Abstract
Background
Posttraumatic stress disorder (PTSD) is a disabling psychiatric disorder that has been associated with lower white matter integrity of tracts connecting the prefrontal cortex with limbic regions. However, previous diffusion tensor imaging (DTI) findings have been inconsistent, showing high variability in the exact location and direction of effects.
Methods
We performed probabilistic tractography of the bilateral uncinate fasciculus, cingulum and superior longitudinal fasciculus (both temporal and parietal projections) in male and female police officers with and without PTSD.
Results
We included 38 (21 men) police officers with and 39 (20 men) without PTSD in our analyses. Compared with trauma-exposed controls, patients with PTSD showed significantly higher mean diffusivity of the right uncinate fasciculus, the major white matter tract connecting the amygdala to the prefrontal cortex (p = 0.012). No other significant between-group or group × sex differences were observed. Mean diffusivity of the right uncinate fasciculus was positively associated with anxiety symptoms (r = 0.410, p = 0.013) in patients with PTSD as well as with amygdala activity (r = 0.247, p = 0.038) and ventromedial prefrontal cortex (vmPFC) activity (r = 0.283, p = 0.016) in all participants in response to happy and neutral faces.
Limitations
Our specific sample of trauma-exposed police officers limits the generalizability of our findings to other PTSD patient groups (e.g., civilian trauma).
Conclusion
Patients with PTSD showed diminished structural connectivity between the amygdala and vmPFC, which was correlated with higher anxiety symptoms and increased functional activity of these brain regions. Our findings provide additional evidence for the prevailing neurocircuitry model of PTSD, postulating that ineffective communication between the amygdala and vmPFC underlies decreased top–down control over fear responses.
Introduction
Approximately 10% of trauma-exposed individuals have posttraumatic stress disorder (PTSD),1 characterized by intrusions and avoidance of reminders of the traumatic event, altered cognitions and mood, and hyperarousal.2 The prevailing neurocircuitry model of PTSD postulates amygdala hyperactivity and ventromedial prefrontal cortex (vmPFC) hypoactivity.3,4 The amygdala plays a pivotal role in fear expression and salience processing,5 whereas the vmPFC is important in top–down control over the fear response.6 Additionally, decreased resting-state functional connectivity between the amygdala and vmPFC has been observed in male patients with PTSD, possibly underlying diminished top–down prefrontal control over the fear response.7
White matter fibres, which make up more than 40% of total brain volume,8 allow effective communication between (cortical and subcortical) brain areas. White matter integrity can be investigated noninvasively with diffusion tensor imaging (DTI), which measures the direction and coherence of water diffusion within myelinated and nonmyelinated tissue.9 The DTI metrics fractional anisotropy (FA) and mean diffusivity (MD) are predominantly used to quantify white matter integrity: FA reflects the extent to which water diffusion is directionally restricted and represents axonal density and coherence, whereas MD (i.e., magnitude of overall diffusion) is sensitive (but not specific) to membrane integrity.10,11
In line with the prevailing neurocircuitry model of PTSD, lower white matter integrity of tracts connecting the prefrontal cortex with limbic regions, including the cingulum bundle and uncinate fasciculus, has been associated with PTSD and its development.12–14 The uncinate fasciculus connects the orbital, medial and lateral prefrontal cortex with rostral temporal areas, including the anterior temporal lobe, parahippocampal gyrus, entorhinal and perirhinal cortex, and amygdala.15 Greater PTSD symptom severity has been associated with lower uncinate fasciculus tract integrity in recently deployed soldiers when measured 2 months after military deployment.12 Additionally, PTSD symptoms after deployment were associated with a decrease in uncinate fasciculus tract integrity from pre- to postdeployment.13 Moreover, vmPFC white matter integrity measured within 2 days posttrauma was lower in trauma-exposed individuals in whom PTSD developed compared with those who did not develop PTSD at 1 and 6 months posttrauma.16 Regarding the cingulum bundle, lower white matter integrity of the cingulum (i.e., connecting the cingulate cortex with the hippocampus and amygdala)17 has been observed in female patients with PTSD compared with trauma-exposed controls, possibly underlying impaired fear extinction learning.14 However, according to a recent meta-analysis on 7 whole-brain DTI studies in patients with PTSD, increased FA of the cingulum bundle has also been observed in patients with PTSD.18 Finally, altered white matter integrity of the left and right superior longitudinal fasciculus, which connects the parietal, temporal and occipital lobes to the ipsilateral frontal cortex,15 was observed in patients with PTSD compared with healthy controls in a quantitative meta-analysis.18
Taken together, the presence and development of PTSD symptoms has been associated with aberrant integrity of several major white matter tracts, including the uncinate fasciculus, cingulum bundle and superior longitudinal fasciculus. However, previous findings have been inconsistent, showing high variability in the location and direction of observed effects. For example, according to the quantitative meta-analysis by Daniels and colleagues18 on whole-brain DTI studies, PTSD was associated with both increased and decreased FA at different locations in the left superior longitudinal fasciculus and at different locations in the bilateral cingulum. These mixed findings may be due to different study designs: patients with PTSD were compared with trauma-exposed14,19–21 or non–trauma-exposed22–24 controls, although trauma-exposure has been found to alter tract integrity.25 Additionally, both male and female patients have been included in the same studies,19,22–24 without specifically investigating or accounting for possible differential effects of sex on tract integrity.26
Therefore, in the present study, we investigated the integrity of several candidate major white matter tracts (i.e., uncinate fasciculus, cingulum bundle, and temporal and parietal projections of the superior longitudinal fasciculus) in patients with PTSD compared with trauma-exposed controls, including both male and female participants to account for potential sex differences. We reconstructed major white matter pathways using an automated and unbiased reconstruction of a priori–determined anatomic white matter tracts using global probabilistic tractography (TRACULA: TRActs Constrained by UnderLying Anatomy).27 Major white matter pathways were reconstructed based on probability distributions of fibre orientations of all voxels along the entire tract, providing mean FA, MD, radial (RD) and axonal diffusivity (AD) values per tract.27 Compared with previously used whole-brain voxel-wise analysis techniques, the number of multiple comparisons is drastically reduced using TRACULA, thereby increasing statistical power to detect relatively subtle differences in tract integrity. Additionally, no intersubject registration is needed, increasing sensitivity to interindividual anatomic variability,28 which may be especially important because PTSD has been associated with grey matter alterations.29
Given previous meta-analytic findings18 and the predominant neurocircuitry model of PTSD,3 we investigated tract integrity of the bilateral uncinate fasciculus, cingulum bundle and superior longitudinal fasciculus (both temporal and parietal projections) in male and female police officers with and without PTSD. We investigated group differences in average FA and MD values of each white matter tract. Additionally, we conducted a segment analysis, in which average FA and MD values were examined at different locations of the white matter tracts. We expected to find diminished white matter integrity of the reconstructed white matter tracts in patients with PTSD compared with trauma-exposed controls. To further characterize findings of altered white matter integrity in patients with PTSD, we investigated clinical correlates (i.e., PTSD symptom severity and subjective anxiety) in patients with the disorder as well as neural (amygdala and vmPFC activity in response to emotional faces) correlates of altered white matter integrity in all participants.
Methods
Participants
We recruited male and female police officers with and without PTSD via advertisements on websites and in journals of the Dutch police and via a psychotrauma diagnostic centre for police personnel (PDC politiepoli, Diemen, the Netherlands; patients only). Control participants were matched to the patients based on age, sex, educational level and years of service.
To be included participants had to be between 18 and 65 years of age, eligible for MRI and free of psychotropic medication. Patients with PTSD had to meet DSM-IV criteria for current PTSD, with a Clinician-Administered PTSD Scale (CAPS for DSM-IV, Dutch translation)30 total score of 45 or higher. Patients were excluded if they had a previously diagnosed personality disorder, current substance-related disorder, severe major depressive disorder (MDD), psychotic disorder, or current suicidal risk. Trauma-exposed controls had to have experienced at least 1 traumatic event according to the DSM-IV PTSD A1 criterion,2 with a CAPS score lower than 15. Controls could not have any current DSM-IV Axis I psychopathology, diagnosed personality disorder or lifetime history of PTSD or MDD. In all participants, we assessed psychopathology (other than PTSD) using the Dutch version of the Mini Neuropsychological Interview (MINI)31 or the Structured Clinical Interview for DSM–IV (SCID;32 for patients of the outpatient clinic).
The Institutional Review Board of the Academic Medical Center, Amsterdam, the Netherlands, approved this study. We conducted the study in accordance with the Declaration of Helsinki and obtained written informed consent from all participants before their enrolment in the study.
Procedure
The present study is part of a double-blind, randomized, placebo-controlled crossover fMRI study on the neural correlates of oxytocin administration in patients with PTSD.33 The study consisted of 3 appointments: 1 intake session, during which inclusion and exclusion criteria were assessed, and 2 MRI sessions. The second session took place, on average, 12.77 ± 14.87 days after the first one. Participants self-administered intranasal oxytocin before one scanning session and placebo before the other, the order of which was randomly assigned and counterbalanced among participants. For the present study, we used functional images acquired under a placebo condition only.
Both MRI sessions started with the acquisition of a structural image. During the first scanning session, we obained a structural T1-weighted image for subcortical and cortical segmentation and registration of the diffusion-weighted images. During the second scanning session, a DTI scan was acquired. Hereafter, functional imaging started in both scanning sessions with an emotional face–matching task34 to elicit robust amygdala activation.33 The task consisted of 1 visuomotor control condition (scrambled faces; 4 blocks) and 2 emotion conditions: 1 with fearful and angry faces (2 blocks) and 1 with happy and neutral faces (2 blocks; see Appendix 1, Fig. S1, available at jpn.ca for the task design).
Prior to each scanning session, we assessed the severity of current depressive and anxiety symptoms in the preceding week using the Hospital Anxiety and Depression Scale (HADS),35 consisting of anxiety (HADS-A) and depression (HADS-D) subscales. We assessed PTSD symptom severity in the preceding week using the revised Impact of Events Scale (IES-R).36 Additionally, participants filled out questionnaires on various demographic variables, alcohol use (Alcohol Use Disorders Identification Test [AUDIT]),37 childhood trauma exposure (the Early Trauma Inventory [ETI-SF])38 and police-related traumatic events (the Police Life Events Scale [PLES]).39
Image acquisition
Participants were scanned with a 3 T Philips Achieva MR system (Best, the Netherlands) using a 32-channel head coil. To acquire a high-resolution anatomic T1-weighted scan, we used a FAST magnetization-prepared rapid gradient-echo sequence (220 slices, voxel size 1 mm3, repetition time [TR] 8.2 s, echo time [TE] 3.8 s, flip angle 8°). Diffusion-weighted images (DWIs) were acquired in 32 directions, with low b (b = 0) images and a b value of 1000 s/mm2 (60 slices, voxel size 3 mm3, TR 7542 ms, TE 86 ms). The functional scans were obtained using an echo planar imaging (EPI) sequence sensitive to the blood-oxygen–level dependent (BOLD) contrast (110 volumes, voxel size 3 mm3, TR 2 s, TE 28 ms, flip angle 76°). The phase-encoding gradient was applied in the anterior–posterior (AP) direction. No correction for susceptibility artifacts was applied.
DTI analysis
Preprocessing of the DWIs was conducted with software developed in house, programmed in Matlab (MathWorks) and executed on the Dutch e-science Grid (www.biggrid.nl), with a web interface to the e-Bioinfra gateway.40,41 We corrected for head movement and deformations induced by eddy currents using affine registration of the DWIs to the non–diffusion-weighted image. Hereafter, the gradient directions were corrected by the rotation component of the transformation. The DWIs were resampled isotropically. Finally, rician noise was reduced using an adaptive noise filtering method, resulting in greater precision of the diffusivity values.42
We estimated voxel-wise probability distributions of diffusion direction on the preprocessed DWIs using the ball-and-stick model, as implemented in BedPostX (Bayesian estimation of diffusion parameters obtained using sampling techniques)43 of the FSL toolbox. Automated cortical and subcortical segmentation of the structural T1-weighted scan was performed with Freesurfer version 5.0, using default options.44 Both BedPostX and Freesurfer analyses were executed on the Dutch e-science Grid.
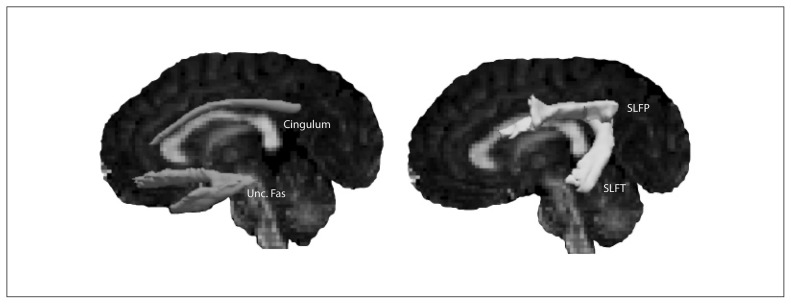
Global probabilistic tractography was performed in TRACULA27 using the estimated diffusion probability distributions as provided by BedPostX and (sub)cortical segmentations as provided by FreeSurfer. We reconstructed the dorsal (but not hippocampal) cingulum bundle, the uncinate fasciculus, and parietal and temporal projections of the superior longitudinal fasciculus. The parietal bundle of the superior longitudinal fasciculus most closely corresponds to the third branch of the superior longitudinal fasciculus (SLF III), whereas the temporal bundle corresponds to the arcuate fasciculus27 (Fig. 1). Additional preprocessing in TRACULA included intrasubject registration of the participant-specific DWIs to the structural T1-weighted image and intersubject registration of the structural T1-weighted images to a common template space (Montreal Neurological Institute [MNI]). Hereafter, cortical and white matter masks were created from Freesurfer reconstructions and transformed from participant-specific structural T1-weighted image space to diffusion-weighted image space, as well as to common template space based on the coregistration transformations described earlier. Next, we conducted tensor fitting for extraction of tensor-based measures (i.e., FA and MD). Finally, anatomic priors for the white matter pathways were computed in template space by combining the individual cortical and white matter masks with the TRACULA atlas, consisting of manually labelled white matter pathways and anatomic segmentations of 30 healthy individuals.27 Anatomic priors are probabilities that the white matter pathway intersects or neighbours subcortical structures and cortical surfaces at each point along the pathway’s trajectory.
Fig. 1.
Trajectories of the uncinate fasciculus (Unc. Fas), cingulum and superior longitudinal fasciculus (temporal [SLFT] and parietal [SLFP] projections) of a representative participant.
For each participant, we reconstructed white matter tracts by simultaneously fitting the shape of each pathway to the individual diffusion probability distributions and to the anatomic priors based on the TRACULA atlas (7 control points, 1000 permutations) in native diffusion image space. For our main analysis, we extracted average weighted FA and MD values for each reconstructed tract. That is, FA and MD values were weighted for the probability of each voxel of belonging to the tract and averaged across all voxels of the tract. For explorative analyses, we additionally extracted weighted FA and MD values for each voxel along each reconstructed tract. As the length of each tract (in number of voxels) was variable among participants, we interpolated the weighted FA and MD values at corresponding positions along the tracts for all participants. To minimize the number of multiple comparisons, FA and MD values were averaged over segments of 5 successive voxels for each tract. Given the varying length of the different tracts, this resulted in 5 segments for the uncinate fasciculus, 6 segments for the cingulum bundle, 4 segments for the parietal projections and 7 segments for the temporal projections of the superior longitudinal fasciculus.
Emotional face matching task
After preprocessing, the emotional face matching conditions (i.e., fearful–angry and happy–neutral faces) were each contrasted with the visuomotor control condition (i.e., scrambled faces) for each participant at the first level.33 These contrast images were fit into a second-level model with group (PTSD v. control) and sex (male v. female) as between-subjects factors, emotion (fearful–angry faces v. happy–neutral faces) as a within-subjects factor and drug order as a covariate. We assessed amygdala and vmPFC activity during emotional face matching for the overall task effect across all participants (see Appendix 1 for fMRI data analysis). Individual contrast estimates of amygdala and vmPFC activity toward fearful–angry and happy–neutral faces were extracted from a 5 mm sphere surrounding the left (MNI coordinates: x, y, z = −20, −8, −16, pFWE < 0.001) and right (MNI coordinates: x, y, z = 24, −10, −14, pFWE < 0.001) amygdala peak task activation and from a 10 mm sphere surrounding the vmPFC peak task activation (MNI coordinates: x, y, z = 6, 48, −2, pFWE = 0.001), all whole brain–corrected.
Statistical analysis
WE conducted statistical analyses using SPSS software version 20.0 (IBM). All variables were first checked for outliers (i.e., standardized value > |2.58|) within each group of participants, and outliers were removed. We checked variables for normality (standardized skewness and/or kurtosis > |2.58|) and transformed them when necessary.
Differences between patients with PTSD and trauma-exposed controls on demographic characteristics, alcohol use and history of trauma exposure were analyzed for men and women separately, using independent t tests for continuous variables and χ2 tests for categorical variables. To investigate tract integrity differences between patients with PTSD and trauma-exposed controls, we conducted repeated-measures analyses of covariance (ANCOVA) for each tract separately, with hemisphere (left v. right) as the within-subjects factor, and group and sex as the between-subjects factors, while controlling for potential confounding variables (as specified in the results). We included tract volume in number of voxels as a covariate in all analyses to control for possible partial volume effects.45 Additionally, we included age as a covariate because (pre-frontal) white matter tract integrity has previously been found to decrease with age.46 For the segment analysis, we additionally included the within-subjects factor segment in the repeated-measures ANCOVAs. Medication order was not included as a covariate in the DTI analyses, as we did not expect any short-term effects of oxytocin administration on white matter integrity. This expectation was confirmed by independent sample t tests assessing differences in white matter integrity (FA and MD) between participants receiving placebo and those receiving oxytocin immediately before DTI scanning for all reconstructed white matter tracts (all p > 0.35).
In total, we conducted 8 repeated-measures ANOVAs (i.e., 4 tracts × 2 DTI metrics), with hemisphere included as a within-subjects factor. Therefore, we adjusted the α for multiple comparisons (n = 8) with a partial Bonferroni correction, taking the high correlation between all tract integrity measures into account and thereby balancing type I and type II errors. Because FA and MD values of all tracts were highly correlated, treating the variables as independent would have resulted in too stringent a correction with a conventional Bonferroni correction. Therefore, we took the correlation between all tract measures (r = 0.484) into account using the Simple Interactive Statistical Analysis Bonferroni tool (www.quantitativeskills.com/sisa/calculations/bonfer.htm),47 rendering a p < 0.017 significant. For the segment analysis, a mean correlation coefficient of r = 0.280 was observed, resulting in a corrected α of < 0.011 for these analyses. In case of significant interaction effects, we corrected post hoc tests for multiple comparisons using a Bonferroni correction (i.e., adjusted α = 0.05/no. of post hoc tests).
We conducted partial correlation analyses between symptom severity and tract integrity in patients with PTSD, correcting for age and tract volume. Additionally, we computed partial correlations between extracted contrast estimates of amygdala and vmPFC activity toward fearful–angry and happy–neutral faces and uncinate fasciculus tract integrity in the entire sample, controlling for age and tract volume.
Results
Participant characteristics
We included 38 (21 men) police officers with PTSD and 39 (20 men) trauma-exposed controls in our analyses. Although study participants were required to be free of psycho-tropic medication, 1 female patient with PTSD started using benzodiazepines daily the week before the second MRI scan; she was not excluded from the DTI analysis, as we did not expect acute medication effects on white matter integrity. Patients and controls did not differ in age, years of service, educational level or alcohol use (Table 1). Male patients with PTSD experienced more types of childhood traumatic events than male trauma-exposed controls (t39 = −2.18, p = 0.037), whereas female trauma-exposed controls experienced more types of work-related traumatic events than female patients with PTSD (t34 = 2.27, p = 0.028).
Table 1.
Demographic and clinical characteristics of study participants
| Characteristic | Group; mean ± SD or no. (%) | Group differences | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| PTSD (n = 38) | Trauma-exposed controls (n = 39) | Men | Women | |||||
|
|
|
|
|
|||||
| Men (n = 21) | Women (n = 27) | Men (n = 20) | Women (n = 19) | Statistic | p value | Statistic | p value | |
| Age, yr | 42.29 ± 9.83 | 38.84 ± 9.70 | 41.35 ± 10.62 | 38.06 ± 9.69 | t39 = −0.293 | 0.36 | t34 = 0.242 | 0.81 |
| Length of police service, yr | 16.29 ± 10.82 | 14.19 ± 10.47 | 18.42 ± 10.05 | 19.05 ± 9.90 | t39 = 0.926 | 0.36 | t34 = 1.692 | 0.10 |
| Educational level | χ2 = 0.928 | 0.12 | χ2 = 0.122 | 0.73 | ||||
| Low | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||||
| Middle | 14 (67) | 14 (88) | 16 (80) | 16 (84) | ||||
| High | 7 (33) | 2 (12) | 4 (20) | 3 (16) | ||||
| Symptom severity | ||||||||
| CAPS total score | 68.05 ± 15.62 | 67.35 ± 11.49 | 4.70 ± 4.79 | 4.68 ± 4.67 | t39 = −17.36 | < 0.001 | t34 = −21.87 | < 0.001 |
| CAPS re-experiencing | 20.95 ± 6.45 | 21.00 ± 5.30 | 0.70 ± 1.17 | 1.10 ± 1.97 | U < 0.001 | < 0.001 | U < 0.001 | < 0.001 |
| CAPS avoidance | 21.57 ± 7.97 | 22.65 ± 5.65 | 1.20 ± 2.35 | 0.68 ± 1.83 | U < 0.001 | < 0.001 | U < 0.001 | < 0.001 |
| CAPS hyperarousal | 25.71 ± 5.79 | 23.59 ± 4.37 | 2.80 ± 3.68 | 3.00 ± 3.07 | U < 0.001 | < 0.001 | U < 0.001 | < 0.001 |
| IES-R total score | 44.57 ± 14.51 | 39.82 ± 23.43 | 1.10 ± 1.85 | 1.72 ± 3.41 | U < 0.001 | < 0.001 | U = 4.000 | < 0.001 |
| Current comorbidity | ||||||||
| MDD | 4 (19) | 4 (24) | 0 (0) | 0 (0) | — | — | — | — |
| Dysthymia | 2 (10) | 1 (6) | 0 (0) | 0 (0) | — | — | — | — |
| Panic disorder | 1 (5) | 0 (0) | 0 (0) | 0 (0) | — | — | — | — |
| Specific phobia | 1 (5) | 0 (0) | 0 (0) | 0 (0) | — | — | — | — |
| HADS anxiety | 11.52 ± 0.71 | 12.47 ± 1.20 | 1.60 ± 0.39 | 3.02 ± 0.42 | t39 = −12.19 | < 0.001 | t34 = −7.41 | < 0.001 |
| HADS depression | 10.40 ± 1.01 | 10.01 ± 1.23 | 0.95 ± 0.35 | 0.47 ± 0.18 | t39 = −9.91 | < 0.001 | t34 = −12.95 | < 0.001 |
| Trauma history and alcohol use | ||||||||
| PLES | ||||||||
| No. of work-related traumatic experiences | 22.50 ± 5.95 | 13.47 ± 9.17 | 20.45 ± 6.42 | 19.74 ± 7.31 | t38 = −1.047 | 0.30 | t34 = 2.279 | 0.029 |
| No. of violent incidents | 9.65 ± 3.45 | 5.06 ± 4.46 | 8.30 ± 3.57 | 6.74 ± 3.63 | t38 = −1.215 | 0.23 | t34 = 1.242 | 0.22 |
| No. of confrontations with tragic incidents | 12.85 ± 3.38 | 8.41 ± 5.32 | 12.15 ± 3.33 | 13.00 ± 4.18 | t38 = −0.660 | 0.51 | t34 = 2.895 | 0.007 |
| ETI no. of different childhood traumatic experiences | 6.09 ± 4.55 | 5.12 ± 5.05 | 3.65 ± 2.35 | 4.42 ± 4.89 | t39 = −2.18 | 0.037 | t34 = −0.457 | 0.65 |
| AUDIT total score | 3.52 ± 3.40 | 4.06 ± 4.64 | 3.40 ± 1.67 | 3.10 ± 1.79 | U = 194 | 0.67 | U = 153.50 | 0.79 |
AUDIT = Alcohol Use Disorder Identification Test; CAPS = Clinician-Administered PTSD Scale; ETI = Early Trauma Inventory; HADS = Hospital Anxiety and Depression Scale; IES-R = Impact of Events Scale – Revised; MDD = major depressive disorder; PLES = Police Life Event Scale; PTSD = posttraumatic stress disorder; SD = standard deviation.
Group differences in tract integrity
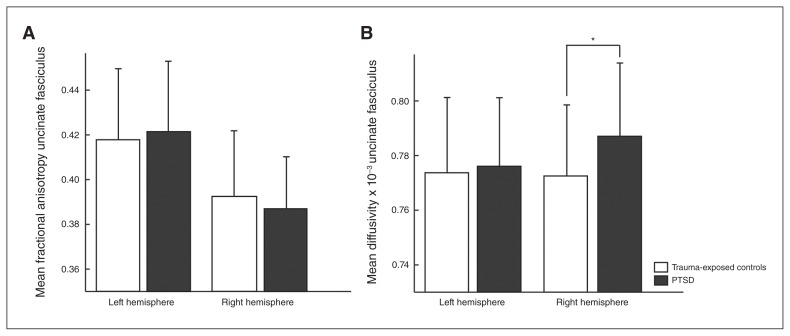
We found a significant hemisphere × group interaction effect for MD of the uncinate fasciculus (F1,67 = 7.575, p = 0.008), in which patients with PTSD showed higher MD of the right uncinate fasciculus (F1,68 = 6.70, p = 0.012, ω2 = 0.0704) than trauma-exposed controls (Table 2 and Fig. 2). These findings were independent of sex (hemisphere × group × sex interaction: F1,67 = 0.297, p = 0.59), and they remained significant after controlling for group differences in childhood and work-related traumatic events (PLES as covariate: F1,67 = 5.29, p = 0.025, ω2 = 0.0546; ETI as covariate: F1,67 = 5.18, p = 0.026, ω2 = 0.0520). We found no significant differences in MD values of the left uncinate fasciculus, FA values of the bilateral uncinate fasciculus, or FA and MD values of the left and right cingulum bundle and the superior longitudinal fasciculus (both temporal and parietal projections) between patients and controls (all p > 0.1). We found significant main effects of sex and hemisphere, showing differences in tract integrity between men and women and/or between the left and right hemisphere (Appendix 1, Table S1).
Table 2.
Results of the repeated-measures ANOVAs on FA and MD values of each tract
| Tract | FA | MD | ||
|---|---|---|---|---|
|
|
|
|||
| F | p value* | F | p value* | |
| Uncinate fasciculus | ||||
| Main effect of group | F1,66 = 0.130 | 0.72 | F1,67 = 3.037 | 0.09 |
| Group × sex interaction | F1,66 = 1.209 | 0.28 | F1,67 = 0.000 | > 0.99 |
| Hemisphere × group interaction | F1,66 = 1.513 | 0.22 | F1,67 = 7.575 | 0.008 |
| Hemisphere × group × sex interaction† | F1,66 = 0.789 | 0.38 | F1,67 = 0.297 | 0.59 |
| Cingulum bundle | ||||
| Main effect of group | F1,68 = 0.521 | 0.47 | F1,67 = 0.269 | 0.61 |
| Group × sex interaction | F1,68 = 0.779 | 0.38 | F1,67 = 0.878 | 0.35 |
| Hemisphere × group interaction | F1,68 = 2.479 | 0.12 | F1,67 = 0.859 | 0.36 |
| Hemisphere × group × sex interaction | F1,69 = 0.327 | 0.57 | F1,67 = 2.802 | 0.10 |
| Superior longitudinal fasciculus, parietal projections | ||||
| Main effect of group | F1,68 = 2.757 | 0.10 | F1,69 = 0.328 | 0.57 |
| Group × sex interaction | F1,68 = 0.090 | 0.77 | F1,69 = 0.037 | 0.85 |
| Hemisphere × group interaction | F1,68 = 0.620 | 0.43 | F1,69 = 0.598 | 0.44 |
| Hemisphere × group × sex interaction | F1,68 = 0.017 | 0.90 | F1,69 = 0.212 | 0.65 |
| Superior longitudinal fasciculus, temporal projections | ||||
| Main effect of group | F1,69 = 2.668 | 0.11 | F1,69 = 1.133 | 0.29 |
| Group × sex interaction | F1,69 = 0.165 | 0.69 | F1,69 = 0.335 | 0.57 |
| Hemisphere × group interaction | F1,69 = 0.096 | 0.76 | F1,69 = 0.742 | 0.39 |
| Hemisphere × group × sex interaction | F1,69 = 0.948 | 0.33 | F1,69 = 0.000 | > 0.99 |
ANOVA = analysis of variance; FA = fractional anisotropy; MD = mean diffusivity.
Results were considered significant for main and interaction effects at p < 0.017.
One-way ANOVA for each hemisphere with group (PTSD – control) and sex (male – female) as between-subject factors (left hemisphere: main effect of group F1,65 = 0.359, p = 0.55; right hemisphere: main effect of group F1,68 = 6.700, p = 0.012; direction: PTSD > controls. For the post hoc test of the group × hemisphere interaction effect, results were considered significant at p < 0.025.
Fig. 2.
Right uncinate fasciculus tract integrity in patients with posttraumatic stress disorder (PTSD) and trauma-exposed controls. Average (A) fractional anisotropy and (B) mean diffusivity for male and female patients with PTSD and trauma-exposed controls. Error bars represent standard deviations of the mean. *p < 0.05.
Segment analysis
We investigated white matter integrity differences between patients with PTSD and trauma-exposed controls for different locations on the uncinate fasciculus, cingulum and the superior longitudinal fasciculus (both parietal and temporal projections). No significant hemisphere × group × segment interactions were found in any of the tracts (all p > 0.01), except for a nominally significant group effect for uncinate fasciculus MD (i.e., PTSD > trauma-exposed controls, p = 0.022). See Appendix 1, Table S2, for significant main effects of segment and segment × sex interactions.
Correlations with symptom severity in patients with PTSD
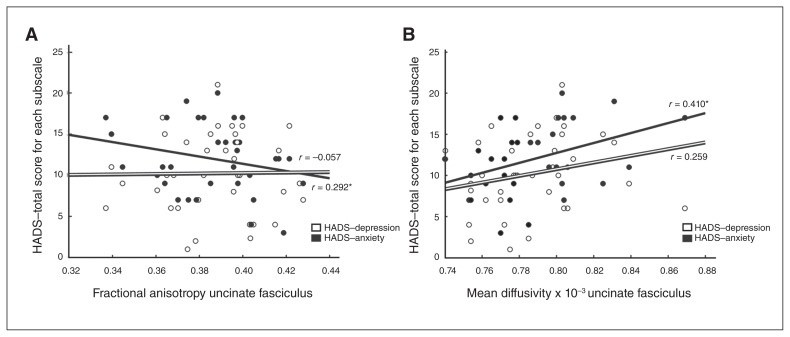
We explored whether right uncinate tract integrity was associated with symptom severity in patients with PTSD. Anxiety symptoms (HADS) positively correlated with MD (r = 0.410, p = 0.013) of the right uncinate fasciculus (Fig. 3), whereas depression symptoms (HADS) and PTSD severity (CAPS total and subscale scores) were not significantly associated with MD of the right uncinate fasciculus (all r < 0.13, all p > 0.1).
Fig. 3.
Correlations between mean diffusivity (MD) of the right uncinate fasciculus and symptom severity in patients with posttraumatic stress disorder (PTSD). Correlations between MD of the right uncinate fasciculus and anxiety (Hospital and Anxiety Depression Scale [HADS] anxiety subscale) and depression symptoms (HADS depression subscale).
Task activation across participants
As expected, the bilateral amygdala was robustly activated, whereas the vmPFC was deactivated when looking at emotional faces (all pFWE < 0.05, whole-brain corrected) across all participants and conditions (Appendix 1, Fig. S1). Exploratory analyses showed significantly less vmPFC deactivation in response to happy–neutral faces in patients with PTSD than in trauma-exposed controls (t72 = −2.689, p = 0.009), whereas no group differences were observed for amygdala activity (Appendix 1, Fig. S1).
Correlations with amygdala and vmPFC activity
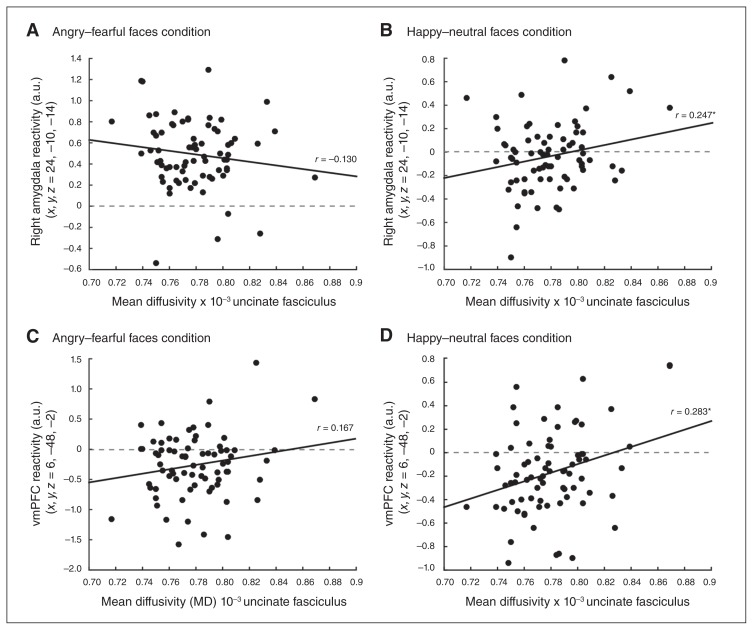
We investigated correlations between right uncinate fasciculus MD and right-sided amygdala and vmPFC activity in response to emotional faces. Across all participants, amygdala and vmPFC activity in response to happy–neutral (but not fearful–angry) faces were significantly positively correlated with right uncinate fasciculus MD (amygdala happy–neutral: r69 = 0.247, p = 0.038; amygdala fearful–angry: r69 = −0.130, p = 0.28; vmPFC happy–neutral: r70 = 0.283, p = 0.016; vmPFC fearful–angry: r70 = 0.167, p = 0.16; Fig. 4). The association between right uncinate fasciculus MD and right amygdala activity in response to happy–neutral faces was significant only in patients with PTSD (PTSD: r32 = 0.416, p = 0.014; trauma-exposed controls: r33 = −0.37, p = 0.83), and the correlation coefficient magnitudes were significantly different between the 2 groups (Z-score difference = 1.96, p = 0.05). On the other hand, the association between right uncinate fasciculus MD and vmPFC activation in response to happy–neutral faces was not significantly different between patients with PTSD and trauma-exposed controls (PTSD: r33 = 0.296, p = 0.08; trauma-exposed controls: r33 = 0.151, p = 0.39; Z-score difference = 0.63, p = 0.53).
Fig. 4.
Correlations between mean diffusivity (MD) of the right uncinate fasciculus and right amygdala reactivity in response to (A) fearful–angry faces and (B) happy–neutral faces compared with the control condition. Contrast estimates in arbitrary units (a.u.) were extracted from a 5 mm sphere surrounding the peak activation effect in the right amygdala (x, y, z = 24, −10, −14). Correlations between MD of the right uncinate fasciculus and ventromedial prefrontal cortex (vmPFC) reactivity in response to (C) fearful–angry faces and (D) happy–neutral faces compared with the control condition. Contrast estimates in arbitrary units were extracted from a 10 mm sphere surrounding the peak activation effect in the vmPFC (x, y, z = 6, 48, −2).
Discussion
We investigated the integrity of major anatomic white matter tracts in male and female police officers with and without PTSD using a global probabilistic tractography method. Compared with trauma-exposed controls, patients with PTSD had a higher MD of the right uncinate fasciculus, which connects the vmPFC and amygdala. In patients with PTSD, right uncinate fasciculus MD values were positively associated with anxiety as well as with amygdala and vmPFC activity toward emotional stimuli.
Our finding of higher uncinate fasciculus MD values in patients with PTSD indicates lower integrity of the major white matter tract connecting the prefrontal cortex to the limbic cortex, which may be characterized by lower membrane integrity (i.e., MD), whereas axonal density and coherence (as represented by FA) appeared unaltered. This observation of lower white matter integrity of the uncinate fasciculus in patients with PTSD is in line with the prevailing neurocircuitry model of PTSD.3 This model postulates diminished communication between the amygdala and vmPFC in patients with PTSD, resulting in decreased inhibitory prefrontal control over the fear response and increased amygdala reactivity toward trauma-related or emotional stimuli.3,4 It has been suggested that the strength of structural and functional connectivity between the amygdala and vmPFC, rather than the activity of either brain region alone, may be especially important in terms of fear regulation and subjective anxiety (see the review by Kim and colleagues48). For example, in healthy women, the frequency of cognitive emotion regulation strategies (i.e., reappraisal) during everyday life was associated with greater left uncinate fasciculus tract integrity.49 Notably, we found a positive association between right uncinate fasciculus MD and anxiety (but not depression and PTSD) severity in patients with PTSD, suggesting that decreased uncinate fasciculus integrity may be associated with severity of anxiety symptoms specifically. Furthermore, the uncinate fasciculus connects the (lateral) prefrontal cortices to the hippocampus, a structure important for contextualizing, including discriminating between threatening and safe contexts.50 Posttraumatic stress disorder has been associated with dysregulations in context processing,51 such as enhanced fear generalization52 and impaired contextual extinction learning3 and safety learning.53 Notably, these processes of contextualization depend on the hippocampus and its communication with the prefrontal cortex.50,54 Furthermore, re-experiencing symptoms in patients with PTSD was previously associated with lower functional resting-state connectivity between the hippocampus and right prefrontal cortex, which was interpreted as underlying impaired contextualization and overgeneralization of trauma cues.55 Therefore, ineffective communication between the hippocampus and prefrontal cortex, as suggested by our finding of decreased uncinate fasciculus tract integrity in patients, may also represent a neural correlate of impaired fear contextualization in patients with PTSD.
Functionally, we found that MD of the right uncinate fasciculus was positively associated with right amygdala and vmPFC activation in response to happy and neutral faces in all participants. Previously, lower uncinate fasciculus integrity was associated with greater amygdala reactivity in response to fearful faces in healthy participants56 and in response to happy and sad faces in children and adolescents (aged 9–19 yr).57 Moreover, lower structural uncinate fasciculus tract integrity in patients with generalized social anxiety disorder (compared with healthy controls) was associated with decreased functional amygdala to vmPFC connectivity.58 In our study, we observed vmPFC deactivation toward emotional faces. The vmPFC is part of the task-negative network or default mode network (DMN), an intrinsic connectivity network that has been associated with internally focused thought59 and that is usually disengaged during tasks requiring attention to stimuli and cognitive processing.59 Notably, PTSD has been associated with difficulties switching between the DMN and central executive areas during task performance.60 In line with this observation, we found significantly diminished vmPFC deactivation toward happy–neutral faces in patients with PTSD compared with trauma-exposed controls. Taken together, decreased uncinate fasciculus white matter integrity was associated with greater amygdala activity and decreased vmPFC deactivation in response to emotional stimuli in the entire sample. Additionally, lower uncinate fasciculus tract integrity (i.e., higher MD) in patients with PTSD was associated with higher self-reported anxiety symptoms on the HADS. Notably, these observations are in the line with the prevailing neurocircuitry model of PTSD, underlining that impaired communication between the vmPFC and amygdala may result in increased (amygdala-mediated) fear responses.
Of note, we observed significant group differences for MD but not FA of the right uncinate fasciculus, which may be explained by differences in neurobiological correlates of these 2fitract integrity measures. Although the exact neurobiological underpinnings of MD and FA are still under debate, FA and MD likely represent complementary aspects of white matter microstructure: whereas FA represents the extent of restricted directionality of diffusion, MD is sensitive to membrane integrity, independent of fibre orientation.10 It has been suggested that MD may be more susceptible to subtle white matter alterations as a consequence of chronic stress exposure: a recent DTI study in elderly men showed a positive association between salivary cortisol levels before a mild cognitive stressor and MD (but not FA) of the uncinate fasciculus.61 Our findings of increased uncinate fasciculus MD in patients with PTSD therefore suggest (subtle) microstructural changes in membrane integrity, reflected by an altered volume of the diffusion tensor (i.e., increased MD), without changes in directionality of diffusion (i.e., unaltered oval shape of the diffusion tensor: FA). Notably, increased MD values could result from higher diffusion parallel to the axon fibres (AD), higher diffusion perpendicular to the axon fibres (RD) or both. Because we did not investigate AD and RD values of reconstructed white matter tracts, we cannot infer whether our finding of increased uncinate fasciculus MD in patients with PTSD resulted from increased parallel or perpendicular diffusion or both, warranting caution when interpreting our findings.
From our study it cannot be concluded whether lower right uncinate fasciculus tract integrity in patients with PTSD may have developed as a consequence of the disorder, or whether it may represent a trait and pre-existing (i.e., pre-trauma) vulnerability factor for PTSD development. In support of the suggestion that it may represent a pretrauma vulnerability factor, lower uncinate fasciculus FA has been found in healthy women with low expression of the serotonin transporter (i.e., 5HTTLPR),62 associated with high anxiety and increased PTSD risk.63 Also, in a prospective imaging study, lower FA and higher MD of vmPFC white matter (measured within 2 days posttrauma) predicted PTSD development at 1 and 6 months posttrauma.16 Alternatively, lower uncinate fasciculus tract integrity in patients with PTSD may be an acquired characteristic. For example, soldiers with increased PTSD symptoms after military deployment showed decreased uncinate fasciculus tract integrity compared with predeployment baseline.13 In addition, higher anxiety symptoms 3–4 months posttrauma were associated with an increase in FA of the uncinate fasciculus and left anterior cingulum from pretrauma to 3–4 months posttrauma.25
Contrary to previous DTI studies, we did not find significant white matter alterations in patients with PTSD in the cingulum bundle and superior longitudinal fasciculus. The cingulum connects the cingulate cortex to the hippocampus and amygdala17 and decreased structural connectivity between these areas was hypothesized to impair fear extinction learning in patients with PTSD.14 Notably, white matter alterations of the cingulum bundle may develop over time with persistent PTSD symptoms: higher FA values of the dorsal cingulum have been found posttreatment in men with persistent PTSD (i.e., who did not recover after treatment), compared with pretreatment baseline, whereas no baseline differences were observed between patients with PTSD and combat-exposed controls.64 However, both increased and decreased FA values were previously found at different locations of the cingulum bundle and superior longitudinal fasciculus in patients with PTSD,18 possibly explaining why we did not find white matter alterations in the tracts we reconstructed using tractography (i.e., averaging over the entire tract, or larger segments along the tracts, compared with whole-brain voxel-wise techniques used in previous studies). Additionally, contradictory results between previous studies and our study may be explained by differences in study designs, such as the control group used (i.e., nontraumatized or trauma-exposed controls), the duration of PTSD and sex-specific effects on tract integrity. We observed lower white matter integrity of all investigated white matter bundles in women than in men, independent of PTSD diagnosis. Previous DTI studies also showed lower FA, but higher MD and RD (i.e., generally indicating lower white matter integrity) in various white matter tracts in healthy women than in men.65 Moreover, although we did not find altered white matter integrity between male and female patients with PTSD, the neurobiology of PTSD may be different for men and women.66 Therefore, it is important to investigate the neurobiological underpinnings of the sex-related white matter alterations, both in healthy individuals and in those with PTSD.
Limitations
Some limitations of our study need to be mentioned. First, we included a specific sample of trauma-exposed police officers, who experienced high levels of work-related traumatic events. Although this provided a homogeneous sample and robust control for neurobiological effects of trauma, it may also have limited the generalizability of our findings to other PTSD groups (i.e., civilian trauma). Second, we included some patients with PTSD who also had (mild to moderate) MDD, which may have influenced our findings.67 However, bilateral uncinate fasciculus tract integrity did not correlate with depression severity in patients with PTSD, suggesting that our findings were not confounded by comorbid depressive symptoms. Finally, our findings need to be interpreted with caution, as the (frontal part of the) uncinate fasciculus may be prone to susceptibility distortions, but we have not corrected for susceptibility artifacts.
Conclusion
We investigated white matter integrity of major white matter tracts in male and female patients with PTSD and trauma-exposed controls using global probabilistic tractography for the first time. Decreased right uncinate fasciculus tract integrity was observed in male and female patients with PTSD compared with trauma-exposed controls, which was associated with higher anxiety symptoms, as well as with higher amygdala activity and reduced vmPFC deactivation in response to emotional stimuli. Although replication is warranted, these findings fit with the prevailing neurocircuitry model of PTSD, suggesting that ineffective communication between the vmPFC and amygdala results in reduced top–down control over fear responses.
Acknowledgements
The authors thank all individuals who participated in this study. The authors thank all personnel of the PDC police outpatient clinic for their valuable help with recruitment of the patients with PTSD. The study is supported by grants from ZonMw, the Netherlands organization for Health Research and Development (grant no. 40-00812-98-10041) and the Academic Medical Center Research Council (110614).
Footnotes
Competing interests: None declared.
Contributors: All authors designed the study. S. Koch and L. Nawijn acquired the data, which all authors analyzed. S. Koch wrote the article, which all authors reviewed and approved for publication.
References
- 1.De Vries G, Olff M. The lifetime prevalence of traumatic events and posttraumatic stress disorder in the Netherlands. J Trauma Stress. 2009;22:259–67. doi: 10.1002/jts.20429. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. DSM-V, Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA, US: American Psychiatric Publishing Inc; 2013. [Google Scholar]
- 3.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of post-traumatic stress disorder and extinction: human neuroimaging research — past, present, and future. Biol Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biol Mood Anxiety Disord. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 6.Milad MR, Wright CI, Orr SP, et al. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Sripada RK, King AP, Garfinkel SN, et al. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37:241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morell P, Norton WT. Myelin. Sci Am. 1980;242:88–92. doi: 10.1038/scientificamerican0580-88. [DOI] [PubMed] [Google Scholar]
- 9.Hagmann P, Jonasson L, Maeder P, et al. Understanding diffusion MR imaging techniques: from scalar diffusion-weighted imaging to diffusion tensor imaging and beyond. Radiographics. 2006;26:S205–23. doi: 10.1148/rg.26si065510. [DOI] [PubMed] [Google Scholar]
- 10.Song S-K, Sun S-W, Ju W-K, et al. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–22. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu C. The basis of anisotropic water diffusion in the nervous system — a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo ME, Jovanovic T, Pham D, et al. White matter microstructure of the uncinate fasciculus is associated with subthreshold post-traumatic stress disorder symptoms and fear potentiated startle during early extinction in recently deployed service members. Neurosci Lett. 2016;618:66–71. doi: 10.1016/j.neulet.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 13.Admon R, Leykin D, Lubin G, et al. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp. 2013;34:2808–16. doi: 10.1002/hbm.22100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fani N, King TZ, Jovanovic T, et al. White matter integrity in highly traumatized adults with and without post-traumatic stress disorder. Neuropsychopharmacology. 2012;37:2740–6. doi: 10.1038/npp.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmahmann JD, Pandya DN, Wang R, et al. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–53. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Wang Z, Ding W, et al. Alterations in white matter microstructure as vulnerability factors and acquired signs of traffic accident-induced PTSD. PLoS One. 2013;8:e83473. doi: 10.1371/journal.pone.0083473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmahmann JD, Pandya DN. Fiber pathways of the brain. New York (NY): Oxford Univ Press; 2006. [Google Scholar]
- 18.Daniels JK, Lamke J-P, Gaebler M, et al. White matter integrity and its relationship to PTSD and childhood trauma–a systematic review and meta-analysis. Depress Anxiety. 2013;30:207–16. doi: 10.1002/da.22044. [DOI] [PubMed] [Google Scholar]
- 19.Abe O, Yamasue H, Kasai K, et al. Voxel-based diffusion tensor analysis reveals aberrant anterior cingulum integrity in posttraumatic stress disorder due to terrorism. Psychiatry Res. 2006;146:231–42. doi: 10.1016/j.pscychresns.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Schuff N, Zhang Y, Zhan W, et al. Patterns of altered cortical perfusion and diminished subcortical integrity in posttraumatic stress disorder: an MRI study. Neuroimage. 2011;54:S62–8. doi: 10.1016/j.neuroimage.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bierer LM, Ivanov I, Carpenter DM, et al. White matter abnormalities in Gulf War veterans with posttraumatic stress disorder: a pilot study. Psychoneuroendocrinology. 2015;51:567–76. doi: 10.1016/j.psyneuen.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Kim MJ, Lyoo IK, Kim SJ, et al. Disrupted white matter tract integrity of anterior cingulate in trauma survivors. Neuroreport. 2005;16:1049–53. doi: 10.1097/00001756-200507130-00004. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Zhang Y, Li L, et al. Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord. 2011;133:294–9. doi: 10.1016/j.jad.2011.03.040. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Jeong D-U, Sim ME, et al. Asymmetrically altered integrity of cingulum bundle in posttraumatic stress disorder. Neuropsychobiology. 2006;54:120–5. doi: 10.1159/000098262. [DOI] [PubMed] [Google Scholar]
- 25.Sekiguchi A, Sugiura M, Taki Y, et al. White matter microstructural changes as vulnerability factors and acquired signs of post-earthquake distress. PLoS One. 2014;9:e83967. doi: 10.1371/journal.pone.0083967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu J-L, Leemans A, Bai C-H, et al. Gender differences and age-related white matter changes of the human brain: a diffusion tensor imaging study. Neuroimage. 2008;39:566–77. doi: 10.1016/j.neuroimage.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011;5:23. doi: 10.3389/fninf.2011.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 29.Kühn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–4. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 30.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV® Axis I Disorders (SCID-I), Clinician Version, Administration Booklet. American Psychiatric Publishing Inc; 2012. [Google Scholar]
- 33.Koch SB, van Zuiden M, Nawijn L, et al. Intranasal oxytocin administration dampens amygdala reactivity towards emotional faces in male and female PTSD patients. Neuropsychopharmacology. 2016;41:1495–504. doi: 10.1038/npp.2015.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hariri AR, Tessitore A, Mattay VS, et al. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–23. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 35.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 36.Weiss DS. The impact of event scale-revised. In: Wilson JP, Keane TM, editors. Assessing psychological trauma and PTSD. New York (NY): Guilford press; 2004. pp. 168–9. [Google Scholar]
- 37.Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C) — an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–95. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 38.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlier I, Gersons B. Development of a scale for traumatic incidents in police work. Psychiatr Fenn. 1992;23:59–70. [Google Scholar]
- 40.Olabarriaga SD, Glatard T, de Boer PT. A virtual laboratory for medical image analysis. IEEE Trans Inf Technol Biomed. 2010;14:979–85. doi: 10.1109/TITB.2010.2046742. [DOI] [PubMed] [Google Scholar]
- 41.Shahand S, Benabdelkader A, Jaghoori MM, et al. A data-centric neuroscience gateway: design, implementation, and experiences. Concurr Comput Pract Exp. 2015;27:489–506. [Google Scholar]
- 42.Caan MWA, Khedoe G, Poot D, et al. Adaptive noise filtering for accurate and precise diffusion estimation in fiber crossings. Med Image Comput Comput Assist Interv. 2010;13:167–74. doi: 10.1007/978-3-642-15705-9_21. [DOI] [PubMed] [Google Scholar]
- 43.Behrens TEJ, Berg HJ, Jbabdi S, et al. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage. 2007;34:144–55. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vos SB, Jones DK, Viergever MA, et al. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. 2011;55:1566–76. doi: 10.1016/j.neuroimage.2011.01.048. [DOI] [PubMed] [Google Scholar]
- 46.Sexton CE, Walhovd KB, Storsve AB, et al. Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci. 2014;34:15425–36. doi: 10.1523/JNEUROSCI.0203-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W, van Tol M-J, Li M, et al. Regional specificity of sex effects on subcortical volumes across the lifespan in healthy aging. Hum Brain Mapp. 2014;35:238–47. doi: 10.1002/hbm.22168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zuurbier LA, Nikolova YS, Ahs F, et al. Uncinate fasciculus fractional anisotropy correlates with typical use of reappraisal in women but not men. Emotion. 2013;13:385–90. doi: 10.1037/a0031163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalisch R, Korenfeld E, Stephan KE, et al. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J Neurosci. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liberzon I, Abelson JL, Aas M, et al. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lissek S, van Meurs B. Learning models of PTSD: theoretical accounts and psychobiological evidence. Int J Psychophysiol. 2015;98:594–605. doi: 10.1016/j.ijpsycho.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jovanovic T, Norrholm SD, Fennell JE, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009;167:151–60. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez RP, Biggs A, Chen G, et al. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. J Neurosci. 2008;28:6211–9. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spielberg JM, Mcglinchey RE, Milberg WP, et al. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry. 2015;78:210–6. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci. 2009;29:11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Swartz JR, Carrasco M, Wiggins JL, et al. Age-related changes in the structure and function of prefrontal cortex-amygdala circuitry in children and adolescents: a multi-modal imaging approach. Neuroimage. 2014;86:212–20. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tromp DPM, Grupe DW, Oathes DJ, et al. Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry. 2012;69:925–34. doi: 10.1001/archgenpsychiatry.2011.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greicius MD, Krasnow B, Reiss AL, et al. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Daniels JK, McFarlane AC, Bluhm RL, et al. Switching between executive and default mode networks in posttraumatic stress disorder: alterations in functional connectivity. J Psychiatry Neurosci. 2010;35:258–66. doi: 10.1503/jpn.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cox SR, Bastin ME, Ferguson KJ, et al. Brain white matter integrity and cortisol in older men: the Lothian Birth Cohort 1936. Neurobiol Aging. 2015;36:257–64. doi: 10.1016/j.neurobiolaging.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pacheco J, Beevers CG, Benavides C, et al. Frontal-limbic white matter pathway associations with the serotonin transporter gene promoter region (5-HTTLPR) polymorphism. J Neurosci. 2009;29:6229–33. doi: 10.1523/JNEUROSCI.0896-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu Y, Garrett ME, Dennis MF, et al. An examination of the association between 5-HTTLPR, combat exposure, and PTSD diagnosis among U.S. veterans. PLoS One. 2015;10:e0119998. doi: 10.1371/journal.pone.0119998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennis M, van Rooij SJH, Tromp DPM, et al. Treatment outcome related white matter differences in veterans with posttraumatic stress disorder. Neuropsychopharmacology. 2015;40:2434–42. doi: 10.1038/npp.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kranz GS, Hahn A, Kaufmann U, et al. White matter microstructure in transsexuals and controls investigated by diffusion tensor imaging. J Neurosci. 2014;34:15466–75. doi: 10.1523/JNEUROSCI.2488-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shvil E, Sullivan GM, Schafer S, et al. Sex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study. Neurobiol Learn Mem. 2014;113:101–8. doi: 10.1016/j.nlm.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Isaac L, Main KL, Soman S, et al. The impact of depression on veterans with PTSD and traumatic brain injury: a diffusion tensor imaging study. Biol Psychol. 2015;105:20–8. doi: 10.1016/j.biopsycho.2014.12.011. [DOI] [PubMed] [Google Scholar]