Abstract
Social anxiety is a form of anxiety characterized by continuous fear of one or more social or performance situations. Although multiple treatment modalities (cognitive behavioral therapy, selective serotonin reuptake inhibitors/selective norepinephrine reuptake inhibitors, benzodiazepines) exist for social anxiety, they are effective for only 60% to 70% of patients. Thus, researchers have looked for other candidates for social anxiety treatment. Our review focuses on the peptide oxytocin as a potential therapeutic option for individuals with social anxiety. Animal research both in nonprimates and primates supports oxytocin's role in facilitation of prosocial behaviors and its anxiolytic effects. Human studies indicate significant associations between social anxiety and oxytocin receptor gene alleles, as well as social anxiety and oxytocin plasma levels. In addition, intranasal administration of oxytocin in humans has favorable effects on social anxiety symptomology. Other disorders, including autism, schizophrenia, and anorexia, have components of social anxiety in their pathophysiology. The therapeutic role of oxytocin for social dysfunction in these disorders is discussed.
Keywords: anxiety, human, oxytocin, primate, rodent, social
Abstract
La ansiedad social es una forma de ansiedad caracterizada por un temor continuo de una o varias situaciones sociales o de rendimiento. Aunque para la ansiedad social existen diversas modalidades terapéuticas (terapia cognitivo conductual, inhibidores selectivos de la recaptura de serotonina, inhibidores selectivos de la recaptura de serotonina y noradrenalina, y benzodiacepinas), ellas resultan efectivas en no más del 60% a 70% de los pacientes. Debido a esto, los investigadores han buscado otras opciones de terapia para la ansiedad social. Esta revisión se enfoca en el péptido oxitocina como una potencial opción terapéutica para sujetos con ansiedad social. La investigación animal tanto de primates como de no primates da soporte al papel de la oxitocina en la facilitación de las conductas pro-sociales y sus efectos ansiolíticos. Los estudios en humanos muestran asociaciones significativas entre la ansiedad social y los alelos del gen del receptor de oxitocina, como también de la ansiedad social y los niveles plasmáticos de oxitocina. Además, la administración de oxitocina intranasal en humanos tiene efectos favorables para la sintomatología de la ansiedad social. Otras patologías, incluyendo el autismo, la esquizofrenia y la anorexia tienen componentes de la ansiedad social en sus fisiopatologías. En este artículo se discute el papel terapéutico de la oxitocina para la disfunción social en estas patologías.
Abstract
L'anxiété sociale est une forme d'anxiété caractérisée par la peur permanente d'une ou plusieurs situations sociales ou de performance. De nombreuses modalités de traitement existent pour l'anxiété sociale, (thérapie cognitive comportementale, inhibiteurs sélectifs de la recapture de la sérotonine, inhibiteurs sélectifs de la recapture de la noradrénaline, benzodiazépines), mais elles ne sont efficaces que pour 60 à 70 % des patients. Des chercheurs ont donc examiné d'autres possibilités de traitement de l'anxiété sociale. Cet article s'intéresse au peptide ocytocine comme traitement éventuel des personnes atteintes d'anxiété sociale. La recherche chez les animaux, à la fois chez les primates et les non-primates, confirme que l'ocytocine facilite les comportements pro-sociaux et a des effets anxiolytiques. D'après des études chez l'homme, il existe des associations significatives entre l'anxiété sociale et les allèles du gène du récepteur de l'ocytocine ainsi qu'entre l'anxiété sociale et les concentrations plasmatiques d'ocytocine. De plus, l'administration intranasale d'ocytocine chez l'homme a des effets bénéfiques sur les symptômes de l'anxiété sociale. Nous analysons ensuite le rôle thérapeutique de l'ocytocine sur le dysfonctionnement social dans l'autisme, la schizophrénie et l'anorexie, des troubles dont la physiopathologie présente une composante d'anxiété sociale.
Introduction
Over the past decade, research has investigated impairments in processing of social information in a broad range of illnesses, including autism, schizophrenia, anorexia, and social anxiety disorder.1-3 Difficulty in social stimuli processing is often evaluated over multiple dimensions and is disease-specific, for instance evaluation of social communication in autism or social fear in social anxiety disorder.3,4 This review specifically discusses the role of oxytocin in social anxiety. Although disorders that contain an aspect of social anxiety differ greatly in symptomatology and etiology, it is important to recognize where similarities exist in the development and functioning of brain regions specific to processing of social stimuli. Thus, treatment beneficial to social anxiety disorder could also be useful for other disorders with social processing dysfunction.
The fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) defines social anxiety disorder as a “persistent fear of one or more social or performance situations in which the person is exposed to unfamiliar people or to possible scrutiny by others.”5 Social anxiety occurs on a wide spectrum ranging from mild adaptive fear to a maladaptive disorder, severely impacting an individual's life. Social anxiety symptomology can exist alone or as a comorbidity with other psychiatric disorders, including, most commonly, eating disorders, major depression, schizophrenia, and other anxiety disorders (generalized anxiety disorder, panic disorder).6,7 However, the connection between symptom and comorbidity is often unclear; a comorbidity can result from the same disease process as the disorder itself. To add to the complexity, the causes of social anxiety are multifactorial, involving genetics, neurobiology, the fetal environment, and the postnatal environment.8
Multiple neurological pathways, neurotransmitters, and brain regions, including the amygdala and anterior cingulate, have been studied in regard to social anxiety, but many remain unknown.9 Current treatment focuses on cognitive behavioral therapy as a first-line treatment and on medications, such as selective serotonin reuptake inhibitors (SSRIs) or selective norepinephrine reuptake inhibitors (SNRIs), as a second-line treatment. Occasionally benzodiazepines are used for short-term relief; less commonly, β-blockers are used in order to control the sympathetic nervous system.8,10 Despite multiple treatment options, treatment is ineffective for 30% to 40% of patients with social anxiety disorder.11 A necessity for more effective treatments exists not only for social anxiety disorder but other disorders with a social impairment component, such as schizophrenia and autism. This need has led researchers to explore other potential mechanisms involved with social anxiety.
Oxytocin is a neuropeptide synthesized primarily in the magnocellular neurons of the paraventricular and supraoptic nuclei of the hypothalamus.12 Oxytocin plays a key role in social cognition, in social behaviors, and in fear conditioning, which are important in social anxiety as well as in other disorders with impaired social functioning. A plethora of studies have uncovered a role for oxytocin in intimacy, social recognition, pair bonding, and anxiety, among others.13 Oxytocin acts centrally within the brain to control behavior, as opposed to its well-known peripheral role in parturition and lactation.13 Oxytocin receptors are distributed throughout the brain, including within the amygdala, ventromedial hypothalamus, brain stem, and nucleus accumbens.14 This review will focus on oxytocin's central role on social anxiety and social processing as a potential target for the treatment of social anxiety and other disorders with dysfunction in social processing.
Animal studies and social anxiety
Animal research investigating oxytocin's role in basic behavioral processes has set the foundation for investigation of oxytocin in human illnesses, including autism, schizophrenia, and anxiety disorders. Animal models of social anxiety-related behaviors are vital for understanding the implications of oxytocin in human social processing and social anxiety. The following sections examine nonprimate and nonhuman primate studies for social behavior and also anxiety components of social anxiety.
Social behavior
Animal studies have examined the role of oxytocin in a variety of mammals, including sheep, mice, and rats.15 Prairie voles have been of particular interest in oxytocin animal research because they demonstrate selective social preference in order to survive and reproduce in their habitats.16,17 Early animal studies demonstrated oxytocin's ability to induce the prosocial behavior of bonding, both maternal bonding with offspring and pair bonding.18-20 In voles, pair bonding is measured by time spent in proximity of a partner versus a stranger, in which more time spent with a partner indicates pair-bond formation.21,22 Pair bonding can be induced in female voles via intracerebroventricular oxytocin injection, or prevented by an oxytocin receptor antagonist in the nucleus accumbens and prefrontal cortex.20,23 Maternal/infant bonding has also been demonstrated in voles.24 Individuals with social anxiety disorder, as well as other disorders such as autism, can struggle with forming and maintaining interpersonal relationships. Oxytocin has the potential to facilitate human “bonding” and should be considered in future studies investigating its effects on relationships in people with social anxiety or autism.
Another aspect of social behavior is social memory, the ability to recognize and differentiate between individuals.25 Central oxytocin administration enhances social memory in male rats,26,27 whereas an oxytocin receptor antagonist blocks social memory in female and male rats.26-28 Oxytocin affects social memory in multiple brain regions, including the olfactory bulb, lateral septum, ventral hippocampus, and amygdala, in nonhuman primates.29-34 These and other studies suggest the potential of oxytocin to also promote prosocial behaviors and enhance social cognition in humans. However, social behavior in humans is distinctively different and more complex than that of nonprimates like voles and rats, which will be discussed below.
Anxiety
Animal research has demonstrated relationships between oxytocin's role in anxiety and social behaviors; for instance, under stress, oxytocin causes rodents to approach and maintain closeness with familiar rodents.35-37 Systemic pretreatment with oxytocin before a stressor (flooded cage) led to a longer amount of time spent with other prairie voles after the event.38 This finding suggests that oxytocin can change stress neural connectivity and promote social cohesion after a stressor that usually would cause dispersal.38 This relationship is not surprising given oxytocin's involvement in brain regions such as the amygdala and paraventricular nucleus of the hypothalamus where oxytocin modulates fear and stress responses.39-42 Specifically, anxiety-provoking stimuli activate the oxytocin system by increasing oxytocin neuronal activity, oxytocin gene expression in the paraventricular and supraoptic nuclei of the hypothalamus, and central and peripheral release of oxytocin.43-48
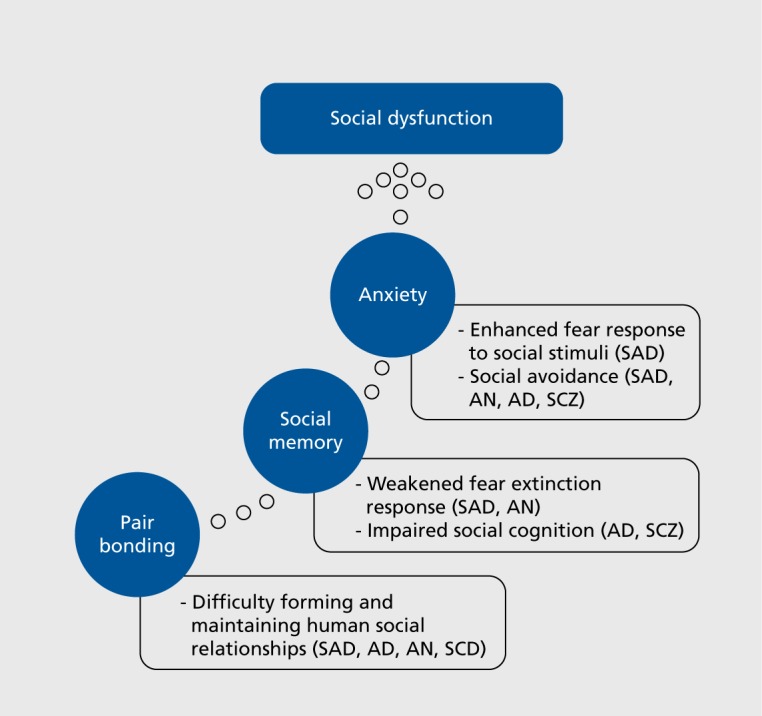
The acute and chronic anxiolytic effects of oxytocin have been demonstrated in a number of rodent studies.49 One method of evaluation used is the elevated plus maze (EPM). More time spent in open space indicates a successful anxiolytic response for the EPM. Central amygdala, prelimbic cortex, and intracerebroventricular administration of oxytocin has demonstrated acute anxiolytic effects in rodents in the EPM test,50-53 whereas administration of oxytocin receptor antagonist induced anxiogenic effects. Similar effects were shown when chronic anxiolysis was evaluated.54-55 Oxytocin's involvement in anxiety-modulating regions of the brain and rodent studies demonstrate its anxiolytic effects and support the potential for using oxytocin in treatment of anxiety disorders. Figure 1 illustrates the human implications of nonprimate animal studies on social functioning and anxiety.
Figure 1. Human implications of nonprimate oxytocin studies on social functioning and anxiety. AD, anxiety disorder; AN, anorexia nervosa; SAD, social anxiety disorder; SCZ, schizophrenia.
Nonhuman primate studies
Rodent studies have provided insight into the central effects of oxytocin. However, rodents display distinctively different social behaviors than that of humans, and this has led animal researchers to use nonhuman primate models of human social behavior. The rhesus macaque has been a central focus of nonhuman primate research because of its complex “human-like” social behaviors, including social imitation, perceptional understanding, and prosocial behaviors.56 Similar to humans, the macaque primarily uses audition and vision for social communication. Oxytocin receptors reside in areas of the brain involved with auditory and sensory stimuli processing of the macaque. These include the superior colliculus, trapezoid body, ventromedial hypothalamus, nucleus basalis of Meynert, and the pedunclopontine tegmental nucleus in the rhesus macaque.57
Rhesus macaques have been studied in several social domains including social development, prosocial choices, and social attention. Oxytocin expression may depend on the presence of a maternal figure in the early stages of life and influence later prosocial behavior development. Macaques reared by mothers have significantly higher baseline oxytocin cerebrospinal fluid levels at 18, 24, and 36 months of age than those raised without a mother.58 Mother-reared macaques also spend more time sitting in close contact and allogrooming (prosocial behaviors), with a significant correlation between these social behaviors and cerebrospinal fluid oxytocin levels.58 Prosocial behaviors have also been studied in adult rhesus macaques. Macaques increase the number of prosocial choices related to “social donation” after 2 hours of oxytocin inhalation. The macaque administered oxytocin made more prosocial choices (providing a juice box to another macaque) than the macaque that did not receive oxytocin.59 These studies support that overall, oxytocin enhances macaque prosocial behavior.56-59
The effects of intranasal oxytocin on gaze patterns and social vigilance in rhesus macaques has been investigated as a component of social attention.60 After oxytocin administration, macaques shifted their gaze patterns; they increased the amount of time spent gazing at eyes and faces of macaque images. In addition, they decreased species-typical social vigilance for images of emotional, dominant, and unfamiliar macaque faces in images. Similar responses were observed to images of negative faces after oxytocin administration, and in addition, there was no observed change in response to neutral faces.61 A possible explanation is that oxytocin reduces activity in brain regions that involve attention and arousal, including the amygdala, which regulates vigilance, properties of faces, and emotional expression.60
Decreased social vigilance can lead to prosocial behavior. These findings are especially relevant to disorders with social-processing dysfunction. Decreased eye contact is a common symptom of multiple psychiatric disorders, including autism and social anxiety disorder.62,63 Increased attention to social situations perceived as threatening is a component of social anxiety disorder.64 Oxytocin is a candidate for both enhancing eye contact and helping to alleviate social vigilance in social anxiety disorder, without affecting social situations viewed as neutral or nonthreatening.
Anxiety has also been investigated in nonhuman primates. Multiple studies support that administration of oxytocin in rhesus macaques decreases cortisol, a glucocorticoid released in response to stress.65 Oxytocin both increases prosocial behaviors and decreases salivary cortisol in macaque infants.66 In addition, mother-reared macaques have decreased plasma cortisol when introduced to a new cage with a companion compared with non-mother-reared macaques.58 As mentioned previously, the mother-reared macaques have increased oxytocin expression. No significant difference between the two groups was found when the macaques were put in a novel cage without a companion. These results indicate an anxiolytic effect in the presence of a companion, relating also to the social effects of oxytocin.
Human studies and social anxiety
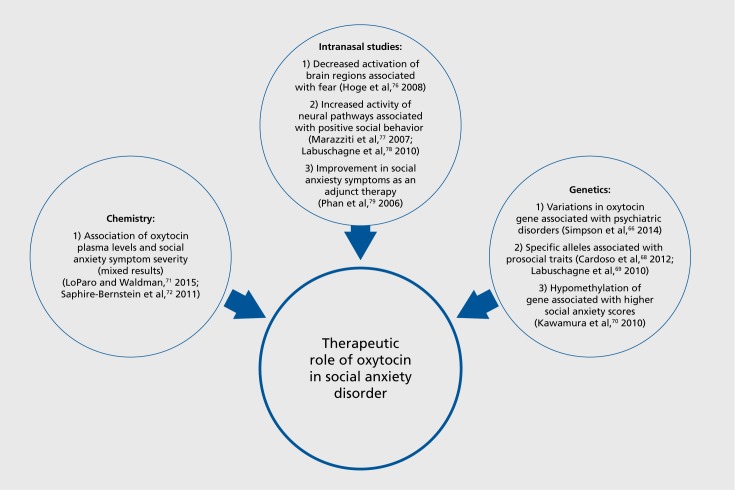
The facilitation of prosocial behaviors and anxiolytic effects of oxytocin observed in animal research has led scientists to investigate oxytocin's role in human psychiatric conditions, such as social anxiety disorder, schizophrenia, and autism. The anxiolytic effects of oxytocin are supported by research demonstrating diminished negative self-judgment during a social task and decreased anxiety in response to social rejection.67,68 Functional magnetic resonance imaging studies implicate underlying anxiolytic neurocircuitry, as decreased fear-associated amygdala activity was observed in response to threatening faces in individuals with generalized social anxiety disorder.69 Research has been conducted at the molecular level, examining oxytocin genes and plasma levels, and at the clinical level, evaluating the effectiveness of intranasal oxytocin as a potential treatment for social anxiety disorder and other disorders with social dysfunction (Figure 2). The impact of oxytocin on social impairment in multiple psychiatric disorders is discussed below.
Figure 2. Studies supporting the therapeutic role of oxytocin in social anxiety disorder.
Genetic and biochemical studies
Genetic variations in the oxytocin receptor gene have been implicated in several psychiatric disorders, including depression, mood disorders, and autism spectrum disorders.70,71 The polymorphism (rs53576) of the oxytocin receptor gene is located on the third intron in three forms: GG, AA, and AG.72 In past studies, the G allele was found to be connected with prosocial traits like empathy, trust, and optimism; whereas the A allele was associated with sensitivity to stress, less optimism, less social skills, and lower self-esteem.73,74 Oxytocin receptor gene methylation occurs in individuals with social anxiety disorder, including hypomethylation at CpG chromosome 3:8809437, which is associated with higher scores on the Social Phobia Scale and Social Interaction Anxiety Scale and a greater degree of amygdala responsiveness to social phobia-related words.75 It is unclear whether the hypomethylation is a cause or result of social anxiety disorder.
Oxytocin plasma levels have also been analyzed in individuals with social anxiety disorder, although results have not been consistent. In one study, plasma oxytocin levels showed a positive correlation with social anxiety symptom severity.76 However, another study did not fully support these results. In individuals in close relationships, they found a positive correlation between oxytocin levels and anxiety but not oxytocin levels and avoidance scale scores.77 Human genetic and biochemical research on the role of oxytocin in social anxiety disorder and other social functioning disorders is still being explored. The studies above suggest an association, but at this time there are no conclusive results.
Intranasal oxytocin studies
Social anxiety disorder
Oxytocin is a 9-amino-acid peptide that is unable to cross the blood brain barrier and enter the central nervous system.13 Intranasal administration has been the primary means of oxytocin deliver in humans thus far. Numerous studies have examined the effects of intranasally administered oxytocin on social anxiety symptoms and as an adjunct treatment for social anxiety disorder.
The amygdala has been a specific focus of human oxytocin research because of its hyperactivity in response to social threats and fear.78,79 Oxytocin inhibits neurons in the amygdala that connect to other brain regions associated with fear, including the anterior cingulate cortex (ACC) and the medial prefrontal cortex (MPC).80,81 Oxytocin reduces increased activation of the ACC and MPC in individuals with social anxiety disorder in response to sad faces.81 Intranasal oxytocin has also been shown to enhance functional connectivity between the amygdala and the bilateral insula and middle cingulate/dorsal cingulate gyrus in individuals with social anxiety disorder when shown fearful faces. These pathways are associated with social/emotional behavior and suggest that oxytocin heightens activity in these regions and dampens activity of pathways related to fear response, thus “normalizing” both pathways.82 A negative correlation has been described between connectivity of the amygdala-anterior cingulate/prefrontal cortex pathway with social anxiety severity at rest. Oxytocin also increases activity in the underactive rostral anterior cingulate cortex/medial frontal cortex pathway in individuals with social anxiety.83
Oxytocin has also been studied as an adjunct to other therapies for social anxiety. In a randomized placebo-controlled study, individuals with social anxiety disorder were administered intranasal oxytocin as an adjunct to exposure therapy.84 Statistically significant improvement in positive evaluation of appearance and speech performance was demonstrated compared with placebo. However, both the placebo and oxytocin groups showed significant symptom reduction and improvement in life impairment scores, indicating a significant placebo effect. Thus, oxytocin as an adjunct may enhance improvement of some characteristics of social anxiety disorder but not others.
Other disorders: schizophrenia, autism, and anorexia
Although the focus has been on social anxiety, it is important to consider the effects of intranasal oxytocin on other disorders with impairments in social function, including schizophrenia and autism. Anorexia will also be discussed briefly because of a high comorbidity with social anxiety disorder (the lifetime prevalence has been reported as 33.9 %).85
Schizophrenia treatment primarily addresses the positive symptoms of the disease but not the negative symptoms, such as asociality and affective flattening. The severity of these negative symptoms is strongly correlated with decline in social function and quality of life, and attempts to diminish this decline have been largely unsuccessful.86 Oxytocin is a potential candidate for the treatment of these negative symptoms. Intranasally administered oxytocin in patients with schizophrenia has demonstrated an overall decrease both in negative and positive symptomology scores.87 Six published clinical trials have reported improvement in negative symptoms with intranasal oxytocin added as an adjunct treatment to atypical antipsychotic medication.88 Specific improvements in social functioning in patients with schizophrenia include increased ability to recognize emotions and improvements in high-level social functioning, such as detection of sarcasm, detection of deception, and empathy.89,90 Individuals with autism also have dysfunction across multiple social domains. They show decreased social motivation, social aloofness, diminished eye contact, decreased social relationship reward, and difficulty maintaining social relationships.91 Multiple studies support the conclusion that administration of intranasal oxytocin in individuals with autism increases eye gaze, enhances feelings of trust, increases recognition of affective speech, and increases scores on the Reading the Mind in the Eyes Test.92-94 Research supports a strong association between autism and schizophrenia,95 and studies are currently investigating the neurological, genetic, developmental, and molecular similarities between these illnesses. Oxytocin and other novel treatments beneficial to one should thus also be considered in the other.
Anorexia differs greatly from schizophrenia and autism, but one commonality is that social impairment can be a marked component of the disorder.6 Oxytocin clinical trials have had mixed results in improvement of anorexia symptoms, and relatively few specific social dysfunction symptoms have been studied.96-98 One study found that oxytocin significantly decreased attention toward “eating” stimuli and “negative body image” stimuli in patients with anorexia. Specific to social dysfunction, these results were most apparent in anorexics with higher levels of autistic-specific traits, indicating that the potential benefit of oxytocin in anorexics could be most effective in those with concurrent social difficulties.94 It remains unclear whether oxytocin plasma levels are reduced in anorexia nervosa at baseline. Some studies have indicated lower levels of oxytocin; however, others have shown increases in plasma oxytocin after eating.99 Future research implications include investigation of the role of oxytocin in anorexics with specific symptoms related to social dysfunction.
Clinical implications for oxytocin in social anxiety treatment/discussion
Researchers investigating the neural impact of oxytocin have primarily used the intranasal route of administration. Because oxytocin is unable to cross the blood-brain barrier, intravenous and oral administration of oxytocin are ineffective.99 Although intranasal administration provides some degree of central access, the bioavailability of exogenous oxytocin in the central nervous system is limited for a number of reasons. First, oxytocin, like any other peptide, will scarcely pass the blood-brain barrier and will be subject to degradation in the living organism. Second, upon intranasal administration, oxytocin can be absorbed through the nasal mucosa in multiple compartments, each of which varies in their absorption capacity and influences the amount of oxytocin that reaches the cerebrospinal fluid versus systemic circulation.100 Thus, the prosocial behaviors or anxiolysis after oxytocin administration could result from multiple pathways: a direct pathway through the olfactory bulb to the cerebrospinal fluid, an indirect peripheral pathway that through a possible feed-forward mechanism influences central nervous system release of endogenous oxytocin, or other routes, such as through the oral mucosa and through a gastroenteric route.101,102 Intranasal oxytocin bioavailability is likely to vary widely because of multiple factors.
Despite these factors, intranasal administration of oxytocin has yielded positive results in improvement of social dysfunction across multiple psychiatric disorders. Until other methods of delivery are developed, these results warrant continuation of intranasal oxytocin research in social anxiety disorder. Currently, researchers are attempting to create small-molecule oxytocin receptor activators that hold promise for exposing the brain to high levels of oxytocin after oral administration. Important to advancement of these future analogs and intranasal oxytocin is evidence of target engagement. Additional studies can investigate the relationship between specific regional receptor binding in the brain and therapeutic outcome at controlled dosages of intranasal oxytocin.103 Future research may also include investigation of other neurotransmitters and neuropeptides implicated in social dysfunction pathophysiology. Multiple such molecules have been suggested to be involved in the pathophysiology of social anxiety. Of particular importance is the neuropeptide vasopressin. Research also supports the role of vasopressin —closely related to oxytocin— in maintaining social relationships and pair bonding.104 One consideration for future investigation of social functioning is whether social dysfunction in psychiatric disorders results from an imbalance in oxytocin and vasopressin neural activity.
In summary, research provides evidence for the role of oxytocin in the pathophysiology of social dysfunction across the span of multiple psychiatric disorders through its impact on social vigilance, positive social evaluation, prosocial behaviors, and anxiolytic effects. Future implications include research supporting oxytocin target engagement, dose relationship of oxytocin with therapeutic effects, and consideration of other neural peptides and neurotransmitters in social functioning pathophysiology.
Acknowledgments
This work was supported by NIH grants R01NS092671 (SB), R01MH110441 (SB), R21DA035592 (CW), R01DA035055 (CW), and R01AA023781 (CW). Ms Jones, Dr Barrera, Dr Wahlestedt, Dr Brothers, and Dr Ring report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Candace Jones, University of Miami Miller School of Medicine, Miami, Florida, USA.
Ingrid Barrera, University of Miami Department of Psychiatry and Behavioral Sciences, Miami, Florida, USA.
Shaun Brothers, University of Miami Department of Psychiatry and Behavioral Sciences, Miami, Florida, USA.
Robert Ring, Drexel University Department of Pharmacology and Physiology, Philadelphia, Pennsylvania, USA.
Claes Wahlestedt, University of Miami Department of Psychiatry and Behavioral Sciences, Miami, Florida, USA.
REFERENCES
- 1.Zucker NL., Losh M., Bulik CM., LaBar KS., Piven J., Pelphrey KA. Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes. Psychol Bull. 2007;133(6):976–1006. doi: 10.1037/0033-2909.133.6.976. [DOI] [PubMed] [Google Scholar]
- 2.de Jong JJ., de Gelder B., Hodiamont PP. Sensory processing, neurocognition, and social cognition in schizophrenia: towards a cohesive cognitive model. Schizophr Res. 2013;146(1-3):209–216. doi: 10.1016/j.schres.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Miskovic V., Schmidt LA. Social tearfulness in the human brain. Neurosci Biobehav Rev. 2012;36:459–478. doi: 10.1016/j.neubiorev.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Valkanova V., Rhodes F., Allan CL. Diagnosis and management of autism in adults. Practitioner. 2013;257(1761):13–16. [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Press. . 2013 [Google Scholar]
- 6.Al-Asadi AM., Klein B. Multiple comorbidities of 21 psychological disorders and relationships with psychosocial variables: a study of the online assessment and diagnostic system within a web-based population. J Med Internet Res. 2015;17(2):e55. doi: 10.2196/jmir.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Achim AM., Maziade M., Raymond E., Oliver D., Merette C., Roy MA. How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull. 2011;37(4):811–821. doi: 10.1093/schbul/sbp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beidel DC., Turner SM. Shy Children, Phobic Adults: Nature and Treatment of Social Anxiety Disorder. 2nd ed. Washington, DC: American Psychological Association. 2013 [Google Scholar]
- 9.Fox AS., Kalin NH. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am J Psychiatry. 2014;171(11):1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pilling S., Mayo-Wilson E., Mavranezouli I., Kew K., Taylor C., Clark DM. Guideline Development Group. Recognition, assessment and treatment of social anxiety disorder: summary of NICE guidance. BMJ. 2013;346:f2541. doi: 10.1136/bmj.f2541. [DOI] [PubMed] [Google Scholar]
- 11.Stein MB., Stein DJ. Social anxiety disorder. Lancet. 2008;371(9618):1115–1125. doi: 10.1016/S0140-6736(08)60488-2. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes CH., Morriell JI., Pfaff DW. Irnmunohistochemical analysis of magnocellular elements in rat hypothalamus: distribution and numbers of cells containing neurophysin, oxytocin, and vasopressin. J Comp Neurol. 1981;198(1):45–64. doi: 10.1002/cne.901980106. [DOI] [PubMed] [Google Scholar]
- 13.Viero C., Shibuya I., Kitamura N., et al Oxytocin: crossing the bridge between basic science and pharmacotherapy. CNS Neurosci Ther. 2010;16(5):e138–e156. doi: 10.1111/j.1755-5949.2010.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HJ., Macbeth AH., Pagani J., Young WS. Oxytocin: the great facilitator of life. Prog Neurobiol. 2009;88(2):127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamasue H., Yee JR., Hurlemann R., Rilling JK., Chen FS., Meyer-Lindenberg A. Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction. J Neurosci. 2012;32(41):14109–14117. doi: 10.1523/JNEUROSCI.3327-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23(8):779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- 17.Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79(3):503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen CA., Prange AJ. Induction of maternal behavior in virgin rats after intracerebroventricular administration of oxytocin. Proc Natl Acad Sci U S A. 1979;76(12):6661–6665. doi: 10.1073/pnas.76.12.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Insel TR., Young L., Wang Z. Molecular aspects of monogamy. Ann N Y Acad Sci. 1997;807:302–316. doi: 10.1111/j.1749-6632.1997.tb51928.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams JR., Insel TR., Harbaugh CR., Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles . (Microtus ochrogaster). J Neuroendocrinol. 1994;6(3):247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- 21.Young LJ., Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7(10):1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- 22.Carter CS., Grippo AJ., Pournajafi-Nazarloo H., Ruscio MG., Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 23.Young LJ., Lim MM., Gingrich B., Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40(2):133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- 24.Ross HE., Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30(4):534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukas M., Neumann ID. Oxytocin and vasopressin in rodent behaviors related to social dysfunctions in autism spectrum disorders. Behav Brain Res. 2013;251:85–94. doi: 10.1016/j.bbr.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Benelli A., Bertolini A., Poggioli R., Menozzi B., Basaglia R., Arletti R. Polymodal dose-response curve for oxytocin in the social recognition test. Neuropeptides. 1995;28(4):251–255. doi: 10.1016/0143-4179(95)90029-2. [DOI] [PubMed] [Google Scholar]
- 27.Engelmann M., Ebner K., Wotjak CT., Landgraf R. Endogenous oxytocin is involved in short-term olfactory memory in female rats. Behav Brain Res. 1998;90(1):89–94. doi: 10.1016/s0166-4328(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 28.Lukas M., Toth I., Veenema AH., Neumann ID. Oxytocin mediates rodent social memory within the lateral septum and the medial amygdala depending on the relevance of the social stimulus: male juvenile versus female adult conspecifics. Psychoneuroendocrinology. 2013;38(6):916–926. doi: 10.1016/j.psyneuen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 29.van Wimersma Greidanus TB., Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713(1-2):153–159. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- 30.Popik P., van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. Eur Neuropsychopharmacol. 1991;1(4):555–560. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- 31.Popik P., Vos PE., Van Ree JM. Neurohypophyseal hormone receptors in the septum are implicated in social recognition in the rat. Behav Pharmacol. 1992;3(4):351–358. [PubMed] [Google Scholar]
- 32.Dluzen DE., Muraoka S., Engelmann M., Landgraf R. The effects of infusion of arginine vasopressin, oxytocin, or their antagonists into the olfactory bulb upon social recognition responses in male rats. Peptides. 1998;19(6):999–1005. doi: 10.1016/s0196-9781(98)00047-3. [DOI] [PubMed] [Google Scholar]
- 33.Larrazolo-Lopez A., Kendrick KM., Aburto-Arciniega M., et al Vaginocervical stimulation enhances social recognition memory in rats via oxytocin release in the olfactory bulb. Neuroscience. 2008;152(3):585–593. doi: 10.1016/j.neuroscience.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Choleris E., Little SR., Mong JA., Puram SV., Langer R., Pfaff DW. Microparticle-based delivery of oxytocin receptor antisense DNA in the medial amygdala blocks social recognition in female mice. Proc Natl Acad Sci U S A. 2007;104(11):4670–4675. doi: 10.1073/pnas.0700670104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engelmann M., Wotjak CT., Neumann I., Ludwig M., Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20(3):341–358. doi: 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 36.Carter CS., Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Ann N Y Acad Sci. 1997;807:164–174. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- 37.Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51(1):18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 38.Olff M., Frijling JL., Kubzansky LD., et al The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38(9):1883–1894. doi: 10.1016/j.psyneuen.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Neumann ID., Krömer SA., Toschi N., Ebner K. Brain oxytocin inhibits the (re)activity of the hypothalamo-pituitary-adrenal axis in male rats: involvement of hypothalamic and limbic brain regions. Regul Pept. 2000;96(1-2):31–38. doi: 10.1016/s0167-0115(00)00197-x. [DOI] [PubMed] [Google Scholar]
- 40.Neumann ID. Involvement of the brain oxytocin system in stress coping: interactions with the hypothalamo-pituitary-adrenal axis. Prog Brain Res. 2002;139:147–162. doi: 10.1016/s0079-6123(02)39014-9. [DOI] [PubMed] [Google Scholar]
- 41.Huber D., Veinante P., Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 42.Viviani D., Charlet A., van den Burg E., et al Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 43.Gibbs DM. Dissociation of oxytocin, vasopressin, and corticotropin secretion during different types of stress. Life Sci. 1984;35(5):487–491. doi: 10.1016/0024-3205(84)90241-8. [DOI] [PubMed] [Google Scholar]
- 44.Williams TD., Carter DA., Lightman SL. Sexual dimorphism in the posterior pituitary response to stress in the rat. Endocrinology. 1985;116(2):738–740. doi: 10.1210/endo-116-2-738. [DOI] [PubMed] [Google Scholar]
- 45.Lang RE., Heil JW., Ganten D., Hermann K., Unger T., Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37(4):314–316. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- 46.Onaka T., Yagi K. Effects of novelty stress on vasopressin and oxytocin secretion by the pituitary in the rat. J Neuroendocrinol. 1993;5(4):365–369. doi: 10.1111/j.1365-2826.1993.tb00496.x. [DOI] [PubMed] [Google Scholar]
- 47.Jezova D., Skultetyova I., Tokarev DI., Bakos P., Vigas M. Vasopressin and oxytocin in stress. Ann N Y Acad Sci. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- 48.Wotjak CT., Ganster J., Kohl G., Holsboer F., Landgraf R., Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85(4):1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]
- 49.Neumann ID., Slattery DA. Oxytocin in general anxiety and social fear: a translational approach. Biol Psychiatry. 2016;79(3):213–221. doi: 10.1016/j.biopsych.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Bale TL., Davis AM., Auger AP., Dorsa DM., McCarthy MM. CNS regions-pecific oxytocin receptor expression: importance in regulation of anxiety and sex behavior. J Neurosci. 2001;21(7):2546–2552. doi: 10.1523/JNEUROSCI.21-07-02546.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blume A., Bosch OJ., Miklos S., et al Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27(8):1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 52.Jurek B., Slattery DA., Maloumby R., et al Differential contribution of hypothalamic MAPK activity to anxiety-like behaviour in virgin and lactating rats. PLoS One. 2012;7(5):e37060. doi: 10.1371/journal.pone.0037060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sabihi S., Durosko NE., Dong SM., Leuner B. Oxytocin in the prelimbic medial prefrontal cortex reduces anxiety-like behavior in female and male rats. Psychoneuroendocrinology. 2014;45:31–42. doi: 10.1016/j.psyneuen.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peters S., Slattery DA., Uschold-Schmidt N., Reber SO., Neumann ID. Dose-dependent effects of chronic central infusion of oxytocin on anxiety, oxytocin receptor binding and stress-related parameters in mice. Psychoneuroendocrinology. 2014;42:225–236. doi: 10.1016/j.psyneuen.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 55.Windle RJ., Shanks N., Lightman SL., Ingram CD. Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology. 1997;138(7):2829–2834. doi: 10.1210/endo.138.7.5255. [DOI] [PubMed] [Google Scholar]
- 56.Chang SW., Platt ML. Oxytocin and social cognition in rhesus macaques: implications for understanding and treating human psychopathology. Brain Res. 2014;1580:57–68. doi: 10.1016/j.brainres.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freeman SM., Inoue K., Smith, Goodman MM., Young LJ. The neuroanatomical distribution of oxytocin receptor binding and mRNA in the male rhesus macaque (Macaca mulatta). Psychoneuroendocrinology. 2014;45:128–141. doi: 10.1016/j.psyneuen.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winslow JT., Noble PL., Lyons CK., Sterk SM., Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 59.Chang SW., Barter JW., Ebitz RB., Watson KK., Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc Natl Acad Sci U S A. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ebitz RB., Watson KK., Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proc Natl Acad Sci U S A. 2013;110(28):11630–11635. doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parr LA., Modi M., Siebert E., Young LJ. Intranasal oxytocin selectively attenuates rhesus monkeys' attention to negative facial expressions. Psychoneuroendocrinology. 2013;38(9):1748–1756. doi: 10.1016/j.psyneuen.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lai MC., Lombardo MV., Baron-Cohen S. Autism. Lancet. 2014;383(9920): 896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 63.Schneier FR., Rodebaugh TL., Blanco C., Lewin H., Liebowitz MR. Fear and avoidance of eye contact in social anxiety disorder. Compr Psychiatry. 2011;52(1):81–87. doi: 10.1016/j.comppsych.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bogels SM., Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clin Psychol Rev. 2004;24(7):827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Hyman SE. Stress and the Brain: the Science of Mental Health. London, UK: Taylor & Francis; 2013 [Google Scholar]
- 66.Simpson EA., Sclafani V., Paukner A., Hamel AF., Novak MA., Meyer JS. Inhaled oxytocin increases positive social behaviors in newborn macaques. Proc Natl Acad Sci U S A. 2014;111(19):6922–6927. doi: 10.1073/pnas.1402471111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alvares GA., Chen NT., Balleine BW., Hickie IB., Guastella AJ. Oxytocin selectively moderates negative cognitive appraisals in high trait anxious males. Psychoneuroendocrinology. 2012;37(12):2022–2031. doi: 10.1016/j.psyneuen.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 68.Cardoso C., Linnen AM., Joober R., Ellenbogen MA. Coping style moderates the effect of intranasal oxytocin on the mood response to interpersonal stress. Exp Clin Psychopharmacol. 2012;20(2):84–91. doi: 10.1037/a0025763. [DOI] [PubMed] [Google Scholar]
- 69.Labuschagne I., Phan KL., Wood A., et al Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kawamura Y., Liu X., Akiyama T., et al The association between oxytocin receptor gene (OXTR) polymorphisms and affective temperaments, as measured by TEMPS-A. J Affect Disord. 2010;127(1-3):31–37. doi: 10.1016/j.jad.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 71.LoParo D., Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015;20(5):640–646. doi: 10.1038/mp.2014.77. [DOI] [PubMed] [Google Scholar]
- 72.Saphire-Bernstein S., Way BM., Kim HS., Sherman DK., Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc Natl Acad Sci U S A. 2011;108(37):15118–151122. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumsta R., Hummel E., Chen FS., Heinrichs M. Epigenetic regulation of the oxytocin receptor gene: implications for behavioral neuroscience. Front Neurosci. 2013;7:83. doi: 10.3389/fnins.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saphire-Bernstein S., Way BM., Kim HS., Sherman DK., Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc Natl Acad Sci U S A. 2011;108(37):15118–15122. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ziegler C., Dannlowski U., Bräuer D., Stevens S., Laeger I., Wittmann H. Oxytocin receptor gene methylation: converging multilevel evidence for a role in social anxiety. Neuropsychopharmacology. 2015;40(6):1528–1538. doi: 10.1038/npp.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoge EA., Pollack MH., Kaufman RE., Zak PJ., Simon NM. Oxytocin levels in social anxiety disorder. CNS Neurosci Ther. 2008;14(3):165–170. doi: 10.1111/j.1755-5949.2008.00051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marazziti D., Baroni S., Catena M., et al A relationship between social anxiety and oxytocin. Eur Psychiatry. 2007;22:S281–S282. [Google Scholar]
- 78.Labuschagne I., Phan KL., Wood A., et al Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Phan KL., Fitzgerald DA., Nathan PJ., Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalised social phobia. Biol Psychiatry. 2006;59(4):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 80.Huber D., Veinante P., Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308(5719):245–248. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 81.Labuschagne I., Phan KL., Wood A., et al Medial frontal hyperactivity to sad faces in generalized social anxiety disorder and modulation by oxytocin. Int J Neuropsychopharmacol. 2012;15(7):883–896. doi: 10.1017/S1461145711001489. [DOI] [PubMed] [Google Scholar]
- 82.Gorka SM., Fitzgerald DA., Labuschagne I., et al Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology. 2015;40(2):278–286. doi: 10.1038/npp.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dodhia S., Hosanagar A., Fitzgerald DA., Labuschagne I., Wood AG., Nathan PJ. Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology. 2014;39(9):2061–2069. doi: 10.1038/npp.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guastella AJ., Howard AL., Dadds MR., Mitchell P., Carson DS. A randomized controlled trial of intranasal oxytocin as an adjunct to exposure therapy for social anxiety disorder. Psychoneuroendocrinology. 2009;34(6):917–923. doi: 10.1016/j.psyneuen.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Levinson CA., Rodebaugh TL. Social anxiety and eating disorder comorbidity: the role of negative social evaluation fears. Eat Behav. 2012;13(1):27–35. doi: 10.1016/j.eatbeh.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Foussias G., Agid O., Fervaha G., Remington G. Negative symptoms of schizophrenia: clinical features, relevance to real world functioning and specificity versus other CNS disorders. Eur Neuropsychopharmacol. 2014;24(5):693–709. doi: 10.1016/j.euroneuro.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 87.Feifel D., Macdonald K., Nguyen A., Cobb P., Warlan H., Galangue B. Adjunctive intranasal oxytocin reduces symptoms in schizophrenia patients. Biol Psychiatry. 2010;68(7):678–680. doi: 10.1016/j.biopsych.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 88.Feifel D., Shilling D., MacDonald K. A review of oxytocin's effects on the positive, negative, and cognitive domains of schizophrenia. Biol Psychiatry. 2015;79(3):222–223. doi: 10.1016/j.biopsych.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Averbeck, Bobin T., Evans S., Shergill SS. Emotion recognition and oxytocin in patients with schizophrenia. Psychol Med. 2012;42(2):259–266. doi: 10.1017/S0033291711001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davis MC., Lee J., Horan WP., et al Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147(2-3):393–397. doi: 10.1016/j.schres.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 91.Preti A., Melis M., Siddi S., Vellante M., Doneddu G., Fadda R. Oxytocin and autism: a systematic review of randomized controlled trials. J Child Adolesc Psychopharmacol. 2014;24(2):54–68. doi: 10.1089/cap.2013.0040. [DOI] [PubMed] [Google Scholar]
- 92.Andari E., Duhamel JR., Zalla T., Herbrecht E., Leboyer M., Sirigu A. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci U S A. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hollander E., Bartz J., Chaplin W., et al Oxytocin increases retention of social cognition in autism. Biol Psychiatry. 2007;61(4):498–503. doi: 10.1016/j.biopsych.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 94.Guastella AJ., Einfeld SL., Gray KM., et al Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biol Psychiatry. 2010;67(7):692–694. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 95.de Lacy N., King BH. Revisiting the relationship between autism and schizophrenia: toward an integrated neurobiology. Annu Rev Clin Psychol. 2013;9:555–587. doi: 10.1146/annurev-clinpsy-050212-185627. [DOI] [PubMed] [Google Scholar]
- 96.Kim YR., Kim CH., Cardi V., Eom JS., Seong Y., Treasure J. Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology. 2014;44:133–142. doi: 10.1016/j.psyneuen.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 97.Kim YR., Kim CH., Park JH., Pyo J., Treasure J. The impact of intranasal oxytocin on attention to social emotional stimuli in patients with anorexia nervosa: a double blind within-subject cross-over experiment. PLoS One. 2014;9(6):e90721. doi: 10.1371/journal.pone.0090721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim YR., Eom JS., Yang JW., Kang J., Treasure J. The impact of oxytocin on food intake and emotion recognition in patients with eating disorders: a double blind single dose within-subject cross-over design. PLoS One. 2015;10(9):e0137514. doi: 10.1371/journal.pone.0137514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Insel TR. Translating oxytocin neuroscience to the clinic: a national institute of mental health perspective. Biol Psychiatry. 2015;79(1):153–154. doi: 10.1016/j.biopsych.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 100.Lawson EA., Holsen LM., Santin M., et al Postprandial oxytocin secretion is associated with severity of anxiety and depressive symptoms in anorexia nervosa. J Clin Psychiatry. 2014;74(5):e451–e457. doi: 10.4088/JCP.12m08154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guastella AJ., Hickie IB., McGuinness MM., et al Recommendations for the standardisation of oxytocin nasal administration and guidelines for its reporting in human research. Psychoneuroendocrinology. 2013;38(5):612–625. doi: 10.1016/j.psyneuen.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 102.Evans SL., Dal Monte O., Noble P., Averbeck BB. Intranasal oxytocin effects on social cognition: a critique. Brain Res. 2014;1580:69–77. doi: 10.1016/j.brainres.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ermisch A., Rühle HJ., Landgraf R., Hess J. Blood-brain barrier and peptides. J Cereb Blood Flow Metab. 1985;5(3):350–357. doi: 10.1038/jcbfm.1985.49. [DOI] [PubMed] [Google Scholar]
- 104.Kelly AM., Goodson JL. Social functions of individual vasopressin-oxytocin cell groups in vertebrates: what do we really know? Front Neuroendocrinol. 2014;35(4):512–529. doi: 10.1016/j.yfrne.2014.04.005. [DOI] [PubMed] [Google Scholar]