Abstract
Background
There is only limited agreement with respect to location, directionality and functional implications of brain structural alterations observed in patients with schizophrenia. Additionally, their link to occurrence of psychotic symptoms remains unclear. A viable way of addressing these questions is to examine populations in an at-risk mental state (ARMS) before the transition to psychosis.
Methods
We tested for structural brain alterations in individuals in an ARMS compared with healthy controls and patients with first-episode psychosis (FEP) using voxel-based morphometry and measures of cortical thickness. Furthermore, we evaluated if these alterations were modified by age and whether they were linked to the observed clinical symptoms.
Results
Our sample included 59 individuals with ARMS, 26 healthy controls and 59 patients with FEP. We found increased grey matter volume and cortical thickness in individuals with ARMS and a similar pattern of structural alterations in patients with FEP. We further found stronger age-related reductions in grey matter volume and cortical thickness in both patients with FEP and individuals with ARMS, linking these alterations to observed clinical symptoms.
Limitations
The ARMS group comprised subgroups with heterogeneous levels of psychosis risk and medication status. Furthermore, the cross-sectional nature of our study and the reduced number of older patients limit conclusions with respect to observed interactions with age.
Conclusion
Our findings on consistent structural alterations in individuals with ARMS and patients with FEP and their link to clinical symptoms have major implications for understanding their time of occurrence and relevance to psychotic symptoms. Interactions with age found for these alterations may explain the heterogeneity of findings reported in the literature.
Introduction
Neuroanatomical studies and meta-analyses in patients with schizophrenia and patients with first-episode psychosis (FEP) have consistently reported evidence of brain structural abnormalities, as measured by structural MRI (sMRI).1–11 Structural alterations commonly found in these studies are volumetric alterations in the anterior cingulate, frontal and temporal regions; hippocampus; amygdala; thalamus; and insula.2,8,12–15 Decreases, increases and negative findings were found, with most studies reporting reduced total and regional grey matter volumes. A comprehensive meta-analysis of these studies indicated the progressive nature of most of these brain structural alterations.3 Additionally, a recent meta-analysis of longitudinal studies found a correlation of these longitudinal brain structural alterations with exposure to antipsychotic treatment, but not with illness duration.16 In contrast, a large cross-sectional meta-analysis found correlations with both factors.17 Another study suggested abnormal nonlinear growth processes as a contributing factor for the observed longitudinal structural alterations.18 Overall, the findings on the directions and location of structural alterations are highly heterogeneous, even across the meta-analyses. It remains largely unclear if and which of the reported structural alterations are linked to specific clinical symptoms and represent an early biomarker or a consequence of psychosis.
A possible way to resolve this question on the time of occurrence of structural alterations in patients with schizophrenia is by examining individuals with a high risk for psychosis (i.e., at-risk mental state, ARMS).19–21 If alterations reported in patients with FEP are a cause or at least a phenotype unrelated to early diagnosis and treatment, one would expect to find similar alterations, though probably less expressed, in the ARMS population. Recent studies aimed to answer this question by comparing individuals with ARMS to healthy controls or patients with FEP.9,15,22–30 Only a subset of those studies compared volumetric information among individuals with ARMS, patients with FEP and healthy controls using unbiased whole-brain approaches.9,22,24,30 Although most of these studies reported prefrontal and temporal decreases in individuals with ARMS compared with healthy controls, the largest study so far failed to find any structural differences among the groups.15 None of the identified studies tested for age-related differences in the identified brain structural alterations among the groups, and only 1 evaluated the link between structural changes found in individuals with ARMS and observed clinical symptoms with a primary focus on cognitive deficits.25 In particular, understanding the associations between observed structural endophenotypes and specific symptoms is essential to establish their relevance as potential treatment biomarkers. Possible reasons for the inconsistent findings with respect to brain structural alterations may include differences in sample sizes, control populations, preprocessing and statistical methodology, demographic factors, or the known heterogeneity of psychotic populations. Correspondingly, from these studies it remains unclear if brain structural alterations in individuals with ARMS can be considered a consequence or an intermediate or predisposing phenotype on the way to manifested psychosis. It also remains unknown whether these structural alterations evolve with age and how they are linked to specific clinical symptoms.
Here we aim to address these questions of early psychosis-related structural brain alterations and their association with age and psychotic symptoms by comparing different structural measures among individuals with ARMS, healthy controls and patients with FEP. We further evaluate if structural alterations observed in patients with FEP and individuals with ARMS are differentially modified by age as compared with each other and with healthy controls. Presence of such interactions between diagnostic groups and age would suggest a differential evolution of brain structural measures over time or different underlying structural pathology in younger and older patients with FEP and individuals with ARMS that might explain the heterogeneity of previous literature findings. Finally, to understand the relevance of the identified structural alterations for specific symptoms we evaluate in subsequent exploratory analyses if the identified brain structural phenotypes are linked to specific psychiatric symptoms observed in those populations.
Methods
Participants
We recruited patients with FEP, individuals with ARMS and healthy controls in the framework of the Basel FePsy study (Früherkennung von Psychosen).31,32 All participants provided written informed consent and received compensation for participating in the present study. The study was approved by the local ethics committee (Ethikkommission Nordwest-und Zentralschweiz [EKNZ]).
Individuals with ARMS and patients with FEP had been seeking help and were assigned to the FePsy study. They were examined by a trained psychiatrist; among other examinations and upon their agreement, MRI scans were obtained as part of the evaluation. Each patient had a case manager who treated them for the duration of the study and who decided if they were able to participate in subsequent examinations, especially if the examinations were not therapeutically relevant (e.g., research sMRI). Participants’ inclusion into the present MRI examination occurred between November 2008 and April 2014. Among the patients who were considered suitable to undergo MRI and who agreed to participate, some were excluded because of medical conditions (acute psychotic symptoms, anxiety and tinnitus), and some decided immediately before the scan not to participate. Before the sMRI section each participant was informed in detail about the procedure and its possible risks and benefits.
The FEP group fulfilled the operational criteria for FEP as described by Yung and colleagues,33 but not the criteria for schizophrenia. Inclusion in the FEP group required scores above 3 on the hallucination item or scores above 4 on the unusual thought content, suspiciousness or conceptual disorganization items of the Brief Psychiatric Rating Scale (BPRS).34 The BRPS was administered on average 9 ± 16 days before or after the MRI examination. In addition, the Scale for Assessment of Negative Symptoms (SANS) was administered in a subsample of participants. The symptoms must have occurred at least several times a week and persisted for more than 1 week.
The ARMS group was defined based on Personal Assessment and Crisis Evaluation (PACE) criteria33 used in previous MRI studies. The very good interrater and prognostic reliability of these criteria have been established in our previous studies.35,36 Inclusion thus required 1 or more of the following: attenuated psychotic symptoms, brief limited intermittent psychotic symptoms (BLIPS), or a first-degree relative with a psychotic disorder plus at least 2 indicators of a clinical change, such as a marked decline in social or occupational functioning. Inclusion because of attenuated psychotic symptoms required scores of 2 or 3 on the hallucination item and scores of 3 or 4 on the unusual thought content or suspiciousness items of the BPRS for at least several times a week and persisting for more than 1 week. Inclusion because of BLIPS required scores of 4 or higher on the hallucination item, or 5 or higher on the unusual thought content, suspiciousness or conceptual disorganization items of the BPRS, with each symptom lasting less than 1 week before resolving spontaneously.
Healthy controls were recruited through local advertisement. To be included, they had to have no history of psychiatric illness, head trauma, neurologic illness, serious medical or surgical illness, or substance dependence, and no family history of any psychiatric disorder, as assessed by an experienced psychiatrist in a detailed clinical interview.
Exclusion criteria for all groups were age younger than 18 years, insufficient knowledge of German, IQ below 70, previous episode of schizophrenic psychosis (treated with major tranquilizers for more than 3 weeks), psychosis due to organic reasons or substance dependence, psychotic symptoms within a clearly diagnosed affective psychosis, or borderline personality disorder.
Structural MRI
All participants were scanned using a Siemens (Erlangen, Germany) MAGNETOM Verio 3 T scanner with a 12-channel radiofrequency head coil. Head movement was minimized by foam padding across the forehead. A whole-brain 3-dimensional T1-weighted magnetization prepared rapid acquisition gradient (MPRAGE) sequence was applied. The acquisition was based on a sagittal matrix of 256 × 256 × 176 and 1 × 1 × 1 mm3 isotropic spatial resolution, with an inversion time of 1000 ms, repetition time of 2 s, echo time of 3.4 ms, flip angle of 8° and bandwidth of 200Hz/pixel. All images were reviewed by trained neuroradiologists and assessed for radiological abnormalities.
Image preprocessing
All image preprocessing and subsequent statistical analyses of imaging data were performed using Statistical Parametric Mapping software version 12 (SPM12) implemented in Matlab R2013a. Image preprocessing comprised segmentation and spatial normalization of sMRI data into Montreal Neurological Institute (MNI) space using NewSegment37 and modulation of the normalized grey matter probability maps using the Jacobian determinants to preserve the total amount of signal (these maps are commonly referred to as voxel-wise grey matter volume). Further, we computed for each participant voxel-based cortical thickness (VBCT) maps using the VBCT toolbox provided for SPM12.38 In brief, the VBCT algorithm assigns to each grey matter voxel a value corresponding to its Euclidean distance to the white matter and cerebrospinal fluid boundary. The VBCT maps were spatially normalized into MNI space using parameters obtained from NewSegment. Both grey matter volume and VBCT maps were smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
Statistical analysis
We compared the clinical and demographic characteristics among the 3 groups using analyses of variance (ANOVAs) and χ2 tests (where appropriate), as implemented in SPSS software version 22. We considered results to be significant at p < 0.05. In case of significant between-group differences in an ANOVA, we performed post hoc Bonferroni-corrected t tests.
Imaging data
Voxel-wise general linear models were computed for both grey matter volume and VBCT maps, including group (FEP, ARMS, control) as a factor and covarying for sex, education and total intracranial volume. Grey matter alterations in the age range covered in our cohort have been shown to be sufficiently approximated by a cubic function.39 Correspondingly, a cubic age regressor was further included in the voxel-wise general linear model analyses. Further, as we were specifically interested in potential differences in age-related alterations, the cubic age regressor was modelled as an interaction term with the diagnosis. We also explored the option of using a linear age term. However, the results did not differ as compared with the cubic model. For both grey matter volume and VBCT we tested for 12 different t contrasts looking for regional relative volumetric between-group differences and for differences in age slopes in the pairwise comparisons of ARMS, FEP and control groups (6 contrasts [control > FEP, control < FEP, control > ARMS, control < ARMS, FEP > ARMS and FEP < ARMS] testing for increases or decreases in pairwise group comparisons, and 6 respective contrasts testing for age × group interactions).
As we were interested in identifying all regions associated with group differences and group-specific age-related alterations rather than peak voxels showing the maximum effects, to evaluate their association with clinical symptoms we used a whole-brain family-wise error (FWE)–corrected cluster threshold of p < 0.05 adjusted for nonstationarity of smoothness combined with a voxel-wise uncorrected threshold of p < 0.05.37,38 For grey matter volume analyses the cluster threshold p values were determined using the nonstationarity correction suggested for this modality.40,41 For VBCT analyses, we determined an exact whole-brain corrected cluster threshold of p < 0.05 using permutation statistics (1000 permutations), as implemented in the AlphaSim function.42 Importantly, as the main purpose of the study was to evaluate the link of structural alterations to clinical symptoms observed in the FEP and ARMS groups, a liberal voxel-wise threshold combined with a whole-brain corrected cluster-wise threshold was chosen to ensure that potentially smaller but more widespread effects were captured for subsequent correlations with clinical symptoms within the clinical groups. Further, to visualize the identified significant associations, we used grey matter volume or VBCT eigenvariates extracted from the significant clusters with the highest respective t values identified in the corresponding contrasts. Eigenvariates obtained from clusters showing differences in direct comparisons between groups were adjusted for all covariates of no interest (age, sex, education and total intracranial volume [TIV]). Eigenvariates extracted from clusters showing significant group × age interactions were adjusted for all covariates of no interest for these contrasts (sex, education and TIV, but not age). The adjusted eigenvariates from regions showing group × age interactions were plotted as a cubic function of age with the corresponding confidence intervals (CIs) separately for each group. Additionally, to provide an estimate of the magnitude of the structural alterations and their similarity between the FEP and ARMS groups, we computed effect sizes (Cohen d) for both ARMS and FEP relative to healthy controls based on cluster eigenvariates from contrasts testing for between-group differences.
Correlations between imaging and clinical measures
To evaluate whether any of the identified structural alterations were associated with psychopathological symptoms observed in patients with FEP or individuals with ARMS we performed an exploratory analysis computing Pearson correlation coefficients between BPRS subscales and extracted eigenvariates. An excellent interrater and a fair longitudinal reliability have been established for the BPRS in previous studies.43,44 Correlations were computed within each group between the extracted eigenvariates from the most significant clusters identified in each contrast (adjusted for covariates of no interest) and clinical symptoms, as measured with the BPRS total scores and its 6 subcomponent (mood disturbance, reality disturbance, activation, apathy, disorganization and somatization) scores.45 Only the patients for whom BPRS scores were available were included in all correlational analyses. All findings from these exploratory correlational analyses are reported at a significance level of p < 0.01. Further, to test if the evaluated BPRS subcomponents or the total score were associated with age, we computed Pearson correlations using a threshold of p < 0.05, uncorrected. To rule out that the identified significant correlations between structural alterations and BPRS subscale scores in the FEP or ARMS groups were confounded by medication status, we additionally computed partial correlations controlling for antipsychotic and antidepressant medication status. Both were entered into these partial correlation analyses as binary (yes/no) variables.
Results
Participants
Of the FePsy participants who were deemed suitable to undergo MRI and who agreed to participate in the present study, 5 (4 individuals with ARMS, 1 patient with FEP) were excluded because of medical conditions, such as acute psychotic symptoms, anxiety and tinnitus, and 4 withdrew immediately before the scan. Additionally, we excluded 5 controls because of medical conditions (incidental findings), resulting in a final sample of 59 patients with FEP, 59 with ARMS and 26 healthy controls with available sMRI scans.
Clinical and demographic findings
The demographic and clinical characteristics of participants are shown in Table 1. The groups did not differ in terms of age or age range. We observed significant between-group differences with respect to sex, education, and alcohol and cannabis consumption. Healthy controls were more often female, they had a higher level of education than patients with FEP (t83 = 3.0, p = 0.004) and individuals with ARMS (t83 = 2.6, p = 0.011), and they had a greater prevalence of moderate alcohol consumption and a lower prevalence of cannabis consumption than either of the patient groups. Correspondingly, sex and education were included as covariates of no interest in all imaging analyses.
Table 1.
Characteristics of the study sample
| Characteristic | Group; mean ± SD* | Statistical test | p value | ||
|---|---|---|---|---|---|
|
| |||||
| Control (n = 26) | ARMS (n = 59) | FEP (n = 59) | |||
| Sex, male:female | 12:14 | 43:16 | 42:17 | χ2 = 6.5 | 0.038 |
| Age, yr [range] | 27.7 ± 4.5 [20–39] | 24.7 ± 5.7 [18–43] | 26.4 ± 6.7 [18–42] | F2 = 2.7 | 0.07 |
| Education, yr | 15.6 ± 3.1 | 13.8 ± 2.9 | 13.4 ± 3.1 | F2 = 4.8 | 0.009 |
| BPRS score (n) | 24.5 ± 1.1 (25) | 39.4 ± 8.6 (49) | 49.7 ± 14.5 (47) | χ2 = 46.3 | < 0.001 |
| SANS (n) | 0 ± 0 (17) | 11.0 ± 11.9 (19) | 18.0 ± 14.8 (21) | χ2 = 11.8 | < 0.001 |
| On antipsychotics, no. | 0 | 0 | 28† | — | |
| On antidepressants, no. | 0 | 23 | 12 | — | |
| Cannabis use, % | 15.4 | 28.1 | 23.9 | χ2 = 1.6 | 0.45 |
| Smoking, % | 26.9 | 51.7 | 59.3 | χ2 = 7.6 | 0.022 |
| Alcohol consumption, %, no:moderate:uncontrolled | 4:89:8 | 21:66:14 | 34:54:12 | χ2 = 11.1 | 0.025 |
ARMS = at-risk mental state; BPRS = Brief Psychiatric Rating Scale; FEP = first-episode psychosis; SANS = Scale for Assessment of Negative Symtpoms; SD = standard deviation.
Unless indicated otherwise.
Antipsychotic medications were aripiprazole (n = 4), quetiapine (n = 11), paliperidone (n = 2), olanzapine (n = 7), risperidone (n = 3) and clozapine (n = 1). The mean chlorpromazine equivalent was 216 ± 267 mg.
Voxel-based morphometry
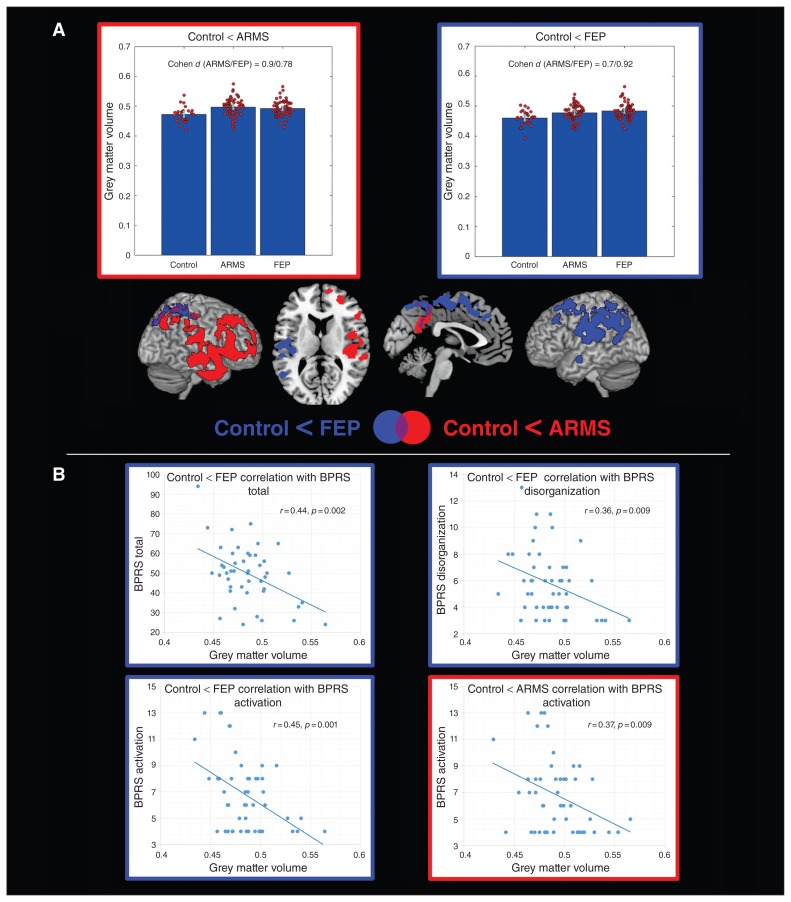
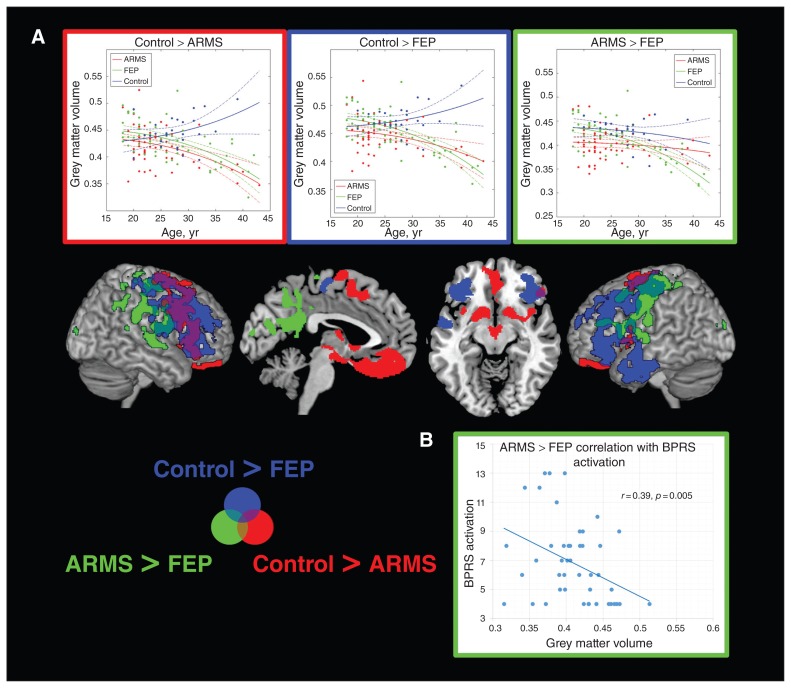
In voxel-wise grey matter volume group comparisons, we found significantly higher grey matter volume in patients with FEP and those with ARMS than in healthy controls in an extensive network covering the left prefrontal temporal, insular, right precuneus and bilateral parietal regions in patients with FEP and the right prefrontal, temporal, parietal, ventral striatal and bilateral precuneus regions in those with ARMS (Fig. 1A, Table 2). No differences in grey matter volume were observed between patients with FEP and those with ARMS. Effect sizes for the differences between healthy controls and either patients with FEP or those with ARMS ranged from 0.7 to 0.92, indicating strong effects (Fig. 1A), and were in general comparable for both the FEP and ARMS groups for all signficant clusters. Significantly higher age-related reductions of grey matter volume were found in both patients with FEP and those with ARMS than in healthy controls (Fig. 2A, Table 2). In individuals with ARMS significantly stronger age-related grey matter volume decreases were observed in the bilateral subgenual, ventral striatal, insular and premotor regions. A similar but more widespread network of stronger age-related decline compared with healthy controls was also observed in patients with FEP, covering bilateral dorsolateral prefrontal, premotor, motor and right temporal regions. Parts of this network covering bilateral premotor and motor but also other regions, including the precuneus and superior temporal regions, showed a significantly faster age-related grey matter volume decline in patients with FEP than in those with ARMS.
Fig. 1.
Voxel-based morphometry group comparisons. (A) Regions showing increased grey matter volume in individuals in an at-risk mental state (ARMS) and in patients with first-episode psychosis (FEP) compared with healthy controls. Combined bar and scatter plots show the grey matter volumes (cluster eigenvariates) for the 3 groups in the depicted clusters. The outline colour of the plots corresponds to the colour of the significant clusters for the corresponding contrast. Error bars represent 95% confidence intervals. (B) Correlations in the FEP group between eigenvariates extracted from significant clusters identified in group comparisons and subcomponents of the Brief Psychiatric Rating Scale (BPRS). Correlation with the eigenvariates of the cluster identified in the contrast control < ARMS is outlined in red. Correlations with the eigenvariates of the cluster identified in the contrast control < FEP are outlined in blue.
Table 2.
Regions showing significant differences in group comparisons and interactions with age
| Modality | Contrast | Cl | Anatomic regions | Cluster size | p value | Peak t value | Peak MNI coordinate (x, y, z) |
|---|---|---|---|---|---|---|---|
| Grey matter volume | ARMS > control | 1 | Right superior, middle and inferior frontal gyrus, temporal pole, superior, middle and inferior temporal gyrus, operculum, anterior insula, putamen, transverse temporal gyrus, triangular gyrus, posterior insula, supramarginal gyrus, pallidum | 14 165 | 0.001 | 3.637 | 62, −26, −15 |
| 2 | Right superior parietal lobule, angular gyrus, postcentral gyrus, cuneus and supramarginal gyrus, bilateral precuneus, posterior cingulate cortex | 5427 | 0.035 | 3.306 | 39, −68, 45 | ||
| Grey matter volume | FEP > control | 1 | Left postcentral gyrus, supramarginal gyrus, angular gyrus, superior parietal lobule, operculum, precentral gyrus, planum temporale, superior and middle temporal gyrus, posterior insula | 8403 | 0.002 | 3.745 | −60, −33, 21 |
| 2 | Right superior parietal lobule, supplementary motor cortex, postcentral gyrus, cuneus, superior occipital gyrus, bilateral precuneus, supplementary motor cortex, precentral gyrus, left superior and middle frontal gyrus | 9256 | 0.005 | 3.316 | 11, −2, 54 | ||
| Grey matter volume | Age interaction: control > ARMS | 1 | Bilateral supplementary motor cortex, superior and middle frontal gyrus, precentral gyrus, right triangular gyrus, operculum, inferior frontal gyrus | 6375 | 0.016 | 3.751 | 50, 18, 33 |
| 2 | Bilateral rectal gyrus, medial frontal cortex, insula, putamen, accumbens, caudate nucleus, basal forebrain, anterior cingulate cortex, thalamus, subcallosal area, right medial orbital gyrus, superior medial frontal gyrus | 8522 | 0.044 | 3.671 | 6, 8, −21 | ||
| Grey matter volume | Age interaction: control > FEP | 1 | Bilateral superior and middle frontal gyrus, precentral gyrus, triangular gyrus, pre- and postcentral gyrus, operculum, insula, orbitofrontal cortex, left superior, middle and inferior temporal gyrus, temporal pole, | 26 513 | < 0.001 | 4.452 | 33, 12, 38 |
| Grey matter volume | Age interaction: ARMS > FEP | 1 | Left pre- and postcentral gyrus, superior and middle frontal gyrus, operculum, superior parietal lobule | 6459 | 0.014 | 3.75 | −36, −20, 62 |
| 2 | Right pre- and postcentral gyrus, supramarginal gyrus, superior parietal lobule, operculum, planum temporale, insula, bilateral posterior cingulate gyrus, cuneus, left superior occipital gyrus, calcalrine cortex | 12 555 | 0.002 | 3.653 | −5, −47, 26 | ||
| VBCT | Control > ARMS | 1 | Bilateral superior and middle frontal and precentral gyrus | 6191 | 0.009 | 3.62 | 11, −11, 77 |
| VBCT | ARMS > control | 1 | Bilateral superior parietal lobule, left inferior occipital gyrus, superior parietal lobule, angular gyrus, middle occipital gyrus, occipital pole, cerebellum exterior | 11 365 | < 0.001 | 3.85 | −17, −95, 11 |
| VBCT | FEP > control | 1 | Left superior, middle and inferior occipital gyrus, occipital pole, fusiform gyrus, cerebellum exterior | 5574 | 0.017 | 4.78 | −17, −96, 5 |
| 2 | Right superior, middle and inferior occipital gyrus, occipital pole, cerebellum exterior, fusiform and lingual gyrus, calcarine cortex | 5493 | 0.020 | 4.53 | 23, −96, 5 | ||
| VBCT | Age interaction: Control > ARMS | 1 | Left cerebellum exterior, fusiform gyrus, superior, middle and inferior temporal gyrus, angular gyrus, inferior and middle occipital gyrus, parahippocampal gyrus, lingual gyrus hippocampus | 8959 | < 0.001 | 4.25 | −54, −53, 6 |
| 2 | Left superior and middle frontal gyrus, frontal pole, triangular gyrus, bilateral medial prefrontal gyrus, anterior cingulate gyrus | 10 262 | < 0.001 | 3.98 | −2, 42, 18 | ||
| 3 | Right superior, middle and inferior temporal gyrus, inferior and middle occipital gyrus, cerebellum exterior, fusiform gyrus | 5626 | 0.017 | 3.53 | 41, −71, −27 | ||
| 4 | Bilateral precuneus, posterior cingulate gyrus, lingual gyrus, vermis, cerebellum exterior, cuneus, lingual gyrus, calcarine cortex | 8066 | < 0.001 | 3.15 | 0, −54, 51 | ||
| VBCT | Age interaction: control > FEP | 1 | Bilateral cerebellum exterior, lingual gyrus, vermis, fusiform gyrus, left parahippocampal gyrus | 5467 | 0.020 | 3.74 | 12, −45, −23 |
ARMS = at-risk mental state; Cl = cluster number; FEP = first-episode psychosis; MNI = Montreal Neurological Institute; VBCT = voxel-based cortical thickness.
Fig. 2.
Voxel-based morphometry interaction analyses with age. (A) Regions showing significant age-related differences between individuals in an at-risk mental state (ARMS), patients with first-episode psychosis (FEP) and healthy controls are displayed with the corresponding age plots for eigenvariates extracted from most significant clusters. The outline colour of the plots corresponds to the colour of the significant clusters for the corresponding contrast. (B) Significant correlation in the FEP group between the cluster showing a significant group × age interaction in the contrast ARMS > FEP and the Brief Psychiatric Rating Scale (BPRS) activation subcomponent.
Voxel-based cortical thickness
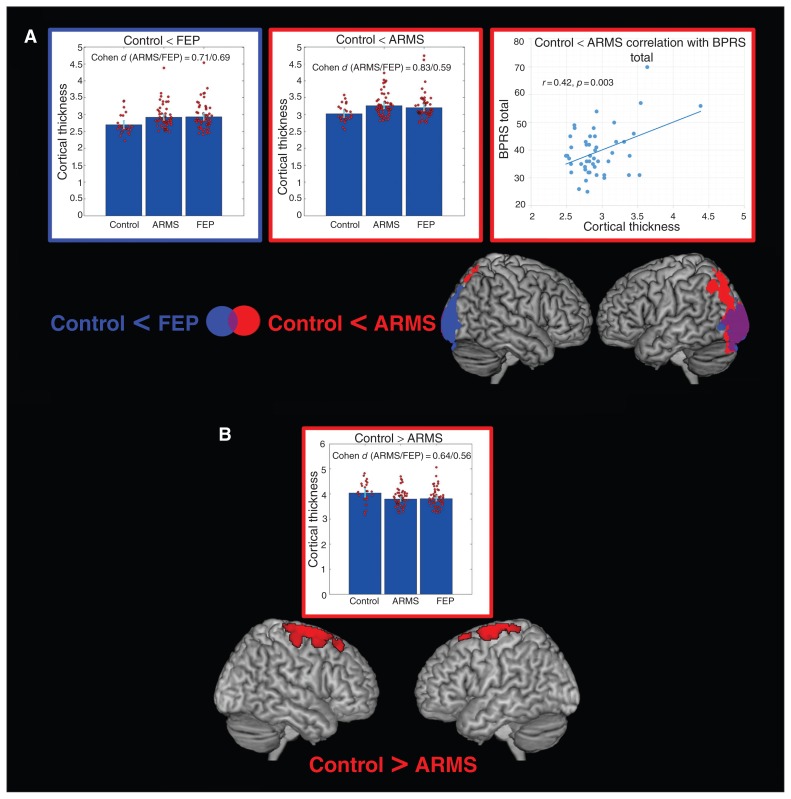
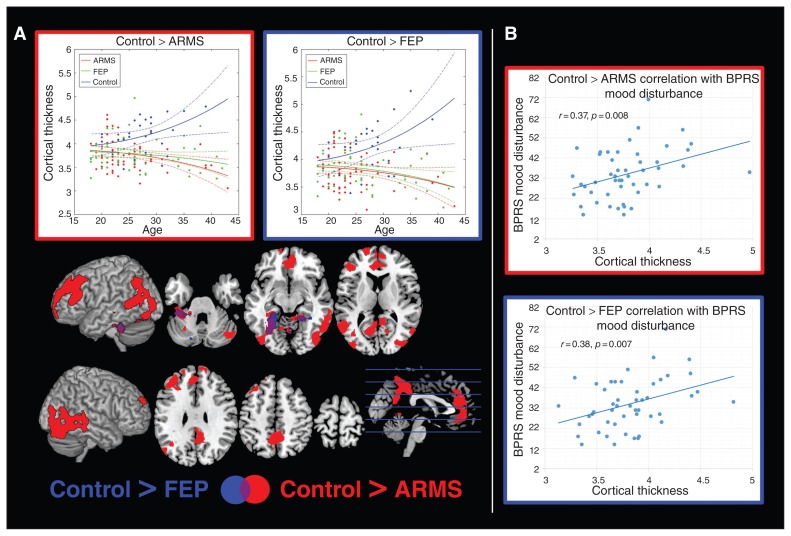
When comparing VBCT across the 3 groups, we found significant increases in both patients with FEP and those with ARMS compared with healthy controls (Fig. 3, Table 2). In both patients with FEP and those with ARMS these increases were observed in bilateral occipital regions. In the ARMS group the significant clusters additionally extended to bilateral parietal cortices. Furthermore, we observed significantly lower VBCT in individuals with ARMS than in healthy controls in bilateral premotor and motor cortices. No significant differences were observed in VBCT between the FEP and ARMS group. The VBCT differences between healthy controls and either patients with FEP or those with ARMS were slightly lower than the VBM differences, ranging from 0.59 to 0.83 (Fig. 1A), and were comparable for both the FEP and ARMS groups for all signficant clusters (Fig. 3). In both patients with FEP and those with ARMS we identified significantly higher age-related cortical thinning than in healthy controls (Fig. 4A, Table 2). In the ARMS group these differences covered an extensive network, including bilateral dorsolateral prefrontal, anterior cingulate, inferior parietal, fusiform, posterior middle and inferior temporal, cerebellar and precuneal regions. In the FEP group the significant differences were more focal, covering bilateral cerebellar and fusiform regions. No differences with respect to age-related cortical alterations were observed in the direct comparisons between the FEP and ARMS groups.
Fig. 3.
Voxel-based cortical thickness group comparisons. (A) Regions showing increased cortical thickness in individuals in an at-risk mental state (ARMS) and patients with first-episode psychosis (FEP) compared with healthy controls. Combined bar and scatter plots show the cortical thickness (cluster eigenvariates) for the 3 groups in the depicted clusters. The outline colour of the plots corresponds to the colour of the significant clusters for the corresponding contrast. Error bars represent 95% confidence intervals. The scatter plots on the right (outlined in red) show the significant correlation in the ARMS group between cortical thickness from the cluster identified in the contrast control < ARMS and the Brief Psychiatric Rating Scale (BPRS) total score. (B) Regions showing reduced cortical thickness in the ARMS group compared with the control group. The combined bar and scatter plot show the cortical thickness (cluster eigenvariates) for the 3 groups in the depicted clusters.
Fig. 4.
Voxel-based cortical thickness interaction analyses with age. (A) Regions showing significant age-related differences between individuals in an at-risk mental state (ARMS), patients with first-episode psychosis (FEP) and healthy controls are shown with the corresponding age plots for eigenvariates extracted from the most significant clusters provided in the top row. The outline colour of the plots corresponds to the colour of the significant clusters for the corresponding contrast. (B) Significant correlations in the FEP group between the Brief Psychiatric Rating Scale (BPRS) mood disturbance subcomponent and cortical thickness (cluster eigenvariates) in regions showing significant group × age interactions in the contrast control > ARMS (outlined in red) and control > FEP (outlined in blue).
Correlations between imaging and clinical measures
None of the BPRS subcomponent scores or the total BPRS score correlated with age (all p > 0.10). In the ARMS group, the only significant positive correlation with BPRS total score was found with VBCT in occipital regions (obtained from the group comparison contrast FEP > control; Fig. 3A). In the FEP group, significant negative correlations were found between grey matter volume from the group comparison contrast FEP > control and BPRS total score, and BPRS activation and disorganization subcomponent scores (Fig. 1B). Furthermore, significant negative correlations were found in patients with FEP between the BPRS activation subcomponent score and prefrontal and parietal grey matter volume from the group comparison contrast ARMS > control and prefrontal grey matter volume from the age interaction contrast FEP < ARMS (Fig. 1B, Fig. 2) In patients with FEP, we further found significant positive correlations between the BPRS mood disturbance subcomponent score and occipitoparietal VBCT from the age interaction contrasts ARMS < control and FEP < control (Fig. 4). All identified correlations between BPRS subscale scores and structural measures remained significant in both the FEP and ARMS groups when controlling for medication status.
Discussion
In the present study we identified structural alterations in individuals with ARMS as a potential early endophenotype of psychosis. We observed extensive structural differences in both patients with FEP and those with ARMS as compared with healthy controls. Both the FEP and ARMS groups showed significantly faster age-related reductions in grey matter volume and VBCT than healthy controls in a largely overlapping anatomic network. We further found these structural alterations in patients with FEP and those with ARMS to be linked to the severity of psychotic symptoms.
In group comparisons of the ARMS and FEP groups with the control group we found increased grey matter volume in both patients with FEP and those with ARMS in a widespread anatomic set of regions covering predominantly left premotor, parietal, insular and temporal regions in patients with FEP and right parietal, prefrontal, insular and temporal regions in those with ARMS. Importantly, there were no significant differences between the FEP and ARMS groups. In addition, the effect size of the structural alterations was similar for both the FEP and ARMS groups relative to the control group for all identified between-group differences. On one hand, these findings indicate that the structural alterations are indeed an early marker of psychosis already present in the ARMS population; on the other hand, the comparable magnitude of these alterations in both patients with FEP and those with ARMS indicates that these alterations are a stable endophenotype potentially predisposing to psychosis. In contrast, the magnitude of those alterations seems not to be linked to conversion from ARMS to FEP. However, this hypothesis remains to be tested in a proper longitudinal setting. In general, the findings of increased grey matter volume in parietal, insular, premotor and temporal regions in patients with schizophrenia and those with ARMS are consistent with previous reports and a recent meta-analysis of VBM studies suggesting an involvement of these regions in patients with schizophrenia and psychosis.9,46–52 However, the directionality of these grey matter volume abnormalities is discussed controversially in the literature.8,9,17,53 In fact, most studies, including our own, conducted in cohorts that did not overlap with the present one found grey matter volume decreases in these patient populations.9,54 A possible explanation for these differential findings across studies and meta-analyses with respect to direction of grey matter volume alterations in patients with psychosis could be due to the identified interactions between diagnosis and age in addition to previously reported treatment, genetic predisposal, ARMS duration or other confounding effects on grey matter structure.17,53,55–57 Among other reasons, including hardware and preprocessing differences, different definitions of the ARMS status could have contributed to the observed heterogeneity. Based on the criteria applied in our study we would, for example, expect our ARMS cohort to mainly comprise individuals with attenuated psychotic symptoms.58 Although the reasons for this divergence remain open, our findings question grey matter volume decreases as a common biomarker in FEP and ARMS populations, suggesting grey matter volume increases also exist in these patient cohorts. Regions showing interactions with age in our study cover an extensive anatomic network overlapping most of the regions also showing an increased grey matter volume in group comparisons. The identified interactions suggest an age-specific differential pattern of grey matter volume differences in these regions in both patients with FEP and those with ARMS compared with healthy controls. These differential findings need to be considered in future imaging studies in patients with schizophrenia and in at-risk populations. Based on these findings, comparison of older patients with ARMS and FEP with healthy controls would likely result in more prevalent structural decreases. Similarly, use of 3 T scanners compared with 1.5 T scanners used in earlier studies along with novel preprocessing pipelines presumably improved segmentation of grey matter structures and may have increased sensitivity to detect grey matter tissue in patients with ARMS and those with FEP. Previous reports suggested, for example, selectively reduced density of oligodendroglia cells in layer VI of patients with schizophrenia.59 Such alterations at the grey and white matter boundary may lead to misleading shifts in classification of grey matter tissue.
In general, our findings of faster age-related grey matter volume reductions in patients with FEP and those with ARMS are in line with the findings of previous longitudinal studies and meta-analyses suggesting accelerated aging in patients with FEP and those with ARMS compared with healthy controls.8 Importantly, the interactions observed in our study are cross-sectional. Correspondingly, the observed age-dependent decreases in grey matter volume in both the FEP and ARMS groups cannot be directly attributed to disease progression, as it is possible that differential disease-related or compensational processes are associated with psychotic symptoms in younger and older patients with FEP and ARMS. The negative correlations we found between grey matter volume increases and symptom severity in patients with FEP support the assumption that compensational processes are linked to the grey matter volume increases in patients with FEP and those with ARMS. In contrast, the finding of a positive correlation between symptom severity and cortical thickness measures in regions of increased cortical thickness in individuals with ARMS support the assumption of disease-related contribution of these structural alterations. Interestingly, we found a gradient from anterior to posterior regions with respect to diagnosis × age interactions observed in grey matter volume. Although in this analysis the lateral prefrontal and premotor regions showed age-related grey matter volume reductions in both the FEP and ARMS groups, the more posterior motor and precuneal regions showed a stronger age-associated reduction only in the FEP group. With the limitation that these findings were obtained in a cross-sectional setting, they support the idea of abnormal nonlinear growth processes as a factor contributing to schizophrenia.18 However, they may also represent evidence of differential mechanisms underlying occurrence or the risk of psychosis in younger and older individuals. The observed negative correlation with the BPRS activation subcomponents comprising motor hyperactivity, excitement and distractibility items is supportive for involvement of grey matter volume abnormalities in these regions in psychotic symptoms. Though such cross-sectional correlations observed between clinical symptoms and brain structural phenotypes identified in patients with FEP and those with ARMS may indicate a dynamic link between brain plasticity in specific regions and the occurrence of respective clinical symptoms. Alternatively, they may also reflect a more static predisposal of patients with specific structural phenotypes for exhibiting more severe and specific clinical symptoms.
When comparing cortical thickness across groups we found bidirectional alterations in the ARMS cohort, with reduced cortical thickness in motor and premotor regions and increased cortical thickness in occipitoparietal areas. These findings of increased cortical thickness in individuals with ARMS are consistent with alterations observed in the FEP cohort and are positively correlated with symptom severity in individuals with ARMS, indicating their potential relevance for emergence of psychotic symptoms. Similar to grey matter volume, both decreases and increases were reported in previous studies evaluating cortical thickness in patients with schizophrenia or psychosis.23,60–63 Our findings suggest that both types of alterations coexist in patients with psychosis, indicating a more complex picture than just global cortical thinning as suggested in previous studies.62,64 Possible explanations for such bidirectional alterations could be a more complex neurodevelopmental misbalance of cortical development or potential compensatory effects across different brain regions. In individuals with ARMS we also found accelerated age-related decreases in cortical thickness in the default mode, theory of mind and working memory–associated regions. These findings are consistent with those of previous studies of longitudinal cortical thickness reductions in the development of psychosis.30 In patients with FEP, cortical thickness in these regions positively correlated with BPRS mood disturbance subcomponents, one of the key psychotic symptoms. Considering that none of the BPRS subcomponents correlated with age, these cross-sectional findings indicate that increased cortical thickness in these regions may be associated with increased risk of psychotic symptoms.
Limitations
The ARMS group comprised subgroups of individuals with different heterogeneous levels of psychosis risk, recruitment strategies65 and consecutive brain abnormalities. Furthermore, the cross-sectional nature of our study does not allow us to make direct conclusions about interactions with age being evidence of altered brain development. Though in line with this theory, our findings can also be interpreted as evidence of differential pathophysiological processes being linked to the emergence of psychotic symptoms in younger and older patients. The reduced number of older patients and controls is a further limitation of our study that might explain the slight age-related increase in grey matter volume and VBCT observed in healthy controls for some of the clusters. In that context, the lower number and the restrictive inclusion criteria for healthy controls (e.g., no family history of any psychiatric disorder) also may have led to biased findings with respect to identified structural alterations in both patient cohorts. As the slope of this increase is not significantly different from zero, these results are consistent with previous literature showing a relatively stable grey matter volume and VBCT in these mostly prefrontal regions in healthy controls for the age range evaluated in our study.66 Furthermore, both the FEP and ARMS groups included a significant number of individuals treated with antidepressants. Though in our correlational analyses we controlled for the potential effects of antidepressants or other medication on clinical symptoms, brain structure might have affected the group comparisons. Importantly, owing to the exploratory nature of the correlational analyses with clinical symptoms, we applied a more lenient statistical threshold. The respective findings should therefore be considered as hypothesis-generating and need to be validated in future studies. Finally, the FEP and ARMS groups had a higher proportion of male participants, and the ARMS group was slightly younger than the FEP and control groups. Both variables were included as control variables or variables of interest in the analyses to minimize their impact. Nonetheless, these differences and other uncontrolled factors, such as psychotic illness duration, subtypes of ARMS might have biased some of our findings.
Conclusion
The present findings of significantly faster age-related decline in grey matter volume and VBCT in the early phase of psychosis development that are linked to clinical symptoms have major implications for understanding the time of occurrence of these alterations. Interactions with age observed for these structural differences may explain the heterogeneity of current imaging findings during the early course of psychosis.
Footnotes
See the commentary by Palaniyappan and colleagues on p. 294
Competing interests: J. Dukart is a full-time employee of F. Hoffmann-La Roche, but received no specific funding for this work. F. Hoffmann-La Roche provided support in the form of salary, but did not have any additional role in the study design, data collection, statistical analysis, decision to publish, or preparation of the manuscript.
Contributors: J. Dukart and S. Borgwardt designed the study. R. Smieskova, F. Harrisberger, C. Lenz, A. Schmidt, A. Walter and A. Simon acquired the data, which J. Dukart, C. Huber, A. Riecher-Rössler, U. Lang, P. Fusar-Poli and S. Borgwardt analyzed. J. Dukart wrote the article, which all authors reviewed and approved for publication.
References
- 1.Wright IC, Rabe-Hesketh S, Woodruff PW, et al. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 2.Ellison-Wright I, Glahn DC, Laird AR, et al. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olabi B, Ellison-Wright I, McIntosh AM, et al. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 4.Baiano M, David A, Versace A, et al. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophr Res. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Job DE, Whalley HC, McConnell S, et al. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–9. [PubMed] [Google Scholar]
- 6.Kubicki M, Shenton ME, Salisbury DF, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–9. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fornito A, Yücel M, Patti J, et al. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophr Res. 2009;108:104–13. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Honea R, Crow TJ, Passingham D, et al. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatry. 2005;162:2233–45. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- 9.Borgwardt SJ, McGuire PK, Aston J, et al. Structural brain abnormalities in individuals with an at-risk mental state who later develop psychosis. Br J Psychiatry. 2007;51:s69–75. doi: 10.1192/bjp.191.51.s69. [DOI] [PubMed] [Google Scholar]
- 10.Fusar-Poli P, Radua J, McGuire P, et al. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophr Bull. 2012;38:1297. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grellmann C, Bitzer S, Neumann J, et al. Comparison of variants of canonical correlation analysis and partial least squares for combined analysis of MRI and genetic data. Neuroimage. 2015;107:289–310. doi: 10.1016/j.neuroimage.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd AM, Matheson SL, Laurens KR, et al. Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry. 2012;72:775–84. doi: 10.1016/j.biopsych.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Van Erp TGM, Hibar DP, Rasmussen JM, et al. Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 2016;21:547–53. doi: 10.1038/mp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke B, Stein JL, Ripke S, et al. Genetic influences on schizophrenia and subcortical brain volumes: large-scale proof of concept. Nat Neurosci. 2016;16:420–31. doi: 10.1038/nn.4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klauser P, Zhou J, Lim JKW, et al. Lack of evidence for regional brain volume or cortical thickness abnormalities in youths at clinical high risk for psychosis: findings from the Longitudinal Youth at Risk Study. Schizophr Bull. 2015;41:1285–93. doi: 10.1093/schbul/sbv012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fusar-Poli P, Smieskova R, Kempton MJ, et al. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci Biobehav Rev. 2013;37:1680–91. doi: 10.1016/j.neubiorev.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haijma SV, Van Haren N, Cahn W, et al. Brain volumes in schizophrenia: a meta-analysis in over 18 000 subjects. Schizophr Bull. 2013;39:1129–38. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexander-Bloch AF, Reiss PT, Rapoport J, et al. Abnormal cortical growth in schizophrenia targets normative modules of synchronized development. Biol Psychiatry. 2014;76:438–46. doi: 10.1016/j.biopsych.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–20. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis — a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34:1207–22. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Bois C, Whalley HC, McIntosh AM, et al. Structural magnetic resonance imaging markers of susceptibility and transition to schizophrenia: a review of familial and clinical high risk population studies. J Psychopharmacol. 2015;29:144–54. doi: 10.1177/0269881114541015. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Takahashi T, Nemoto K, et al. Gray matter changes in subjects at high risk for developing psychosis and first-episode schizophrenia: a voxel-based structural MRI study. Front Psychiatry. 2013;18:16. doi: 10.3389/fpsyt.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller S, Borgwardt SJ, Schindler C, et al. Can cortical thickness asymmetry analysis contribute to detection of at-risk mental state and first-episode psychosis? A pilot study. Radiology. 2009;250:212–21. doi: 10.1148/radiol.2501072153. [DOI] [PubMed] [Google Scholar]
- 24.Nenadic I, Dietzek M, Schönfeld N, et al. Brain structure in people at ultra-high risk of psychosis, patients with first-episode schizophrenia, and healthy controls: a VBM study. Schizophr Res. 2015;161:169–76. doi: 10.1016/j.schres.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 25.Koutsouleris N, Patschurek-Kliche K, Scheuerecker J, et al. Neuro-anatomical correlates of executive dysfunction in the at-risk mental state for psychosis. Schizophr Res. 2010;123:160–74. doi: 10.1016/j.schres.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Modinos G, Mechelli A, Ormel J, et al. Schizotypy and brain structure: a voxel-based morphometry study. Psychol Med. 2010;40:1423–31. doi: 10.1017/S0033291709991875. [DOI] [PubMed] [Google Scholar]
- 27.Borgwardt SJ, McGuire PK, Aston J, et al. Reductions in frontal, temporal and parietal volume associated with the onset of psychosis. Schizophr Res. 2008;106:108–14. doi: 10.1016/j.schres.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 28.Fusar-Poli P, Borgwardt S, Crescini A, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–85. doi: 10.1016/j.neubiorev.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Mechelli A, Riecher-Rössler A, Meisenzahl EM, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–95. doi: 10.1001/archgenpsychiatry.2011.42. [DOI] [PubMed] [Google Scholar]
- 30.Cannon TD, Chung Y, He G, et al. Progressive reduction in cortical thickness as psychosis develops: a multisite longitudinal neuro-imaging study of youth at elevated clinical risk. Biol Psychiatry. 2015;77:147–57. doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riecher-Rössler A, Gschwandtner U, Aston J, et al. The Basel early-detection-of-psychosis (FEPSY)-study–design and preliminary results. Acta Psychiatr Scand. 2007;115:114–25. doi: 10.1111/j.1600-0447.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 32.Riecher-Rössler A, Pflueger MO, Aston J, et al. Efficacy of using cognitive status in predicting psychosis: a 7-year follow-up. Biol Psychiatry. 2009;66:1023–30. doi: 10.1016/j.biopsych.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 33.Yung AR, Phillips LJ, McGorry PD, et al. Prediction of psychosis: a step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172:14–20. [PubMed] [Google Scholar]
- 34.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 35.Riecher-Rössler A, Aston J, Borgwardt S, et al. Vorhersage von Psychosen durch stufenweise Mehrebenenabklärung–Das Basler FePsy (Früherkennung von Psychosen)-Projekt. Fortschr Neurol Psychiatr. 2013;81:265–75. doi: 10.1055/s-0033-1335017. [DOI] [PubMed] [Google Scholar]
- 36.Riecher-Rössler A, Aston J, Ventura J, et al. Das Basel Screening Instrument für Psychosen (BSIP): Entwicklung, Aufbau, Reliabilität und Validität. Fortschr Neurol Psychiatr. 2008;76:207–16. doi: 10.1055/s-2008-1038155. [DOI] [PubMed] [Google Scholar]
- 37.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Hutton C, De Vita E, Ashburner J, et al. Voxel-based cortical thickness measurements in MRI. Neuroimage. 2008;40:1701–10. doi: 10.1016/j.neuroimage.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walhovd KB, Westlye LT, Amlien I, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–32. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Worsley KJ, Andermann M, Koulis T, et al. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayasaka S, Phan KL, Liberzon I, et al. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 42.Song X-W, Dong Z-Y, Long X-Y, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bell M, Milstein R, Beam-Goulet J, et al. The Positive and Negative Syndrome Scale and the Brief Psychiatric Rating Scale: reliability, comparability, and predictive validity. J Nerv Ment Dis. 1992;180:723–8. doi: 10.1097/00005053-199211000-00007. [DOI] [PubMed] [Google Scholar]
- 44.Heaton RK, Gladsjo JA, Palmer BW, et al. Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- 45.Zanello A, Berthoud L, Ventura J, et al. The Brief Psychiatric Rating Scale (version 4.0) factorial structure and its sensitivity in the treatment of outpatients with unipolar depression. Psychiatry Res. 2013;210:626–33. doi: 10.1016/j.psychres.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Honea RA, Meyer-Lindenberg A, Hobbs KB, et al. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry. 2008;63:465–74. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–16. doi: 10.1176/appi.ajp.2013.12091148. [DOI] [PubMed] [Google Scholar]
- 48.Cooper D, Barker V, Radua J, et al. Multimodal voxel-based meta-analysis of structural and functional magnetic resonance imaging studies in those at elevated genetic risk of developing schizophrenia. Psychiatry Res Neuroimaging. 2014;221:69–77. doi: 10.1016/j.pscychresns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Heinze K, Reniers RL, Nelson B, et al. Discrete alterations of brain network structural covariance in individuals at ultra-high risk for psychosis. Biol Psychiatry. 2015;77:989–96. doi: 10.1016/j.biopsych.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 50.Koutsouleris N, Riecher-Rössler A, Meisenzahl EM, et al. Detecting the psychosis prodrome across high-risk populations using neuroanatomical biomarkers. Schizophr Bull. 2015;41:471–82. doi: 10.1093/schbul/sbu078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutsouleris N, Meisenzahl EM, Borgwardt S, et al. Individualized differential diagnosis of schizophrenia and mood disorders using neuroanatomical biomarkers. Brain. 2015;138:2059–73. doi: 10.1093/brain/awv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maillard AM, Ruef A, Pizzagalli F, et al. The 16p11. 2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry. 2015;20:140–7. doi: 10.1038/mp.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smieskova R, Fusar-Poli P, Aston J, et al. Insular volume abnormalities associated with different transition probabilities to psychosis. Psychol Med. 2012;42:1613–25. doi: 10.1017/S0033291711002716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borgwardt SJ, Riecher-Rössler A, Dazzan P, et al. Regional gray matter volume abnormalities in the at risk mental state. Biol Psychiatry. 2007;61:1148–56. doi: 10.1016/j.biopsych.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Dukart J, Regen F, Kherif F, et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proc Natl Acad Sci U S A. 2014;111:1156–61. doi: 10.1073/pnas.1321399111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harrisberger F, Smieskova R, Vogler C, et al. Impact of polygenic schizophrenia-related risk and hippocampal volumes on the onset of psychosis. Transl Psychiatry. 2016;6:e868. doi: 10.1038/tp.2016.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smieskova R, Allen P, Simon A, et al. Different duration of at-risk mental state associated with neurofunctional abnormalities. A multimodal imaging study. Hum Brain Mapp. 2012;33:2281–94. doi: 10.1002/hbm.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fusar-Poli P, Cappucciati M, Borgwardt S, et al. Heterogeneity of psychosis risk within individuals at clinical high risk: a meta-analytical stratification. JAMA Psychiatry. 2016;73:113–20. doi: 10.1001/jamapsychiatry.2015.2324. [DOI] [PubMed] [Google Scholar]
- 59.Uranova NA, Vostrikov VM, Orlovskaya DD, et al. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 60.Knöchel C, Reuter J, Reinke B, et al. Cortical thinning in bipolar disorder and schizophrenia. Schizophr Res. 2016;172:78–85. doi: 10.1016/j.schres.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Rimol LM, Nesvåg R, Hagler DJ, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–60. doi: 10.1016/j.biopsych.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 62.Goldman AL, Pezawas L, Mattay VS, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Arch Gen Psychiatry. 2009;66:467–77. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Swam C, Federspiel A, Hubl D, et al. Possible dysregulation of cortical plasticity in auditory verbal hallucinations — a cortical thickness study in schizophrenia. J Psychiatr Res. 2012;46:1015–23. doi: 10.1016/j.jpsychires.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Oertel-Knöchel V, Knöchel C, Rotarska-Jagiela A, et al. Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb Cortex. 2013;23:61–70. doi: 10.1093/cercor/bhr380. [DOI] [PubMed] [Google Scholar]
- 65.Fusar-Poli P, Schultze-Lutter F, Cappucciati M, et al. The dark side of the moon: meta-analytical impact of recruitment strategies on risk enrichment in the clinical high risk state for psychosis. Schizophr Bull. 2016;42:732–43. doi: 10.1093/schbul/sbv162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziegler G, Dahnke R, Jäncke L, et al. Brain structural trajectories over the adult lifespan. Hum Brain Mapp. 2012;33:2377–89. doi: 10.1002/hbm.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]