Abstract
Objectives
Uterine serous carcinoma (USC) is an aggressive subtype of endometrial cancer that carries an extremely poor prognosis. Up to 35% of USC may overexpress the epidermal growth factor receptor-2 (HER2/Neu) at strong (i.e., 3 +) level by immunohistochemistry (IHC) or harbor HER2/Neu gene amplification by fluorescence in situ hybridization (FISH). In this study we assessed the sensitivity of a panel of USC cell lines with and without HER 2/neu gene amplification to dacomitinib (PF-00299804), an irreversible pan–human epidermal growth factor receptor tyrosine kinase inhibitor.
Materials and Methods
Eight primary cell lines (i.e., four harboring HER 2/neu gene amplification by FISH and four FISH− cell lines), all demonstrating similar in vitro growth rates, were evaluated in viability/proliferation assays. The effect of dacomitinib on cell growth, cell-cycle distribution and signaling was determined using flow cytometry-based assays.
Results
Dacomitinib caused a significantly stronger growth inhibition in HER2/neu FISH+ USC cell lines when compared to FISH− USC (dacomitinib IC50 mean ± SEM = 0.02803 ± 0.003355μM in FISH+ versus 1.498 ± 0.2209μM in FISH− tumors, P<0.0001). Dacomitinib growth-inhibition was associated with a significant and dose-dependent decline in phosphorylated HER2/neu and S6 transcription factor and a dose-dependent and time-dependent cell cycle arrest in G0/G1 in FISH+ USC.
Conclusions
Dacomitinib is remarkably effective against chemotherapy resistant HER2/neu gene amplified USC. Clinical studies with Dacomitinib in HER2/neu FISH+ USC patients resistant to standard salvage chemotherapy are warranted.
Keywords: Dacomitinib, Endometrial cancer, USC, HER2/neu amplicication, PF-00299804
Objectives
Uterine serous carcinoma (USC) is a highly aggressive subtype of endometrial cancer [1]. While it represents less than 10% of all endometrial cancer cases, it accounts for over 40% of recurrences and deaths [2, 3]. Despite aggressive adjuvant therapy, the recurrence rate of USC is approximately 50%, which is higher than its endometrioid counterpart [4], and even in early stage (Stage I or II), recurrence rates occur in 31–80% [5]. Stage by stage, uterine serous carcinoma, compared to endometrioid adenocarcinoma, portends a worse 5-year survival rate [4] [6]. Given the biologically aggressive nature and high recurrence and mortality rates, new treatments are desperately needed for USC patients. Proto-oncogenes are a group of normal genes that play important roles in the regulation of cell proliferation. Abnormalities in the expression, structure, or activity of proto-oncogene products contribute to the development and maintenance of the malignant phenotype. The human HER2/neu (c-erbB2) gene product, like the epidermal growth factor receptor, is a transmembrane receptor protein that includes a cysteine rich extracellular ligand binding domain, a hydrophobic membrane spanning region, and an intracellular tyrosine kinase domain [7]. With no direct ligand identified to date, HER2/neu functions as a preferred partner for heterodimerization with other members of the EGFR family (namely HER-1 or ErbB1, HER-3 or ErbB3 and HER-4 or ErbB4), and thus plays an important role in coordinating the complex ErbB signaling network that is responsible for regulating cell growth and differentiation [7]. In recent Gynecologic Oncology Group (GOG) studies (i.e., GOG 177), HER2 overexpression (either 2+ (moderate) or 3+ (strong) by immunostaining) and HER2 gene amplification (a ratio of HER2 copies to chromosome 17 (CEP17) copies > or = 2) were detected in 44% (104 of 234; 58 were 2+ and 46 were 3+) and 12% (21 of 182) of the endometrial cancer specimens tested, respectively [8]. In these studies a significant increased frequency of HER2 overexpression was found in USC vs. the other subtypes (23 of 38, 61% vs. 81 of 196, 41%, respectively, P=0.03). The results of GOG 177 confirmed previous studies documenting amplification of HER2/neu in 26–62% of USC cases, and suggest HER2/neu as a potential target for novel therapies in this biologically aggressive variant of endometrial cancer [9]. Dacomitinib (PF-00299804) is an oral, irreversible pan-ErbB small-molecule tyrosine kinase inhibitor (TKI), currently in clinical trials in patients with advanced non-small cell lung cancer (NSCLC) [10–14]. Unlike previous HER2 specific compounds, which targeted extracellular receptor domains (i.e., trastuzumab or pertuzumab), dacomitinib works by irreversibly inhibiting the kinase activity of HER1/EGFR, HER2, and HER4 through binding covalently to the receptor tyrosine kinase domains and preventing autophosphorylation, thereby inhibiting downstream signaling and leading to tumor-growth inhibition and apoptosis[15]. Our preclinical study represents the first evaluation of dacomitinib against primary HER2/neu amplified vs. non-amplified USC cell lines in vitro as well as a molecular evaluation of the down-stream effects of dacomitinib on the intracellular signaling pathway and cell cycle distribution. This understanding may lead to novel therapies for patients harboring this aggressive and deadly subset of endometrial tumors.
Methods
Collection of tumors, establishment of primary USC cell lines, and assessment of HER2/neu Status
Patients were consented for tumor banking prior to surgery and samples of each tumor were collected under approval of the institutional review board (IRB). These tumors were processed such that portions of the tumor were saved for genetic analysis, immunohistochemistry and a portion homogenized and reconstituted in petri dishes to establish primary cell lines. Cell lines were grown and established in a culture media of RPMI 1640 as described previously [16]. Primary USC cell lines (4 with HER2 gene amplification and 4 without) demonstrating similar in vitro growth rates were selected for analysis.
Drug
Dacomitinib (PF-00299804), an irreversible inhibitor of HER-1 (EGFR), HER2 and HER-4 tyrosine kinase, was provided by Pfizer Inc. (Peapack, NY). It was diluted to a solution of 10mM in DMSO (Sigma-Aldrich, St. Louis, MO) to create a stock solution..
Chemo-response assays
The effect of Dacomitinib on the IC50 of cells was determined using flow cytometry assays. Tumor cells of the 8 primary USC cell lines were harvested during the exponential growth phase and plated in 6 well plates for chemo-response assays. HER2 amplified and non-amplified cell lines were grown in a monolayer at the starting concentration of 15,000 cells/ml using RPMI 1640 with 10% FBS and 1% penicillin streptomycin and 1% amphotericin B. After 24 hours of incubation the cells were treated with scalar concentrations of dacomitinib ranging from 0.001μM to 2 μM for 72 hours. Concentrations up to 4.0 μM were used against the more resistant USC cell lines in additional experiments. At the 72-hour time point, the entirety of the samples were collected and centrifuged. The effect on cell viability was determined by staining each sample with propidium iodide (PI) (2μl of 500μg/ml stock solution in PBS with 1% azide and 2% fetal bovine serum, Biotium) and reading it using the FACSCalibur flow cytometry platform (Becton, Dickinson & Company, San Jose, CA). This allowed for quantification of the percent viable cells as a mean +/−SEM relative to vehicle treated cells as 100% viable controls. The IC50 for each cell line was then calculated using GraphPad Prism6 (GraphPad Software, Inc., San Diego, CA). Each experiment was repeated a minimum of 3 times per cell line.
Cell-cycle analysis
Cells were plated in 6 well plates at a concentration of 20,000 cells/ml. They were allowed to incubate for 24 hours. Scalar amounts of dacomitinib were then placed in each well. Cultures were allowed to incubate for up to 48 hours and harvested at the time point of 12hours, 24 hours and 48 hours for analysis. Cells were stained as previously described [17]. Data were analyzed using FlowJo software (FlowJo X 10.0.7, Ashland, OR).
HER2/neu and S6 phosphorylation
Cells were plated at 100,000 cells per ml of RPMI into 6 well plates. They were allowed to incubate for 24 hours. Cells were then treated with dacomitinib at concentrations of 0.010μM, 0.120μM, and 0.240μM. After incubation for 2, 4, 8, 10, 12, 16 and 24 hours they were harvested. Cells were fixed using 4% paraformaldehyde for 10 minutes at 37°C and then washed with PBS and permeabilized with 90% methanol for more than 30min. They were suspended in incubation buffer (PBS containing 0.5%BSA), blocked for 10 minutes, and aliquoted into 3 tubes. Cells were allowed to incubate with primary rabbit monoclonal antibodies: Phospho-HER2/ErbB2 (Y1221/1222 Cell Signaling Technologies, Inc., Danvers, MA), Phospho-S6 Ribosomal Protein (Ser235/236 Cell Signaling Technology, Inc., Danvers, MA) and no primary antibody for one hour on ice. Cells were then washed in incubation buffer 2 times and allowed to incubate with fluorescein conjugated goat anti-rabbit immunoglobulin (AQ132F) secondary fluorescein conjugated antibody (Millipore, Temecula, California) for half an hour on ice. Samples were then washed, suspended in PBS, and read by flow cytometry. Mean fluorescent intensities (MFI) were then calculated using FlowJo software. The difference in MFI between groups and treatments were then compared.
Statistical analysis
Statistical analysis was completed using GraphPad Prism6 (GraphPad Software, Inc., San Diego, CA). All data in different experimental groups were expressed as the mean±SEM. Data shown in the study were obtained from at least three independent experiments. All IC50 data were analyzed by first normalizing the number of live cells in each well treated with drug to the number of live cells in the untreated control. Using Prism6, normalized data were fit to nonlinear 3-parameter logistic dose response curves against the base-10 logarithms of dose in micromolar. The resulting parameter estimates were used to calculate the IC50 in log10 units for each experiment. One way analysis of variance was used to compare the IC50 data of HER2 amplified vs. non-amplified cell lines, while group means were compared using student’s t-test. Unpaired two-sided student’s t-tests were used to compare the cell cycle changes after removal from the analysis of the aggregates of apoptotic/dead tumor cells (i.e., sub-G1 phase) as well as the differences in the mean fluorescent intensities (MFI) pHER2 and pS6. Two-way ANOVA test was used for multiple comparisons of the MFI changes in phosphoprotein pHER2 and pS6 levels before and after the exposure to dacomitinib. Differences in all comparisons were considered statistically significant if P<0.05. The significance of difference is indicated as *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001.
Results
Selection of cell lines and determination of sensitivity to dacomitinib
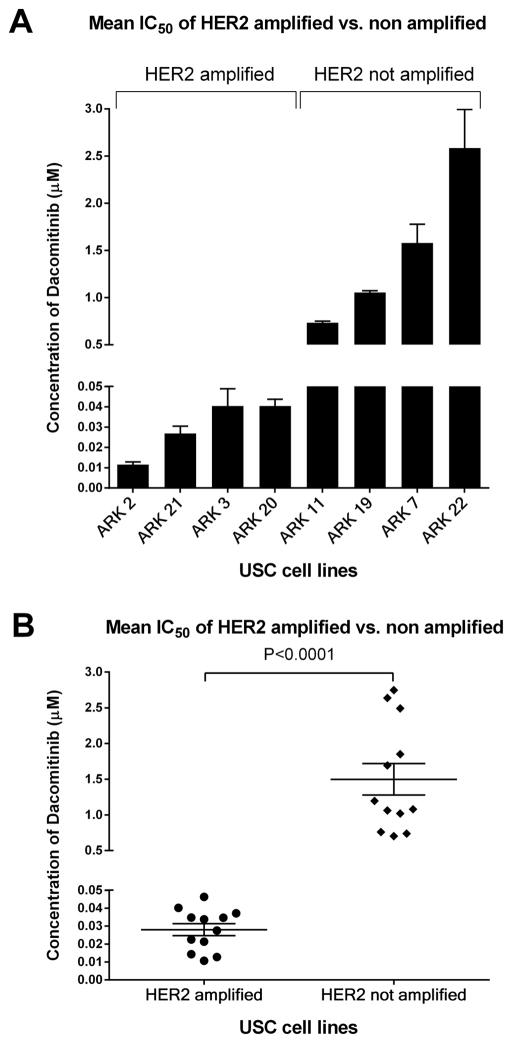
Cell lines were selected based on differential expression of HER2/neu detected by immunohistochemistry (IHC) and confirmed by FISH analysis based upon our previously published data [16, 18]. Four of fifteen established cell lines that showed amplification of HER2/neu were selected as experimental cell lines because of their similar growth rates [16]. Four more cell lines that were not HER2/neu amplified were selected as controls. The characteristics of the cell lines and patient’s tumors from which they were established are described in Table 1. First, we evaluated the cell lines response to dacomitinib in viability/proliferation flow cytometry-based assays. As representatively shown in Fig. 1A, we found dacomitinib to cause a significantly stronger differential growth inhibition in FISH+ USC cell lines when compared to FISH−. For example, FISH+ cell lines, ARK-2 and ARK-21, were the most sensitive to dacomitinib, with a mean inhibitory concentration (IC50) ± standard error of mean (SEM) of 0.01153 ± 0.00130 μM and 0.02687 ± 0.00360 μM, respectively. Within the FISH− cell lines, ARK-7 and ARK-22 were found to be the least sensitive, with IC50 values of 1.58000 ± 0.19720 μM and 2.629±0.05258 μM, respectively (Fig. 1A). Final analysis of FISH+ cell lines revealed that they were more than 50 fold more sensitive to dacomitinib in vitro than their non-amplified counterpart (dacomitinib IC50 mean ± SEM = 0.02803± 0.003355μM in c-erbB2 amplified versus 1.498±0.2209μM in c-erbB2 not amplified tumors, P<0.0001, Fig. 1B). These data suggest that uterine serous carcinoma cell lines that overexpress HER2/neu are exquisitely sensitive to dacomitinib in vitro.
Table 1.
Patient Characteristics and HER2 status in primary USC cell lines.
| Cell line | Age | Race | FIGO Stage | HER2 IHC score | C-erbB2 FISH |
|---|---|---|---|---|---|
| ARK-2 | 63 | B | IV | 3+ | Amplified |
| ARK3 | 59 | B | IV | 3+ | Amplified |
| ARK7 | 75 | W | IIc | 2+ | Not Amplified |
| ARK-11 | 80 | B | IIIc | 1+ | Not Amplified |
| ARK19 | 65 | W | Ia | 2+ | Not Amplified |
| ARK-20 | 42 | W | II | 3+ | Amplified |
| ARK-21 | 70 | W | Ia | 3+ | Amplified |
| ARK-22 | 60 | W | IVb | 0 | Not Amplified |
FIGO, International Federation of Gynecology and Obstetrics stage 1988; B = black; W = white.
Figure 1.
Comparison of mean IC50 values between HER2 amplified and not amplified USC cell lines.
A. Comparison of mean IC50 values for each HER2 amplified and non-amplified cell line run in triplicate.
B. Comparison of the overall mean IC50 values for HER2 Neu amplified and non-amplified cell lines each represented in triplicate.
Alterations in cell cycle distribution with dacomitinib treatment
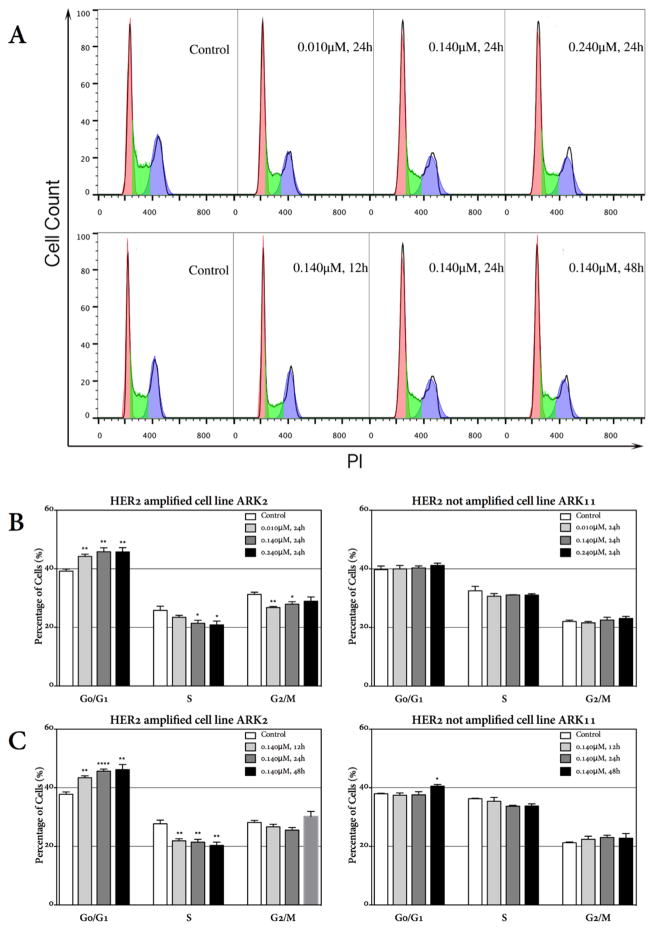
The effect of dacomitinib on HER2 amplified uterine serous carcinoma cell lines in vitro was further examined through cell cycle analysis by PI staining. As shown in Figure 2A, treatment with dacomitinib induced G0/G1 cell cycle arrest in HER2 amplified cell lines in dose-dependent and time-dependent manners. As representatively shown for ARK-2 cells in Figure 2A, the percentage of cells in the G0/G1 phase of cell cycle significantly increased to 44.23% (P=0.0027), 45.85% (P=0.0044) and 45.78% (P=0.0064) at the concentration of 0.010μM, 0.140μM and 0.240μM of dacomitinib, respectively, after 24 hours incubation when compared to 39.20% in the control cells. ARK-2 cells obtained maximum S phase inhibition at 0.240μM (Figure 2B). When ARK-2 cells were incubated with 0.140μM of dacomitinib for a different time course (i.e., from 12 hrs to 48 hrs), the percentage of USC cells arrested in G0/G1 significantly increased from 37.76% in the untreated control to 43.40% (P=0.0037), 45.69% (P<0.0001) and 46.20% (P=0.0024) at 12hours, 24hours and 48 hours, respectively (Figure 2C). As representatively demonstrated for ARK-11 in Figure 3C, no such effect was observed in any of the HER2 tested FISH− USC cell lines. These results suggest that dacomitinib may potently inhibit the rapid progression through the cell cycle of HER2 amplified cell lines.
Figure 2.
Changes in cell cycle distribution in HER2 amplified and non-amplified cell lines after treatment with dacomitinib. Dacomitinib induces cell cycle arrest at G0/G1 for the HER2 amplified cell line ARK-2.
A. ARK-2 cells were treated with 0.010μM, 0.140μM and 0.240μM dacomitinib for 24hours (upper panel) and treated with 0.140μM dacomitinib for 12 hours, 24 hours and 48 hours (lower panel). The experiments were performed at least 3 times, and a representative experiment is shown.
B. Cell cycle analysis of HER2 amplified cell line ARK-2 24 hours post different concentrations of dacomitinib demonstrating a significant G0/G1 arrest and a decrease in the S-phase fractions in a dose-dependent manner as compared with the HER2 not amplified cell line ARK-11.
C. Cell cycle analysis of HER2 amplified cell line ARK-2 12hours, 24hours and 48 hours post 0.140μM dacomitinib treatment demonstrating G0/G1 arrest in a time-dependent manner. No such effect was observed in HER2 not amplified cell line ARK-11.
Figure 3.
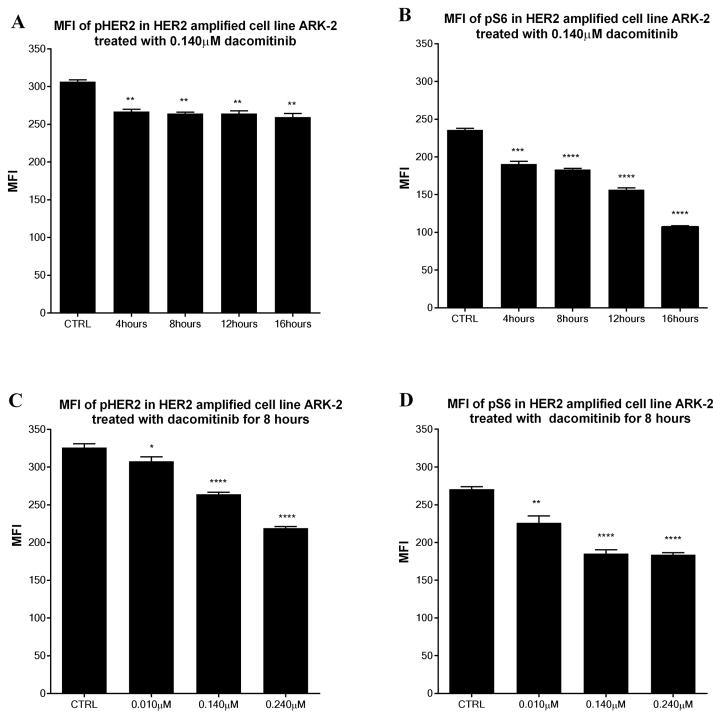
Comparison of the mean fluorescent intensity (MFI) in HER2 amplified cell line ARK 2 after treatment with dacomitinib at a concentration of 0.140μM in different time course for phosphorylated HER2 (a) and S6 (b). Comparison of the MFI in cell line ARK-2 after 8 hours exposure to different dose of dacomitinib for phosphorylated HER2 (c) and S6 (d).
Alterations in HER2/neu and S6 phosphorylation after treatment with dacomitinib
The USC cell line ARK-2 was chosen as the representative HER2 amplified cell line while ARK-11 was chosen as the non-amplified control cell line due to their differential expression of HER2/neu and nearly identical doubling time (ARK-2 vs. ARK-11, 18.2 hours vs. 23.1 hours, data not shown). In experiments performed at different time points we found a clear effect of daconitinib on the phosphorylation of HER2 and S6 after 8 hours exposure in the HER2 amplified cell lines (i.e., ARK-2) but not in the FISH− cell line (i.e., ARK-11) using 0.010μM of dacomitinib. Thus, 8 hours was selected as the initial time point for the analysis of changes in HER2 and S6 phosphorylation. These experiments were replicated in ARK-2 using doses of dacomitinib based on data regarding Ctrough (the human serum concentration prior to dose administration at steady state, approximately 0.140 μM) and Cmax (the maximum plasma concentration, approximately 0.240μM), achieved in humans after cycle 1 day 7 and multiple dosing of 45mg of dacomitinib daily [19], these values were consistent with those previously reported [20, 21]. Time course experiments were first performed using 0.140μM of dacomitinib. Data revealed that there was a decrease in the phosphorylation of HER2 and S6 with the time course extension while reaching its nadir at 16 hours (Figure 3a, 3b). Furthermore, a dose response assay was then completed at the 8-hour time point using the IC50 of the two cell lines and Ctrough and Cmax in humans after cycle 1 day 7 and multiple dosing of 45mg of dacomitinib daily (0.010 μM, 0.140μM and 0.240 μM). After incubation of ARK-2 and ARK-11 for 8 hours with dacomitinib, cells were harvested and the phosphorylation of HER2 and S6 was analyzed (not shown). Comparisons of mean fluorescent intensities for phospho-HER2 revealed that there was a significant decrease in phosphorylation at 8 hours with 0.010 μM (MFI 307.7 ± 6.0, P=0.038), 0.140μM (MFI 264.0 ±2.9, P<0.0001), and 0.240 μM (MFI 219.2±2.1, P<0.0001) when compared to untreated ARK-2 (MFI 326.1±4.8, Figure 3c). Similarly, analysis of phospho-S6 revealed a significant decrease in the MFI at 8 hours with 0.010 μM (MFI 226.2 ± 9.1, P=0.0017), 0.140μM (MFI 185.4 ±5.0 P<0.0001), and 0.240 μM (MFI 183.9±2.5, P<0.0001) when compared to untreated ARK-2 (MFI 270.8±3.1, Figure 3d). However, while a decrease in phosphorylation of HER2 and S6 was detected after the exposure to dacomitinib in HER2 not amplified cell line ARK-11, there was no significant difference in all the MFI for HER2 and S6 with 0.010 μM, 0.140μM or 0.240 μM when compared with the untreated cells. Further analysis using a two-way ANOVA accounting for both HER2 amplification status and dacomitinib treatment found that HER2 amplified cell line ARK-2 showed a significant decrease in HER2 and S6 phosphorylation (P<0.0001) compared with the not amplified USC cell line ARK-11 after treatment with dacomitinib. Similar results were obtained using the remaining USC amplified vs non-amplified control cell lines (data not shown). These data suggest that tumors reliant upon HER2 amplification as a driver for proliferation are indeed susceptible to treatment with dacomitinib in vitro.
Discussion
Development of novel effective therapies against recurrent chemotherapy resistant USC remains an unmet medical need. Although recent comprehensive genetic studies (i.e., whole exome sequencing) from The Cancer Genome Atlas (TCGA) Network and our own research group have reported that 21% to 44% of USC may harbor HER2/Neu gene amplifications [18, 22] and this is associated with aggressive disease and poor prognosis in multiple human tumors including USC [23–26], currently no FDA approved drugs are available for the treatment of endometrial cancer patients harboring chemotherapy resistant/recurrent disease overexpressing HER2/neu. Trastuzumab is a humanized anti-HER2/neu MAb endowed with significant clinical activity in HER2 amplified breast cancer patients [27, 28]. However, despite encouraging case reports [29–31], when trastuzumab was evaluated as a single-agent by the GOG in a heterogeneous group of endometrial cancer patients overexpressing HER2/neu (i.e., GOG-181B), it failed to demonstrate significant activity [28]. While ongoing clinical trials are assessing the efficacy of trastuzumab in combination with chemotherapy in a more homogenous group of patients with HER2/neu amplified USC (i.e., NCT01367002) certain characteristics of irreversible pan-erbB receptor tyrosine kinase inhibitors such as dacomitinib, suggest potential higher clinical activity against USC. For example, reports in breast cancer have shown that trastuzumab may bind intermittently with HER2 or with less affinity due to receptor conformational variants [32]. These interactions lead to inhibition of signaling through the HER2 pathway but are potentially less efficient at inhibiting proliferation pathways than might be expected in HER2 amplified tumors. Moreover, retrospective data in patients with HER2 amplified breast cancer revealed that patients with truncated p95HER2 were less responsive to HER2 therapy with trastuzumab [32]. Consistent with these mechanisms of trastuzumab resistance being active in USC, our group has recently demonstrated that p95HER2 shedding commonly occurs in vitro and in vivo in patients harboring USC with HER2/neu amplification [33]. The release of free floating receptor from the tumor surface competes for trastuzumab and decreases the bioavailability for cell membrane associated tumor receptors. Importantly, the small tyrosine kinase inhibitor dacomitinib, may potentially circumvent these problems, and preclinical data in breast cancer lends support to this notion. Indeed, dacomitinib molecular design allows it to bind in the ATP pocket of the ErbB 1 and ErbB2 receptor associated tyrosine kinase through the targeting of a cysteine residue [34]. This residue is conserved between these two receptors, which gives the daconitinib shared specificity [35]. Thus, inhibition of these receptors through an irreversible covalent modification of the intracellular ATP pocket may provide more robust antitumor effects compared to trastuzumab in HER2/neu amplified uterine serous carcinoma. Consistent with this view, our experimental results suggest that dacomitinib is remarkably active against primary HER2 amplified uterine serous carcinoma cell lines. Indeed, our in vitro results exposing multiple fully sequenced primary USC cell lines with or without c-erbB2 gene amplifications to dacomitinib clearly demonstrated a dramatic (i.e., about 100 fold-difference in IC50) higher sensitivity of the HER2/neu amplified USC to the exposure of the irreversible HER2/neu inhibitor. Moreover, HER2/neu amplified uterine serous carcinomas treated with dacomitinib showed significant inhibition of HER2 auto-phosphorylation and a significant decrease in the phosphorylation of the transcription factor S6. These changes in cell signaling, related to the inhibition of the tumors driver pathway HER2/neu, confer a significant build up in the G0/G1 phase of the cell cycle. Cell cycle arrest in G1 leads to decreased proliferation and is likely to lead to apoptosis. Our results in primary USC cell lines are similar to data published in preclinical studies using dacomitinib against HER2-amplified breast cancer [36] and lung cancer cell lines [37]. Phase I clinical trials in cancer patients based on the aforementioned preclinical data demonstrated dacomitinib to be generally safe and well tolerated at the maximum dose of 45 mg daily in humans. In these studies showing encouraging signs of antitumor activity in gefitinib/erlotinib treated NSCLC patients [21] the dose-limiting toxicities included stomatitis and skin toxicities while the most common adverse events were mild and mainly related to skin toxicities, fatigue, and gastrointestinal side-effects including diarrhea, nausea, and vomiting. Data from a randomized phase II study of dacomitinib showed that, compared with the reversible EGFR inhibitor erlotinib, dacomitinib demonstrated significantly improved median progression-free survival (PFS) from 1.91 months treated with erlotinib to 2.86 months in patients with advanced non–small-cell lung cancer (NSCLC)[20]. Skin effect and diarrhea were again the most prominent adverse events and such events were mild or moderate in severity and manageable. In conclusion, the novel preclinical data presented in this study suggests that dacomitinib is endowed with remarkable activity against HER2 amplified USC in vitro and, accordingly, it may represent a novel treatment option for a significant subset of USC patients with disease resistant to chemotherapy. Phase II studies with dacomitinib in USC overexpressing HER2/neu are warranted.
Acknowledgments
We wish to thank Pfizer for providing dacomitinib for our experiments. This work was supported in part by R01 CA154460-01 and U01 CA176067-01A1 grants from NIH, the Deborah Bunn Alley Foundation, the Tina Brozman Foundation, The Discovery to Cure Foundation and the Guido Berlucchi Foundation to ADS. This investigation was also supported by NIH Research Grant CA-16359 from the NCI.
Footnotes
Conflicts of interest: The authors report no conflicts of interest
References
- 1.Hendrickson M, Ross J, Eifel P, Martinez A, Kempson R. Uterine papillary serous carcinoma: A highly malignant form of endometrial adenocarcinoma. The American journal of surgical pathology. 1982;6:93–108. doi: 10.1097/00000478-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, Powell MA, Hendrickson MR, Kapp DS, Chan JK. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. British journal of cancer. 2006;94:642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boruta DM, 2nd, Gehrig PA, Groben PA, Bae-Jump V, Boggess JF, Fowler WC, Jr, Van Le L. Uterine serous and grade 3 endometrioid carcinomas: Is there a survival difference? Cancer. 2004;101:2214–2221. doi: 10.1002/cncr.20645. [DOI] [PubMed] [Google Scholar]
- 4.Acharya S, Hensley ML, Montag AC, Fleming GF. Rare uterine cancers. The lancet oncology. 2005;6:961–971. doi: 10.1016/S1470-2045(05)70463-0. [DOI] [PubMed] [Google Scholar]
- 5.del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: A review of the literature. Gynecologic oncology. 2012;127:651–661. doi: 10.1016/j.ygyno.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Goff BA. Uterine papillary serous carcinoma: What have we learned over the past quarter century? Gynecologic oncology. 2005;98:341–343. doi: 10.1016/j.ygyno.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Hynes NE, Lane HA. Erbb receptors and cancer: The complexity of targeted inhibitors. Nature reviews Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 8.Grushko TA, Filiaci VL, Mundt AJ, Ridderstrale K, Olopade OI, Fleming GF Gynecologic Oncology G. An exploratory analysis of her-2 amplification and overexpression in advanced endometrial carcinoma: A gynecologic oncology group study. Gynecologic oncology. 2008;108:3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.English DP, Roque DM, Santin AD. Her2 expression beyond breast cancer: Therapeutic implications for gynecologic malignancies. Molecular diagnosis & therapy. 2013;17:85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarden Y, Sliwkowski MX. Untangling the erbb signalling network. Nature reviews Molecular cell biology. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 11.Kim SM, Kwon OJ, Hong YK, Kim JH, Solca F, Ha SJ, Soo RA, Christensen JG, Lee JH, Cho BC. Activation of il-6r/jak1/stat3 signaling induces de novo resistance to irreversible egfr inhibitors in non-small cell lung cancer with t790m resistance mutation. Molecular cancer therapeutics. 2012;11:2254–2264. doi: 10.1158/1535-7163.MCT-12-0311. [DOI] [PubMed] [Google Scholar]
- 12.Pecorelli S, Zigliani L, Odicino F. Revised figo staging for carcinoma of the cervix. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2009;105:107–108. doi: 10.1016/j.ijgo.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD Herceptin Adjuvant Trial Study T. Trastuzumab after adjuvant chemotherapy in her2-positive breast cancer. The New England journal of medicine. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 14.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nukiwa T North-East Japan Study G. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated egfr. The New England journal of medicine. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 15.Gonzales AJ, Hook KE, Althaus IW, Ellis PA, Trachet E, Delaney AM, Harvey PJ, Ellis TA, Amato DM, Nelson JM, Fry DW, Zhu T, Loi CM, Fakhoury SA, Schlosser KM, Sexton KE, Winters RT, Reed JE, Bridges AJ, Lettiere DJ, Baker DA, Yang J, Lee HT, Tecle H, Vincent PW. Antitumor activity and pharmacokinetic properties of pf-00299804, a second-generation irreversible pan-erbb receptor tyrosine kinase inhibitor. Molecular cancer therapeutics. 2008;7:1880–1889. doi: 10.1158/1535-7163.MCT-07-2232. [DOI] [PubMed] [Google Scholar]
- 16.English DP, Bellone S, Cocco E, Bortolomai I, Pecorelli S, Lopez S, Silasi DA, Schwartz PE, Rutherford T, Santin AD. Oncogenic pik3ca gene mutations and her2/neu gene amplifications determine the sensitivity of uterine serous carcinoma cell lines to gdc-0980, a selective inhibitor of class i pi3 kinase and mtor kinase (torc1/2) American journal of obstetrics and gynecology. 2013;209:465e461–469. doi: 10.1016/j.ajog.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Schwab CL, Bellone S, English DP, Roque DM, Lopez S, Cocco E, Nicoletti R, Bortolomai I, Bonazzoli E, Ratner E, Silasi DA, Azodi M, Schwartz PE, Rutherford TJ, Santin AD. Afatinib demonstrates remarkable activity against her2-amplified uterine serous endometrial cancer in vitro and in vivo. British journal of cancer. 2014 doi: 10.1038/bjc.2014.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S, Choi M, Overton JD, Bellone S, Roque DM, Cocco E, Guzzo F, English DP, Varughese J, Gasparrini S, Bortolomai I, Buza N, Hui P, Abu-Khalaf M, Ravaggi A, Bignotti E, Bandiera E, Romani C, Todeschini P, Tassi R, Zanotti L, Carrara L, Pecorelli S, Silasi DA, Ratner E, Azodi M, Schwartz PE, Rutherford TJ, Stiegler AL, Mane S, Boggon TJ, Schlessinger J, Lifton RP, Santin AD. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reckamp KL, Giaccone G, Camidge DR, Gadgeel SM, Khuri FR, Engelman JA, Koczywas M, Rajan A, Campbell AK, Gernhardt D, Ruiz-Garcia A, Letrent S, Liang J, Taylor I, O’Connell JP, Janne PA. A phase 2 trial of dacomitinib (pf-00299804), an oral, irreversible pan-her (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer. 2014;120:1145–1154. doi: 10.1002/cncr.28561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramalingam SS, Blackhall F, Krzakowski M, Barrios CH, Park K, Bover I, Seog Heo D, Rosell R, Talbot DC, Frank R, Letrent SP, Ruiz-Garcia A, Taylor I, Liang JQ, Campbell AK, O’Connell J, Boyer M. Randomized phase ii study of dacomitinib (pf-00299804), an irreversible pan-human epidermal growth factor receptor inhibitor, versus erlotinib in patients with advanced non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:3337–3344. doi: 10.1200/JCO.2011.40.9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janne PA, Boss DS, Camidge DR, Britten CD, Engelman JA, Garon EB, Guo F, Wong S, Liang J, Letrent S, Millham R, Taylor I, Eckhardt SG, Schellens JH. Phase i dose-escalation study of the pan-her inhibitor, pf299804, in patients with advanced malignant solid tumors. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17:1131–1139. doi: 10.1158/1078-0432.CCR-10-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Research N. Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright C, Angus B, Nicholson S, Sainsbury JR, Cairns J, Gullick WJ, Kelly P, Harris AL, Horne CH. Expression of c-erbb-2 oncoprotein: A prognostic indicator in human breast cancer. Cancer research. 1989;49:2087–2090. [PubMed] [Google Scholar]
- 24.Berchuck A, Kamel A, Whitaker R, Kerns B, Olt G, Kinney R, Soper JT, Dodge R, Clarke-Pearson DL, Marks P, et al. Overexpression of her-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer research. 1990;50:4087–4091. [PubMed] [Google Scholar]
- 25.Hetzel DJ, Wilson TO, Keeney GL, Roche PC, Cha SS, Podratz KC. Her-2/neu expression: A major prognostic factor in endometrial cancer. Gynecologic oncology. 1992;47:179–185. doi: 10.1016/0090-8258(92)90103-p. [DOI] [PubMed] [Google Scholar]
- 26.Tanner M, Hollmen M, Junttila TT, Kapanen AI, Tommola S, Soini Y, Helin H, Salo J, Joensuu H, Sihvo E, Elenius K, Isola J. Amplification of her-2 in gastric carcinoma: Association with topoisomerase iialpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 27.Santin AD, Bellone S, Gokden M, Palmieri M, Dunn D, Agha J, Roman JJ, Hutchins L, Pecorelli S, O’Brien T, Cannon MJ, Parham GP. Overexpression of her-2/neu in uterine serous papillary cancer. Clinical cancer research: an official journal of the American Association for Cancer Research. 2002;8:1271–1279. [PubMed] [Google Scholar]
- 28.Fleming GF, Sill MW, Darcy KM, McMeekin DS, Thigpen JT, Adler LM, Berek JS, Chapman JA, DiSilvestro PA, Horowitz IR, Fiorica JV. Phase ii trial of trastuzumab in women with advanced or recurrent, her2-positive endometrial carcinoma: A gynecologic oncology group study. Gynecologic oncology. 2010;116:15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Villella JA, Cohen S, Smith DH, Hibshoosh H, Hershman D. Her-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2006;16:1897–1902. doi: 10.1111/j.1525-1438.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 30.Jewell E, Secord AA, Brotherton T, Berchuck A. Use of trastuzumab in the treatment of metastatic endometrial cancer. International journal of gynecological cancer: official journal of the International Gynecological Cancer Society. 2006;16:1370–1373. doi: 10.1111/j.1525-1438.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 31.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing her2/neu. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2008;102:128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, Di Cosimo S, Matias-Guiu X, Ramon y Cajal S, Arribas J, Baselga J. Expression of p95her2, a truncated form of the her2 receptor, and response to anti-her2 therapies in breast cancer. Journal of the National Cancer Institute. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 33.Todeschini P, Cocco E, Bellone S, Varughese J, Lin K, Carrara L, Guzzo F, Buza N, Hui P, Silasi DA, Ratner E, Azodi M, Schwartz PE, Rutherford TJ, Pecorelli S, Santin AD. Her2/neu extracellular domain shedding in uterine serous carcinoma: Implications for immunotherapy with trastuzumab. British journal of cancer. 2011;105:1176–1182. doi: 10.1038/bjc.2011.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wissner A, Wissner E, Overbeek M, Reich MB, Floyd B, Johnson N, Mamuya E, Rosfjord C, Discafani R, Davis X, Shi S, Rabindran B, Gruber F, Ye W, Hallett R, Nilakantan R, Shen Y-F, Wang L, Greenberger H-R, Tsou Synthesis and structure–activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of the tyrosine kinase activity of the epidermal growth factor receptor (egfr) and the human epidermal growth factor receptor-2 (her-2) Journal of medicinal chemistry. 2003;46:49–63. doi: 10.1021/jm020241c. [DOI] [PubMed] [Google Scholar]
- 35.Tsou HR, Overbeek-Klumpers EG, Hallett WA, Reich MF, Floyd MB, Johnson BD, Michalak RS, Nilakantan R, Discafani C, Golas J, Rabindran SK, Shen R, Shi XQ, Wang YF, Upeslacis J, Wissner A. Optimization of 6,7-disubstituted-4-(arylamino)quinoline-3-carbonitriles as orally active, irreversible inhibitors of human epidermal growth factor receptor-2 kinase activity. Journal of Medicinal Chemistry. 2005;48:1107–1131. doi: 10.1021/jm040159c. [DOI] [PubMed] [Google Scholar]
- 36.Kalous O, Conklin D, Desai AJ, O’Brien NA, Ginther C, Anderson L, Cohen DJ, Britten CD, Taylor I, Christensen JG, Slamon DJ, Finn RS. Dacomitinib (pf-00299804), an irreversible pan-her inhibitor, inhibits proliferation of her2-amplified breast cancer cell lines resistant to trastuzumab and lapatinib. Molecular cancer therapeutics. 2012;11:1978–1987. doi: 10.1158/1535-7163.MCT-11-0730. [DOI] [PubMed] [Google Scholar]
- 37.Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov GN, Bradner JE, Althaus IW, Gandhi L, Shapiro GI, Nelson JM, Heymach JV, Meyerson M, Wong KK, Janne PA. Pf00299804, an irreversible pan-erbb inhibitor, is effective in lung cancer models with egfr and erbb2 mutations that are resistant to gefitinib. Cancer research. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]