measuring locomotor activity. Some telemetry systems are wireless, enabling continuous (24/7) measurement of EEG, EMG, activity, and temperature in freely moving mice or rats for periods up to several weeks. Sleep architecture can be quantified with information from spectral EEG power and EMG activity. These data allow derivation of metrics such as REM, non-REM or SWS, and active wakefulness,99 as well as circadian rhythmicity of locomotor activity and body temperature. Early work shows that some of these metrics are sensitive to stress or administration of stress peptides (Figure 2), providing proof-of -principle that the study of sleep architecture may represent a valuable new approach to the study of anxiety-related disorders in rodents, with end points that are similar—if not identical—to those that can be collected in humans. Wireless systems add ethological elements to these studies: they allow free-range of movement (unlike tethered systems) and allow animals to be group-housed while data is collected. There are other translationally relevant metrics that can be quantified, including hypothermic/hyperthermic responses, epileptiform activity, and EEG spectral power.100 Finally, constant collection of data over a period of weeks allows for within-subject experimental designs that can provide detailed insight on the onset, recovery, and persistence of anxiety-related changes in sleep and facilitate the development of new hypotheses testable in humans with the same end points.
Figure 2. Electroencephalogram/electromyogram (EEG/EMG) telemetry in rodents as a potential index of stress. The use of telemetry in mice and rats allows derivation of numerous metrics, including sleep architecture and circadian rhythm. (A) C57BL/6 mouse next to a transmitter (manufacturer: Data Sciences International) that is subcutaneously implanted to allow remote measurements: EEG/EMG, activity, and body temperature. (B) Representative example of a brief period of EEG and EMG signal from awake, slow-wave sleep, or rapid eye movement (REM) sleep. (C) Regular circadian fluctuations of activity and temperature in C57BL/6J mice, where 0-12 h is the light phase and 12-24 h is the dark phase. These cycles can be dysregulated by stress. (D) Effects of the stress peptide pituitary adenylate cyclase-activating polypeptide (PACAP; administered intracerebroventricularly [ICV]) on exploratory behavior in the elevated plus maze (EPM) (left) and REM sleep (right) in C57BL/6J mice. PACAP decreased exploratory behavior and increased REM during the dark phase, au, arbitrary units; ZT, zeitgeber time.
Summary
There are numerous methods for study of anxietyrelated disorders in rodents. Many do not provide direct insight into the signs and symptoms of anxiety in humans; rather, they have been developed and refined to maximize sensitivity to standard pharmacological agents and thus are more accurately conceptualized as assays rather than models. Caution is needed when interpreting the data and making conclusions in the context of human anxiety disorders. The development of novel methodologies that allow the collection of analogous in vivo end points in rodents and humans is a high priority and represents the type of advances needed to match the ever-increasing sophistication of molecular approaches useful for the study and treatment of psychiatric illness.
Acknowledgments
Kimberly R. Lezak, PhD, Galen Missig, PhD, and William A. Carlezon Jr, PhD have no conflicts of interest to disclose.
Contributor Information
Kimberly R. Lezak, Behavioral Genetics Laboratory, Department of Psychiatry, McLean Hospital, Harvard Medical School, Belmont, Massachusetts, USA.
Galen Missig, Behavioral Genetics Laboratory, Department of Psychiatry, McLean Hospital, Harvard Medical School, Belmont, Massachusetts, USA.
William A. Carlezon Jr, Behavioral Genetics Laboratory, Department of Psychiatry, McLean Hospital, Harvard Medical School, Belmont, Massachusetts, USA.
REFERENCES
- 1.Hyman S. Mental health: depression needs large human-genetics studies. Nature. 2014;515(7526):189–191. doi: 10.1038/515189a. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbert BN., Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walker DL., Toufexis DJ., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463(1-3):199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 4.Davis M., Walker DL., Miles L., Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker DL., Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213(1-2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 6.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44(12):1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 7.Walker DL., Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct Funct. 2008;213(1-2):29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- 8.Walker DL., Miles LA., Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourin M. Animal models for screening anxiolytic-like drugs: a perspective. Dialogues Clin Neurosci. 2015;17(3):295–303. doi: 10.31887/DCNS.2015.17.3/mbourin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crawley JN. Neuropharmacology specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol Biochem Behav. 1981;15(5):695–699. doi: 10.1016/0091-3057(81)90007-1. [DOI] [PubMed] [Google Scholar]
- 11.Crawley J., Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13(2):167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- 12.Bourin M., Hascoet M. The mouse light/dark box test. Eur J Pharmacol. 2003;463(1-3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 13.Handley SL., Mithani S. Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of 'fear'-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol. 1984;327(1):1–5. doi: 10.1007/BF00504983. [DOI] [PubMed] [Google Scholar]
- 14.Pellow S., File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24(3):525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 15.File SE., Hyde JR. Can social interaction be used to measure anxiety? Br J Pharmacol. 1978;62(1):19–24. doi: 10.1111/j.1476-5381.1978.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.File SE., Seth P. A review of 25 years of the social interaction test. Eur J Pharmacol. 2003;463(1-3):35–53. doi: 10.1016/s0014-2999(03)01273-1. [DOI] [PubMed] [Google Scholar]
- 17.File SE. Animal models for predicting clinical efficacy of anxiolytic drugs: social behaviour. Neuropsychobiology. 1985;13(1-2):55–62. doi: 10.1159/000118163. [DOI] [PubMed] [Google Scholar]
- 18.Deacon RM. Hyponeophagia: a measure of anxiety in the mouse. J Vis Exp. 2011;(51):doi:10.3791–2613. doi: 10.3791/2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulawa SC., Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29(4-5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Bourin M., Petit-Demouliere B., Dhonnchadha BN., Hascoet M. Animal models of anxiety in mice. Fundam Clin Pharmacol. 2007;21(6):567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 21.Dielenberg RA., McGregor IS. Defensive behavior in rats towards predatory odors: a review. Neurosci Biobehav Rev. 2001;25(7-8):597–609. doi: 10.1016/s0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard RJ., Blanchard DC. Attack and defense in rodents as etho-experimental models for the study of emotion. Prog Neuropsychopharmacol Biol Psychiatry. 1989;13(suppl):S3–S14. doi: 10.1016/0278-5846(89)90105-x. [DOI] [PubMed] [Google Scholar]
- 23.Zangrossi H., Jr File SE. Behavioral consequences in animal tests of anxiety and exploration of exposure to cat odor. Brain Res Bull. 1992;29(34):381–388. doi: 10.1016/0361-9230(92)90072-6. [DOI] [PubMed] [Google Scholar]
- 24.Cattarelli M., Chanel J. Influence of some biologically meaningful odorants on the vigilance states of the rat. Physiol Behav. 1979;23(5):831–838. doi: 10.1016/0031-9384(79)90186-0. [DOI] [PubMed] [Google Scholar]
- 25.Waddell J., Morris RW., Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120(2):324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 26.Davis M., Walker DL., Lee Y. Roles of the amygdala and bed nucleus of the stria terminalis in fear and anxiety measured with the acoustic startle reflex. Possible relevance to PTSD. Ann N Y Acad Sci. 1997;821:305–331. doi: 10.1111/j.1749-6632.1997.tb48289.x. [DOI] [PubMed] [Google Scholar]
- 27.Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychon Bull Rev. 1994;1(4):429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 28.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl). 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 29.Willner P. The chronic mild sterss (CMS) model of depression: history, evaluation and usage. Neurobiol Stress. 2017;(6):78–93. doi: 10.1016/j.ynstr.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammack SE., Cheung J., Rhodes KM., et al Chronic stress increases pituitary adenylate cyclase-activating peptide (PACAP) and brain-derived neurotrophic factor (BDNF) mRNA expression in the bed nucleus of the stria terminalis (BNST): roles for PACAP in anxiety-like behavior. Psychoneuroendocrinology. 2009;34(6):833–843. doi: 10.1016/j.psyneuen.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 32.Yamamoto S., Morinobu S., Takei S., et al Single prolonged stress: toward an animal model of posttraumatic stress disorder. Depress Anxiety. 2009;26(12):1110–1117. doi: 10.1002/da.20629. [DOI] [PubMed] [Google Scholar]
- 33.Liberzon I., Krstov M., Young EA. Stress-restress: effects on ACTH and fast feedback. Psychoneuroendocrinology. 1997;22(6):443–453. doi: 10.1016/s0306-4530(97)00044-9. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi T., Morinobu S., Iwamoto Y., Yamawaki S. Effect of paroxetine on enhanced contextual fear induced by single prolonged stress in rats. Psychopharmacology (Berl). 2006;189(2):165–173. doi: 10.1007/s00213-006-0545-6. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto S., Morinobu S., Fuchikami M., Kurata A., Kozuru T., Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology. 2008;33(9):2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- 36.Miczek KA., Yap JJ., Covington HE, 3rd. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120(2):102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berton O., McClung CA., Dileone RJ., et al Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 38.Golden SA., Covington HE, 3rd., Berton O., Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6(8):1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iniguez SD., Riggs LM., Nieto SJ., et al Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress. 2014;17(3):247–255. doi: 10.3109/10253890.2014.910650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinsey SG., Bailey MT., Sheridan JF., Padgett DA., Avitsur R. Repeated social defeat causes increased anxiety-like behavior and alters splenocyte function in C57BL/6and CD-1 mice. Brain Behav Immun. 2007;21(4):458–466. doi: 10.1016/j.bbi.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donahue RJ., Muschamp JW., Russo SJ., Nestler EJ., Carlezon WA Jr. Effects of striatal ΔFosB overexpression and ketamine on social defeat stress-induced anhedonia in mice. Biol Psychiatry. 2014;76(7):550–558. doi: 10.1016/j.biopsych.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lupien SJ., McEwen BS., Gunnar MR., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 43.Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(suppl 1):18–28. [PubMed] [Google Scholar]
- 44.Nishi M., Horii-Hayashi N., Sasagawa T. Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci. 2014;8:166. doi: 10.3389/fnins.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pryce CR., Ruedi-Bettschen D., Dettling AC., Feldon J. Early life stress: long-term physiological impact in rodents and primates. News Physiol Sci. 2002;17:150–155. doi: 10.1152/nips.01367.2001. [DOI] [PubMed] [Google Scholar]
- 46.van Oers HJ., de Kloet ER., Levine S. Early vs. late maternal deprivation differentially alters the endocrine and hypothalamic responses to stress. Brain Res Dev Brain Res. 1998;111(2):245–252. doi: 10.1016/s0165-3806(98)00143-6. [DOI] [PubMed] [Google Scholar]
- 47.Molet J., Maras PM., Avishai-Eliner S., Baram TZ. Naturalistic rodent models of chronic early-life stress. Dev Psychobiol. 2014;56(8):1675–1688. doi: 10.1002/dev.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ivy AS., Brunson KL., Sandman C., Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunson KL., Kramar E., Lin B., et al Mechanisms of late-onset cognitive decline after early-life stress. J Neurosci. 2005;25(41):9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dalle Molle R., Portella AK., Goldani MZ., et al Associations between parenting behavior and anxiety in a rodent model and a clinical sample: relationship to peripheral BDNF levels. Transl Psychiatry. 2012;2:e195. doi: 10.1038/tp.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huizink AC., de Medina PG., Mulder EJ., Visser GH., Buitelaar JK. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41(9):1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 52.de Weerth C., van Hees Y., Buitelaar JK. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74(2):139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 53.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32(6):1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Estes ML., McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353(6301):772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Babri S., Doosti MH., Salari AA. Strain-dependent effects of prenatal maternal immune activation on anxiety- and depression-like behaviors in offspring. Brain Behav Immun. 2014;37:164–176. doi: 10.1016/j.bbi.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 56.Giovanoli S., Engler H., Engler A., et al Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–1099. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 57.Haskell SG., Gordon KS., Mattocks K., et al Gender differences in rates of depression, PTSD, pain, obesity, and military sexual trauma among Connecticut War Veterans of Iraq and Afghanistan. J Womens Health (Larchmt). 2010;19(2):267–271. doi: 10.1089/jwh.2008.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ressler KJ., Mercer KB., Bradley B., et al Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramikie TS., Ressler KJ. Stress-related disorders, pituitary adenylate cyclase-activating peptide (PACAP)ergic system, and sex differences. Dialogues Clin Neurosci. 2016;18(4):403–413. doi: 10.31887/DCNS.2016.18.4/kressler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimamoto A., Debold JF., Holly EN., Miczek KA. Blunted accumbal dopamine response to cocaine following chronic social stress in female rats: exploring a link between depression and drug abuse. Psychopharmacology (Berl). 2011;218(1):271–279. doi: 10.1007/s00213-011-2364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trainor BC., Pride MC., Villalon Landeros R., et al Sex differences in social interaction behavior following social defeat stress in the monogamous California mouse (Peromyscus californicus). PLoS One. 2011;6(2):e17405. doi: 10.1371/journal.pone.0017405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gruene TM., Flick K., Stefano A., Shea SD., Shansky RM. Sexually divergent expression of active and passive conditioned fear responses in rats. Elife. 2015;4 doi:07554eLife:11352. doi: 10.7554/eLife.11352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bale TL., Epperson CN. Sex as a biological variable: who, what, when, why, and how. Neuropsychopharmacology. 2017;42(2):386–396. doi: 10.1038/npp.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ducottet C., Belzung C. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: evidence from inbred strains of mice. Behav Brain Res. 2005;156(1):153–162. doi: 10.1016/j.bbr.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 65.van Bogaert MJ., Groenink L., Oosting RS., Westphal KG., van der Gugten J., Olivier B. Mouse strain differences in autonomic responses to stress. Genes Brain Behav. 2006;5(2):139–149. doi: 10.1111/j.1601-183X.2005.00143.x. [DOI] [PubMed] [Google Scholar]
- 66.Sanford LD., Yang L., Wellman LL., Dong E., Tang X. Mouse strain differences in the effects of corticotropin releasing hormone (CRH) on sleep and wakefulness. Brain Res. 2008;1190:94–104. doi: 10.1016/j.brainres.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jennifer C., Chan AH., Bale TL. Strained in planning your mouse background? Using the HPA stress axis as a biological readout for backcrossing strategies. Neuropsychopharmacology. 2017 Mar 31. Epub ahead of print. doi:10.1038/npp. 2017.:66. doi: 10.1038/npp.2017.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alexandrov V., Brunner D., Hanania T., Leahy E. High-throughput analysis of behavior for drug discovery. Eur J Pharmacol. 2015;750:82–89. doi: 10.1016/j.ejphar.2014.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiltschko AB., Johnson MJ., Iurilli G., et al Mapping sub-second structure in mouse behavior. Neuron. 2015;88(6):1121–1135. doi: 10.1016/j.neuron.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuzesi T., Daviu N., Wamsteeker Cusulin Jl., Bonin RP., Bains JS. Hypothalamic CRH neurons orchestrate complex behaviours after stress. Nat Commun. 2016;7:11937. doi: 10.1038/ncomms11937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamm AO., Richter J., Pane-Farre CA. When the threat comes from inside the body: a neuroscience based learning perspective of the etiology of panic disorder. Restor Neurol Neurosci. 2014;32(1):79–93. doi: 10.3233/RNN-139011. [DOI] [PubMed] [Google Scholar]
- 72.Davis M., Antoniadis EA., Amaral DG., Winslow JT. Acoustic startle reflex in rhesus monkeys: a review. Rev Neurosci. 2008;19(2-3):171–185. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- 73.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association. . 2013 [Google Scholar]
- 74.Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52(10):958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 75.Walker DL., Davis M. Light enhanced startle: futher pharmacological and behavioral characterization. Psychopharmacology. 2002;159:304–310. doi: 10.1007/s002130100913. [DOI] [PubMed] [Google Scholar]
- 76.Walker DL., Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.de Jongh R., Groenink L., van Der Gugten J., Olivier B. The light-enhanced startle paradigm as a putative animal model for anxiety: effects of chlordiazepoxide, flesinoxan and fluvoxamine. Psychopharmacology (Berl). 2002;159(2):176–180. doi: 10.1007/s002130100914. [DOI] [PubMed] [Google Scholar]
- 78.Grillon C., Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. IntJ Psychophysiol. 1998;28(3):223–231. doi: 10.1016/s0167-8760(97)00072-x. [DOI] [PubMed] [Google Scholar]
- 79.Muhlberger A., Wieser MJ., Pauli P. Darkness-enhanced startle responses in ecologically valid environments: a virtual tunnel driving experiment. Biol Psychol. 2008;77(1):47–52. doi: 10.1016/j.biopsycho.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 80.Bocker KB., Baas JM., Kenemans JL., Verbaten MN. Stimulus-preceding negativity induced by fear: a manifestation of affective anticipation. Int J Psychophysiol. 2001;43(1):77–90. doi: 10.1016/s0167-8760(01)00180-5. [DOI] [PubMed] [Google Scholar]
- 81.Morgan CA. 3rd, Southwick SM., Grillon C., Davis M., Krystal JH., Charney DS. Yohimbine-facilitated acoustic startle reflex in humans. Psychopharmacology (Berl). 1993;110(3):342–346. doi: 10.1007/BF02251291. [DOI] [PubMed] [Google Scholar]
- 82.Holmes AJ., Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Arch Gen Psychiatry. 2008;65(2):179–188. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Elliott R., Sahakian BJ., McKay AP., Herrod JJ., Robbins TW., Paykel ES. Neuropsychological impairments in unipolar depression: the influence of perceived failure on subsequent performance. Psychol Med. 1996;26(5):975–989. doi: 10.1017/s0033291700035303. [DOI] [PubMed] [Google Scholar]
- 84.Beats BC., Sahakian BJ., Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26(3):591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- 85.Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl). 2002;163(3-4):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 86.Van't Veer A., Yano JM., Carroll FI., Cohen BM., Carlezon WA Jr. Corticotropin-releasing factor (CRF)-induced disruption of attention in rats is blocked by the kappa-opioid receptor antagonist JDTic. Neuropsychopharmacology. 2012;37(13):2809–2816. doi: 10.1038/npp.2012.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Donahue RJ., Venkataraman A., Carroll FI., Meloni EG., Carlezon WA Jr. Pituitary adenylate cyclase-activating polypeptide disrupts motivation, social interaction, and attention in male sprague dawley rats. Biol Psychiatry. 2016;80(12):955–964. doi: 10.1016/j.biopsych.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Paine TA., Tomasiewicz HC., Zhang K., Carlezon WA Jr. Sensitivity of the five-choice serial reaction time task to the effects of various psychotropic drugs in Sprague-Dawley rats. Biol Psychiatry. 2007;62(6):687–693. doi: 10.1016/j.biopsych.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 89.Beard C., Donahue RJ., Dillon DG., et al Abnormal error processing in depressive states: a translational examination in humans and rats. Transl Psychiatry. 2015;5:e564. doi: 10.1038/tp.2015.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boland EM., Ross RJ. Recent advances in the study of sleep in the anxiety disorders, obsessive-compulsive disorder, and posttraumatic stress disorder. Psychiatr Clin North Am. 2015;38(4):761–776. doi: 10.1016/j.psc.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Pace-Schott EF., Germain A., Milad. MR. Sleep and REM sleep disturbance in the pathophysiology of PTSD: the role of extinction memory. Biol Mood Anxiety Disord. 2015;5:3. doi: 10.1186/s13587-015-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palagini L., Baglioni C., Ciapparelli A., Gemignani A., Riemann D. REM sleep dysregulation in depression: state of the art. Sleep Med Rev. 2013;17(5):377–390. doi: 10.1016/j.smrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Rampin C., Cespuglio R., Chastrette N., Jouvet M. Immobilisation stress induces a paradoxical sleep rebound in rat. Neurosci Lett. 1991;126(2):113–118. doi: 10.1016/0304-3940(91)90532-x. [DOI] [PubMed] [Google Scholar]
- 94.Bonnet C., Leger L., Baubet V., Debilly G., Cespuglio R. Influence of a 1 h immobilization stress on sleep states and corticotropin-like intermediate lobe peptide (CLIP or ACTH18-39, Ph-ACTH18-39) brain contents in the rat. Brain Res. 1997;751(1):54–63. doi: 10.1016/s0006-8993(96)01390-x. [DOI] [PubMed] [Google Scholar]
- 95.Moreau JL., Scherschlicht R., Jenck F., Martin JR. Chronic mild stressinduced anhedonia model of depression; sleep abnormalities and curative effects of electroshock treatment. Behav Pharmacol. 1995;6(7):682–687. [PubMed] [Google Scholar]
- 96.Cheeta S., Ruigt G., van Proosdij J., Willner P. Changes in sleep architecture following chronic mild stress. Biol Psychiatry. 1997;41(4):419–427. doi: 10.1016/S0006-3223(96)00058-3. [DOI] [PubMed] [Google Scholar]
- 97.Vazquez-Palacios G., Velazquez-Moctezuma J. Effect of electric foot shocks, immobilization, and corticosterone administration on the sleepwake pattern in the rat. Physiol Behav. 2000;71(1-2):23–28. doi: 10.1016/s0031-9384(00)00285-7. [DOI] [PubMed] [Google Scholar]
- 98.Pawlyk AC., Morrison AR., Ross RJ., Brennan FX. Stress-induced changes in sleep in rodents: models and mechanisms. Neurosci Biobehav Rev. 2008;32(1):99–117. doi: 10.1016/j.neubiorev.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mang GM., Franken P. Sleep and EEG phenotyping in mice. Curr Protoc Mouse Biol. 2012;2(1):55–74. doi: 10.1002/9780470942390.mo110126. [DOI] [PubMed] [Google Scholar]
- 100.Lundt A., Wormuth C., Siwek ME., et al EEG radiotelemetry in small laboratory rodents: a powerful state-of-the art approach in neuropsychiatry, neurodegenerative, and epilepsy research. Neural Plast. 2016;2016:821–3878. doi: 10.1155/2016/8213878. [DOI] [PMC free article] [PubMed] [Google Scholar]



