Abstract
Copper is a nutritional metal required for brain development and function. Wilson’s disease (WD), or hepatolenticular degeneration, is an inherited human copper metabolism disorder caused by mutation of ATP7B gene. Many WD patients present with variable neurological and psychiatric symptoms, which may be related to neurodegeneration secondary to copper metabolism imbalance. The objective of this study is to explore feasibility and use of copper-64 chloride ([64C]CuCl2) as a tracer for noninvasive assessment of age-dependence changes of cerebral copper metabolism in WD using an Atp7b−/− knockout mouse model of WD and a positron emission tomography/computed tomography (PET/CT) scanner. Continuing from recent study of biodistribution and radiation dosimetry of [64C]CuCl2 in Atp7b−/− knockout mice, PET quantitative analysis revealed low 64Cu radioactivity in the brains of Atp7b−/− knockout mice at 7th week of age, compared with the 64Cu radioactivity in the brains of age and gender-matched wild type C57BL/6 mice, at 24 hour (h) post intravenous injection of [64C]CuCl2 as a tracer. Furthermore, age-dependent increase of 64Cu radioactivity was detected in the brains of Atp7b−/− knockout mice from 13th to 21th week of age, using the data derived from a longitudinal [64C]CuCl2-PET/CT study of Atp7b−/− knockout mice with orally administered [64Cu]CuCl2 as a tracer. The findings of this study support the use of [64Cu]CuCl2-PET/CT as a tool for noninvasive assessment of age-dependent changes of cerebral copper metabolism in WD patients presenting with variable neurological and psychiatric symptoms.
Keywords: copper metabolism, Wilson’s disease, Atp7b gene, Neurodegeneration, Positron emission tomography, Copper-64 chloride
Introduction
Copper is an essential nutrient required for development and function of human brain [1, 2]. Wilson’s disease (WD) is an inherited copper metabolism disorder caused by mutation of ATP7B gene encoding a copper-transporting P-type ATPase functioning as an efflux copper transporter [3–5]. The ATP7B efflux copper transporter is highly expressed in liver and plays an important role in delivery and biliary excretion of copper from liver. Malfunction of ATP7B efflux copper transporter causes accumulation of excess amount of copper ions in the liver, resulting hepatic metabolic changes and liver tissue damage. The ATP7B protein is also expressed in the brain and plays an important role in maintenance of cerebral copper homeostasis. Development of neurological and behavioral disorders in some of WD patients is likely related to disruption of copper homeostasis in the brain due to malfunction of ATP7B protein. The molecular mechanism of variable presentation of neuropsychiatric symptoms in WD patients remains unclear, largely due to lack of a tool for real-time assessment of changes of cerebral copper metabolism related to neurodegeneration within the brain of WD patients. Positron emission tomography (PET) is a useful technology for functional imaging of cerebral metabolic activity in vivo based on its high sensitivity and quantification capability. Recently, biodistribution and radiation dosimetry of [64Cu]CuCl2 in Atp7b−/− knockout mice, a well-established mouse model of WD [6], was determined by PET/CT analysis [7–9]. Exploring feasibility and use of [64Cu]CuCl2 as a tracer for noninvasive assessment of changes of cerebral copper metabolism in WD, this study aimed to assess age-dependent changes of 64Cu radioactivity in the brains of Atp7b−/− knockout mice using PET/CT, following intravenous injection of [64Cu]CuCl2 as a tracer. Positron emitting 2-deoxy-2-[F-18]-fluoro-D-glucose ([18F]FDG) is a glucose analogue [10], which is widely used as a tracer for assessment of changes of cerebral glucose metabolism in neurological and psychiatric disorders with PET. In order to determine whether [64Cu]CuCl2 can be used as a tracer for assessment of cerebral copper metabolism, a sequential PET/CT imaging was conducted to compare biodistribution and cerebral [18F]FDG and 64Cu radioactivity in mice intravenously injected with [18F]FDG and [64Cu]CuCl2 as a tracer, respectively. Continuing from recent studies of 64Cu biodistribution and radiation dosimetry in the Atp7b−/− knockout mice administered with [64Cu]CuCl2 intravenously [7] or orally [8, 9], further PET quantitative analysis was performed to measure age-dependent changes of 64Cu radioactivity in the brains of Atp7b−/− knockout mice. The data from this preclinical study support further development of [64Cu]CuCl2 as a new tracer for assessment of age-dependent changes of copper metabolism in WD patients presented with neuropsychiatric symptoms using a PET or PET/CT scanner.
Materials and Methods
Small animals and Radiopharmaceuticals
Balb/c mice were purchased from the animal resource center of the University of Texas Southwestern Medical Center (Dallas, TX). Free access to copper-adequate food and drinking water was provided routinely by animal housing facility, at UT Southwestern Medical Center at Dallas. The tracer [64Cu]CuCl2 was purchased from Washington University (St Louis, MO), which was produced via 64Ni(p,n)64Cu on a biomedical cyclotron and supplied in a form of [64Cu]CuCl2 in 0.1M HCl solution. The specific activity of 64Cu was 6.9 ± 2.5 Ci/μmol. All small animal experiments were conducted according to the protocol approved by the Institutional Animal Care and Use Committee, UT Southwestern Medical Center, Dallas, TX.
[18F]FDG PET/CT imaging
[18F]FDG PET/CT imaging of Balb/c mice was performed using a method described previously [11, 12]. In short, Balb/c mice were anesthetized by inhalation of 3% isoflurane in 100% oxygen (3 L/min) at room temperature, using an isoflurane vaporizer (Summit Anesthesia Solutions, Salt Lake City, UT) and scanned using a Siemens Inveon PET/CT multimodality system (Siemens Medical Solutions). The mice were transferred to spread-supine position on the imaging bed and subjected to inhalation of 2% isoflurane in 100% oxygen (3 L/min) during the PET/CT procedure. After intravenous injection of [18F]FDG (2 μCi (74 kBq)/g body weight) via tail vein, the mice were subjected to PET/CT imaging at 30 minutes and 2 h post injection (p.i.), respectively. Dynamic whole body data acquisition was started for 30 minutes with the head in the center of the field of view (FOV), followed by static imaging for 15 minutes at 2 h p.i. PET/CT images were reconstructed using the ordered subsets expectation maximization 3D algorithm (OSEM3D), and data were analyzed using the Inveon Research Workplace (IRW) software (Siemens) which allows fusion of CT and PET image volumes.
[64Cu]CuCl2-PET/CT imaging
[64Cu]CuCl2-PET/CT imaging of Balb/c mice was performed using a method described previously [7–9, 13]. Briefly, Balb/c mice were anesthetized by 3% isoflurane in 100% oxygen (3 L/min) at room temperature, using an isoflurane vaporizer (Summit Anesthesia Solutions, Salt Lake City, UT). The mice were positioned in a spread-supine position on the imaging bed and subjected to inhalation of 2% isoflurane in 100% oxygen (3 L/min) during the PET/CT procedure. PET/CT imaging was then initiated following intravenous injection of [64Cu]CuCl2 (2 μCi (74 kBq)/g body weight) diluted in a volume of 100 μL normal saline (0.9% sodium chloride) via tail vein. Dynamic whole body data acquisition was started for 30 minutes (5 minute frames) with the head in the center of the field of view (FOV), followed by static imaging for 15 minutes at 2 and 24 h p.i, respectively. PET/CT images were reconstructed using the ordered subsets expectation maximization 3D algorithm (OSEM3D), and data were analyzed using the Inveon Research Workplace (IRW) software (Siemens) which allows fusion of CT and PET image volumes.
Quantification of copper ions in mouse tissues by ICP-MS
To investigate correlation of 64Cu radioactivity measured by [64Cu]CuCl2-PET/CT with copper ions present in mouse organs or tissues, mouse tissue copper ions in another group of Blab/c mice (n=5, female, 8 weeks of age) were quantified by Inductively coupled plasma mass spectrometry (ICP-MS) in a method similar to those previously described [9, 14, 15]. For sample preparation, mouse tissues were harvested after the Balb/c mice were euthanized under anesthesia. Tissues and organs were frozen for further study. Before copper concentration determination, tissues were collected and weighted. 200 μL of Fresh aqua regia (a mixture of nitric acid and hydrochloric acid nitric in a ratio of 1:3, tracer metal grade) purchased from Fisher scientific (Pittsburgh, PA) were added to these tissues, followed by heating to 80 °C for 20 min. Ultrasound was assisted to digested these tissues. After overnight digestion, all of the sample solutions were clear, without visible residues. The solution was diluted with Milli-Q water (Millipore gradient, USA) for ICP-MS determination. Copper analysis was performed with 7700 series ICP-MS instrument (Agilent technologies, CA). Calibration was performed using 6 to 8 standards and stable copper isotopes were measured with instrumental parameters. Copper concentration was recorded as ng/mg wet weight and values presented as means ± standard deviation (SD).
Quantification of 64Cu radioactivity in the brain of Atp7b−/− knockout mice
Using the data acquired from our recent studies of biodistribution and radiation dosimetry of [64Cu]CuCl2 in Atp7b−/− knockout mice [7, 8], 64Cu radioactivity in the brain of Atp7b−/− knockout mice was analyzed using the Inveon Research Workplace (IRW) software (Siemens). In order to measure 64Cu radioactivity in the different regions of a mouse brain, 9 regions of interest (ROIs) were defined manually on the PET/CT images with reference to an MR imaging–based atlas of mouse brain anatomy [16] as shown on Fig 2. Regions included were: ROI 1 for olfactory bulb (OB), ROI 2 for frontal cortex (FC), ROI 3 for posterior cortex (PC), ROI 4 for hippocampus (HC), ROI 5 for basal ganglia (BG), ROI 6 for thalamus (T), ROI 7 for middle brain (MB), ROI 8 for brain stem (BS), ROI 9 for cerebellum (CB). The quantity of 64Cu radiotracer activity was obtained and recorded as a percentage of injected dose per gram tissue (%ID/g).
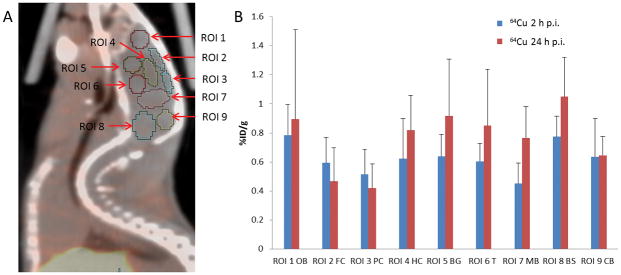
Fig 2. PET quantification of 64Cu radioactivity in the brain of Balb/c mice after intravenous injection of [64Cu]CuCl2 as a tracer.
A. Schematic presentation of 9 ROIs manually drawn within the region of brain on PET/CT imaging of Balb/c mice. B. 64Cu radioactivity in the different regions of mouse brain by PET quantitative analysis. 64Cu radioactivity in the cortex at 24 h p.i was lower than the cortical 64Cu radioactivity at 2 h p.i. In contrast, 64Cu radioactivity in the other regions of brain at 24 h p.i. was higher than 64Cu radioactivity in the brain at 2 h p.i.
ROI, region of interest; OB, olfactory bulb; FC, frontal cortex; PC, posterior cortex; HC, hippocampus, BG, basal ganglia; T, thalamus; MB, middle brain; BS, brain stem; CB, cerebellum. %ID/g, percentage of injected dose per gram; h, hour; p.i., post intravenous injection.
Statistical analysis
Friedman rank based ANOVA was used to test the difference in median cerebral 64Cu radioactivity between Atp7b−/− knockout mice and control C57BL/6 mice for each organ and for each time point (2 h and 24 h p.i., respectively). P value of less than 0.05 was considered as statistically significant.
RESULTS
Biodistribution of [18F]FDG and 64Cu radioactivity in mice
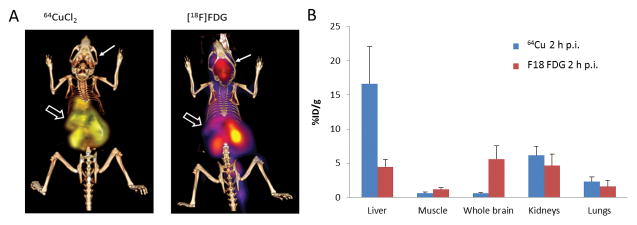
Sequential PET/CT imaging was performed to compare the biodistribution of [18F]FDG and 64Cu radioactivity in mice (Blab/c, n = 5, female, 8 weeks of age) after intravenous injection of [8F]FDG and [64Cu]CuCl2, respectively. There was different biodistribution of 18FDG and 64Cu radioactivity in Blab/c mice (Fig 1, Table 1). In contrast to abundant [18F]FDG in the brain of Balb/c mice injected with [18F]FDG, there was relatively low 64Cu radioactivity in the brain of Balb/c mice injected with [64C]CuCl2 intravenously. Moreover, regional analysis of the brain showed decreased cortical 64Cu radioactivity at 24 h p.i. as compared to the 64Cu radioactivity at 2 h p.i. (Fig 2, Table 2). With respect to all other brain regions, 64Cu radioactivity at 24 h p.i. was higher than the 64Cu radioactivity at 2 h p.i., except for unchanged 64Cu radioactivity in the cerebellum (Fig 2, Table 2).
Fig 1. Biodistribution of [64Cu]CuCl2 and [18F]FDG in Balb/c mice by PET/CT imaging.
A. Low 64Cu radioactivity in the brain and intense 64Cu radioactivity in the liver were visualized on the PET/CT image of Balb/c mice at 30 minutes p.i. of [64Cu]CuCl2. In contrast, abundant physiological [18F]FDG radioactivity was visualized in the brain of Balb/c mice at 30 minutes p.i. of [18F]FDG. An open thick arrow points to the liver, whereas a thin arrow points to the brain. B. Biodistribution of 64Cu and [18F]FDG in Balb/c mice by PET quantitative analysis, showing high 64Cu radioactivity in the liver of Balb/c mice at 2 h p.i. of [64Cu]Cl2 and abundant physiological 18FDG radioactivity in the brains of Balb/c mice at 2 h p.i. of 18FDG. %ID/g, percentage of injected dose per gram; p.i., post intravenous injection.
Table 1.
Biodistribution of 64Cu and [18F]FDG in Balb/c mice by PET/CT after intravenous injection of [64Cu]CuCl2 and [18F]FDG, respectively.
| [64Cu]CuCl2 0.5 h p.i. |
[64Cu]CuCl2 2 h p.i. |
[64Cu]CuCl2 24 h p.i. |
[18F]FDG 0.5 h p.i. |
[18F]FDG 2 h p.i. |
|
|---|---|---|---|---|---|
| Liver | 11.8 ± 7.4* | 16.6 ± 5.4 | 16.74 ± 6.3 | 4.73 ± 1.02 | 4.43 ± 1.12 |
| heart | 1.7 ± 1.3 | 1.96 ± 0.59 | 2.12 ± 0.62 | 29.4 ± 14.0 | 30.9 ± 11.9 |
| Muscle | 0.51 ± 0.37 | 0.59 ± 0.17 | 0.66 ± 0.31 | 0.29 ± 0.35 | 1.16 ± 0.31 |
| brain | 0.57 ± 0.41 | 0.58 ±0.17 | 0.80 ± 0.34 | 7.13 ± 2.02 | 5.62 ± 1.92 |
| Kidney | 5.7 ± 4.0 | 6.14 ±1.37 | 8.28 ± 3.26 | 11.9 ± 1.74 | 4.65 ±1.71 |
| Lung | 2.1 ± 1.6 | 2.32 ±0.70 | 2.26 ± 0.69 | 2.48 ± 1.65 | 1.58 ± 0.92 |
%ID/g, percentage of injected dose per gram, mean ± standard deviation (SD)
, h, hour; p.i., post intravenous injection.
Table 2.
Biodistribution of [64Cu]CuCl2 and [18F]FDG (%ID/g) in the brain of Balb/c mice by PET/CT after intravenous injection of the tracers.
| [64Cu]CuCl2 0.5 h p.i. |
[64Cu]CuCl2 2 h p.i. |
[64Cu]CuCl2 24 h p.i. |
[18F]FDG 0.5 h p.i. |
[18F]FDG 2 h p.i. |
|
|---|---|---|---|---|---|
| ROI 1 OB | 0.36 ± 0.31 | 0.79 ± 0.21 | 0.89 ± 0.62 | 6.5 ± 1.7 | 6.3 ± 1.0 |
| ROI 2 FC | 0.39 ± 0.31 | 0.59 ± 0.18 | 0.47 ± 0.23 | 5.8 ± 1.7 | 4.9 ± 1.1 |
| ROI 3 PC | 0.31 ± 0.21 | 0.52 ± 0.17 | 0.41 ±0.17 | 6.1 ± 2.0 | 4.8 ± 1.3 |
| ROI 4 HC | 0.64 ± 0.50 | 0.63 ± 0.28 | 0.82 ± 0.24 | 7.8 ± 2.4 | 5.7 ± 2.0 |
| ROI 5 BG | 0.67 ± 0.59 | 0.64 ± 0.15 | 0.92 ± 0.24 | 6.9 ± 2.2 | 6.1 ± 1.8 |
| ROI 6 T | 0.63 ± 0.49 | 0.60 ± 0.12 | 0.85 ± 0.39 | 7.6 ± 2.3 | 5.5 ± 1.9 |
| ROI 7 MB | 0.50 ± 0.38 | 0.45 ± 0.14 | 0.77 ± 0.22 | 9.2 ± 3.0 | 5.9 ± 2.0 |
| ROI 8 BS | 0.86 ± 0.68 | 0.77 ± 0.14 | 1.05 ± 0.27 | 8.2 ± 2.6 | 6.3 ± 2.4 |
| ROI 9 CB | 0.50 ± 0.41 | 0.64 ± 0.26 | 0.64 ± 0.13 | 8.5 ± 2.5 | 5.8 ± 2.1 |
%ID/g, percentage of injected dose per gram, mean ± standard deviation (SD)
ROI, region of interest; h, hour; p.i., post intravenous injection.
Copper concentration of mouse tissues determined by ICP-MS
Copper concentration in tissues of Balb/c mice (n = 5, female, 8 weeks of age) was quantified by ICP-MS. High concentration of copper ions was detected in the heart (7.49 ± 1.04 ng/mg), liver (6.51 ± 1.22 ng/mg), brain (5.66 ± 0.97), stomach (5.46 ± 1.57 ng/mg), and kidney (4.27 ± 0.93 ng/mg), compared with relatively lower concentration of copper ions in lungs (3.23 ± 0.88 ng/mg), muscle (2.31 ± 0.79 ng/mg), intestine (2.39 ± 0.49 ng/mg), and spleen (1.68 ± 0.68 ng/mg). High copper concentration of liver tissue was correlated with abundant hepatic 64Cu radioactivity measured by [64Cu]CuCl2-PET/CT imaging (Table 1, Fig 3). Despite the high concentration of endogenous copper ions in brain tissue determined by ICP-MS, there was low 64Cu radioactivity in the brain of Balb/c mice measured by [64Cu]CuCl2-PET/CT imaging (Fig 1 and Fig 3). These findings suggest that [64Cu]CuCl2-PET/CT is a useful tool for tracking copper fluxes in vivo, but is limited or not useful for indirect estimation of the concentration of endogenous copper ions in mouse tissues.
Fig. 3. Biodistribution of 64Cu radioactivity determined by [64Cu]CuCl2 PET/CT imaging and concentration of copper ions in Balb/c mice measured by ICP-MS.

Abundant 64Cu radioactivity in liver and kidneys (A) corresponded with high concentration of endogenous copper ions determined by ICP-MS (B). In contrast, low 64Cu radioactivity in the brain and heart tissue corresponded high concentration of copper ions in these tissues determined by ICP-MS. %ID/g, percentage of injected dose per gram; h, hour; p.i., post intravenous injection.
Decreased 64Cu radioactivity in the brain of 7-week-old Atp7b−/− knockout mice after intravenous injection of [64C]CuCl2
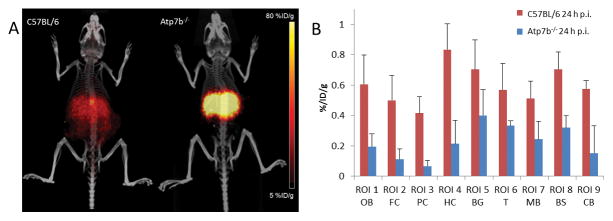
Quantitative analysis was performed to study 64Cu radioactivity in the brain of Atp7b−/− knockout mice injected with 64CuCl2 intravenously, using the data from recent study of 64Cu biodistribution and radiation dosimetry in Atp7b−/− knockout mice [7]. At 7th week of age, 64Cu radioactivity at 24h p.i. in the brain of Atp7b−/− knockout mice (0.34 ± 0.08 %ID/g) was significantly lower than the 64Cu radioactivity in the brain of age-matched C57BL/6 mice (0.61 ± 0.09 %ID/g, p < 0.03) (Fig 4, Table 3). Moreover, whereas the brain 64Cu radioactivity of control C57BL/6 mice increased between 2 and 24 h p.i., it was decreased between 2 and 24 h p.i. in the brain of Atp7b−/− knockout mice. These results demonstrate the potential of 64CuCl2 PET/CT imaging for tracking copper fluxes in the mouse brain.
Fig. 4. Decreased 64Cu radioactivity in the brains of Atp7b−/− mice at 7th week of age compared with physiologic 64Cu radioactivity in the brains of age-matched C57BL/6 mice.
(A) Increased accumulation of 64Cu was visualized in the liver on PET/CT images of Atp7b−/− knockout mice after intravenous injection of [64Cu]CuCl2, compared with relatively low 64Cu radioactivity in the liver of age-matched wild type C57BL/6 mice. (B) PET quantitative analysis demonstrated that 64Cu radioactivity in the brains of Atp7b−/− knockout mice was significantly lower than the 64Cu radioactivity in the brains of control C57BL/6 mice at 24 h p.i of [64Cu]CuCl2.
ROI, region of interest; OB, olfactory bulb; FC, frontal cortex; PC, posterior cortex; HC, hippocampus, BG, basal ganglia; T, thalamus; MB, middle brain; BS, brain stem; CB, cerebellum. %ID/g, percentage of injected dose per gram; h, hour; p.i., post intravenous injection.
Table 3.
64Cu uptake (%ID/g) in the brains of Atp7b−/− and C58BL/6 mice by PET/CT after intravenous injection of [64Cu]CuCl2 as a tracer.
|
Atp7b−/− 2 h p.i. |
Atp7b−/− 24 h p.i. |
C57BL/6 2 h p.i. |
C57BL/6 24 h p.i. |
|
|---|---|---|---|---|
| ROI 1 (OB) | 0.53 ± 0.18 | 0.19 ± 0.08 | 0.49 ± 0.11 | 0.61 ± 0.19 |
| ROI 2 (FC) | 0.39 ± 0.10 | 0.11 ± 0.07 | 0.45 ± 0.12 | 0.50 ± 0.16 |
| ROI 3 (PC) | 0.36 ± 0.11 | 0.07 ± 0.04 | 0.48 ± 0.09 | 0.41 ± 0.11 |
| ROI 4 (HC) | 0.45 ± 0.16 | 0.21 ± 0.16 | 0.54 ± 0.15 | 0.83 ± 0.17 |
| ROI 5 (BG) | 0.66 ± 0.23 | 0.40 ± 0.17 | 0.50 ± 0.08 | 0.70 ± 0.20 |
| ROI 6 (T ) | 0.41 ± 0.17 | 0.33 ± 0.03 | 0.48 ± 0.04 | 0.57 ± 0.17 |
| ROI 7 (MB) | 0.33 ± 0.06 | 0.24 ± 0.12 | 0.30 ± 0.07 | 0.51 ± 0.11 |
| ROI 8 (BS) | 0.33 ± 0.11 | 0.32 ± 0.08 | 0.63 ± 0.21 | 0.70 ± 0.11 |
| ROI 9 (CB) | 0.43 ± 0.14 | 0.15 ± 0.18 | 0.45 ± 0.12 | 0.57 ± 0.05 |
%ID/g, percentage of injected dose per gram, mean ± standard deviation (SD)
ROI, region of interest; h, hour; p.i., post intravenous injection.
Age-dependent changes of 64Cu radioactivity in the brain of Atp7b−/− knockout mice determined by longitudinal PET/CT imaging after oral administration of [64Cu]CuCl2
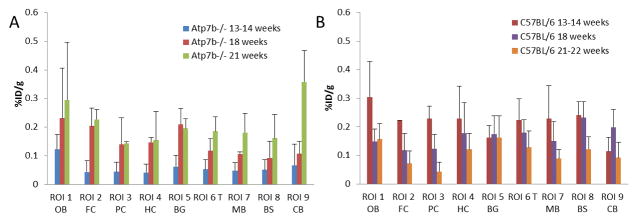
In order to determine whether there are age-dependent changes of cerebral copper metabolism in Atp7b−/− knockout mice, a quantitative analysis of 64Cu accumulation in the brain of Atp7b−/− knockout mice was performed using the data from recent longitudinal PET/CT imaging of Atp7b−/− knockout mice after oral administration of [64Cu]CuCl2 [8, 9]. Age-dependent changes of 64Cu radioactivity were identified in the brain of Atp7b−/− knockout mice (n = 4, female) between 13 and 21 weeks of age (Table 4, Fig 5). At 13th week of age, 64Cu radioactivity in the brain of Atp7b−/− knockout mice (0.05 ± 0.04 %ID/g) was lower than the 64Cu radioactivity in the brains of control C57BL/6 mice (0.11 ± 0.10 %ID/g, p = 0.048). Subsequently, there was an age-dependent increase of 64Cu radioactivity in the brain of Atp7b−/− knockout mice. By 21th week of age, 64Cu radioactivity in the brain of Atp7b−/− knockout mice (0.17 ± 0.09 %ID/g) was significantly higher than the 64Cu uptake in the brain of age-matched control C57BL/6 mice (0.09 ± 0.05 %ID/g, p = 0.042). Moreover, quantitative analysis revealed significant increase of 64Cu radioactivity in the region of hippocampus (p = 0.042), thalamus (p = 0.002), brain stem (p = 0.001), and cerebellum (p = 0.014) of the brains of Atp7b−/− knockout mice, compared with those in the corresponding brain regions of control C57BL/6 mice (Table 4). No significant increase of 64Cu radioactivity was identified in the regions of olfactory bulb (p = 0.474), frontal cortex (p = 0.61), posterior cortex (p = 0.072), and basal ganglia (p= 0.256) of the brains of Atp7b−/− knockout mice, compared with 64Cu radioactivity in the corresponding brain regions of control C57BL/6 mice (Table 4).
Table 4.
Age-dependent changes of 64Cu uptake (%ID/g) in the brains of Atp7b−/− mice by longitudinal PET/CT at 24 h after oral administration of [64Cu]CuCl2 as a tracer
| Atp7b−/− 3th week |
Atp7b−/− 18th week |
Atp7b−/− 21th week |
C57BL/6 13th week |
C57BL/6 18th week |
C57BL/6 21th week |
|
|---|---|---|---|---|---|---|
| ROI 1 OB | 0.12 ± 0.14 | 0.23 ± 0.18 | 0.30 ± 0.20 | 0.30 ± 0.12 | 0.15 ± 0.04 | 0.15 ± 0.05 |
| ROI 2 FC | 0.04 ± 0.04 | 0.20 ± 0.06 | 0.23 ± 0.03 | 0.22 ± 0.01 | 0.12 ± 0.06 | 0.06 ± 0.05 |
| ROI 3 PC | 0.04 ± 0.03 | 0.14 ± 0.09 | 0.14 ± 0.00 | 0.23 ± 0.04 | 0.12 ± 0.05 | 0.04 ± 0.03 |
| ROI 4 HC | 0.04 ± 0.03 | 0.15 ± 0.02 | 0.15 ± 0.10 | 0.23 ± 0.11 | 0.18 ± 0.11 | 0.12 ± 0.07 |
| ROI 5 BG | 0.06 ± 0.04 | 0.21 ± 0.05 | 0.20 ± 0.03 | 0.16 ± 0.04 | 0.17 ± 0.06 | 0.16 ± 0.07 |
| ROI 6 T | 0.05 ± 0.03 | 0.12 ± 0.04 | 0.19 ± 0.05 | 0.22 ± 0.07 | 0.18 ± 0.05 | 0.13 ± 0.06 |
| ROI 7 MB | 0.05 ± 0.03 | 0.11 ± 0.01 | 0.18 ± 0.07 | 0.23 ± 0.11 | 0.15 ± 0.07 | 0.09 ± 0.03 |
| ROI 8 BS | 0.05 ± 0.04 | 0.09 ± 0.06 | 0.16 ± 0.08 | 0.24 ± 0.05 | 0.23 ± 0.06 | 0.12 ± 0.04 |
| ROI 9 CB | 0.07 ± 0.07 | 0.11 ± 0.04 | 0.36 ± 0.11 | 0.11 ± 0.05 | 0.20 ± 0.06 | 0.09 ± 0.05 |
%ID/g, percentage of injected dose per gram, mean ± standard deviation (SD)
ROI, region of interest; h, hour.
Fig. 5. Age-dependent changes of 64Cu radioactivity in brains of Atp7b−/− knockout mice and control C57BL/6 mice by longitudinal PET/CT imaging after oral administration of [64Cu]CuCl2 as a tracer.
A Age-dependent increases of 64Cu radioactivity were detected in all brain regions in Atp7b−/− knockout mice at 24 h p.o. of [64Cu]CuCl2 as a tracer. Longitudinal PET/CT imaging was performed at 13th, 18th, and 21th week of age. (B) In contrast, age-dependent decrease of 64Cu radioactivity were detected in all brain regions in C57BL/6 mice at 24 h p.o. of 64CuCl2 as a tracer. Longitudinal PET/CT imaging was performed at 13th, 18th, and 21th week of age.
ROI, region of interest; OB, olfactory bulb; FC, frontal cortex; PC, posterior cortex; HC, hippocampus, BG, basal ganglia; T, thalamus; MB, middle brain; BS, brain stem; CB, cerebellum. %ID/g, percentage of injected dose per gram; h, hour; p.o., post oral administration.
Discussion
Copper is required for development and function of brain, and copper metabolism imbalance secondary to malfunction of ATP7B efflux copper transporter causes neurodegeneration in WD [17, 18]. Some of WD patients present with symptoms of neurological and/or psychiatric disorders, while many of WD patients present with mainly symptoms of hepatic disease without significant symptoms of neuropsychiatric disorders. The cause of this phenotypic diversity in WD is not clear, that may be related to effect of ATP7B gene mutation on cellular localization and function of ATP7B efflux copper transporter and cellular copper tolerance [19] and effect of other modification factors [20]. Animals with genetic defects in Atp7b gene have been a remarkably useful tool in study of molecular mechanisms of WD pathophysiology. The Atp7b−/− knockout mouse is a well-established mouse model of WD, developing hepatic pathology caused by accumulation of excess amount of copper ions in the liver [6, 21]. In contrast to neuroinflammatory and behavioral changes observed in toxic milk mouse model of WD [22, 23], no significant neuropsychiatric symptoms were observed in adult Atp7b−/− knockout mice although neurological symptoms (tremor, ataxia, abnormal locomotor behavior) were observed in Atp7b−/− knockout pups younger than 2 weeks of age and nursed by homozygous Atp7b−/− mothers [21]. The cause of absence of neuropsychiatric symptoms in adult Atp7b−/− knockout mice is not clear, that may be related to age-dependent changes of cerebral copper metabolism. Cerebral copper deficiency present at infant age is no longer present in the adult Atp7b−/− knockout mice when there is improvement of availability and delivery of copper to the brain tissues. It remains to be determined whether old Atp7b−/− knockout mice will develop neuropsychiatric symptoms when there is neuron loss caused by neurotoxicity of excess amount of copper ions accumulated in the brain tissues.
The data from sequential [18F]FDG and [64Cu]CuCl2-PET/CT imaging of Balb/c mice (Table 1, 2 and Fig 1, 2) demonstrated the capability of 64Cu to diffuse across the blood brain barrier, as previously described [24], supporting use of [64Cu]CuCl2 as a tracer for noninvasive assessment of cerebral copper metabolism in vivo by quantification of 64Cu radioactivity using PET or PET/CT imaging. Our findings are in line with previous studies that applied [64Cu]CuCl2 as a tracer for investigation of cerebral copper metabolism in aging mice with autoradiography [25], study of changes of cerebral copper metabolism in a mouse model of Menkes disease [26], as well as diagnostic imaging of traumatic brain injury [13]. To determine whether 64Cu radioactivity measured by PET/CT is correlated with the concentration of endogenous copper ions in mouse tissues, copper concentration of mouse tissues was determined by ICP-MS and correlated with 64Cu biodistribution in Balb/c mice on [64Cu]CuCl2-PET/CT imaging (Fig 3). Intense 64Cu radioactivity in the liver is correlated with a large quantity of hepatic endogenous copper ions measured by ICP-MS, but there is no direct correlation between relatively low cerebral 64Cu radioactivity determined by PET/CT and high concentration of copper ions in brain tissues determined by ICP-MS (Fig 3). This suggests that [64Cu]CuCl2-PET/CT measures dynamic fluxes and accumulation of 64Cu in the mouse brain tissues, while ICP-MS measures total amount of endogenous copper ions in the mouse brain tissues, regulated by a network of copper transporters and chaperons [27–29]. It is important to study both of dynamic copper fluxes and quantity of endogenous copper ions in brain tissues in dissecting the role of disruption of copper homeostasis, either copper deficiency or accumulation of excess amount of copper ions, in pathophysiology of WD [14, 15, 30] and other neurological disease associated with copper metabolism disorders [28].
Continuing from recent biodistribution and radiation dosimetry study in Atp7b−/− knockout mice injected with [64Cu]CuCl2 intravenously [7], further PET quantitative analysis of current study demonstrated that 64Cu radioactivity in the brains of 7-week-old Atp7b−/− knockout mice was significantly lower than the 64Cu radioactivity in the brains of age-matched control C57BL/6 mice (Table 3, and Fig 4). Low 64Cu radioactivity in the brains of Atp7b−/− knockout mice was likely related to sequestration of 64Cu in the liver due to malfunction of Atp7b efflux copper transporter. On the other hand, low 64Cu radioactivity in the Atp7b−/− knockout mice at 7th week of age could also be related to reduced copper influx mediated by human copper transporter 1 or changes of copper transport mediated by other copper transporters or chaperons [27–29]. Compared with physiological 64Cu radioactivity in the brains of C57BL/6 mice, low 64Cu radioactivity in the brains of Atp7b−/− knockout mice at 7th week of age may represent copper deficiency in the mouse brain tissues or decreased availability of copper from blood pool. It remains to be determined whether low 64Cu radioactivity detected in the brain of Atp7b−/− knockout mice at 7th week of age is associated with development of neurological and/or psychiatric disorders. Based on further PET quantitative analysis of the data acquired from recent longitudinal PET/CT imaging of Atp7b−/− knockout mice after oral administration of [64Cu]CuCl2 [8, 9], age-dependent changes of 64Cu radioactivity was detected in the brain of Atp7b−/− knockout mice [Table 4 and Fig 5]. There was gradual increase of 64Cu radioactivity in the brain of Atp7b−/− knockout mice from 13th to 21th week of age, likely due to increased availability of 64Cu from blood pool when liver sequestration of 64Cu is reduced or increased retention of 64Cu in the brain tissues caused by malfunction of Atp7b copper efflux transporter. Increased 64Cu radioactivity was detected in all of brain regions in Atp7b−/− knockout mice by 21th week of age, including regions of cortex, basal ganglia, thalamus, midbrain and brain stem and cerebellum, with highest 64Cu radioactivity detected in cerebellum [Fig 5]. It will be useful to correlate 64Cu radioactivity detected by [64Cu]CuCl2 PET/CT with abnormalities detected by other imaging modalities, such as abnormal fiber projection between subcortical nuclei [31], for delineation of molecular mechanism of brain tissue damage in WD patients with neuropsychiatric disorders.
Addressing limitations of this study, the data of sequential [18F]FDG and [64Cu]CuCl2 PET/CT imaging (Fig 1, 2, 3A) and mouse tissue copper concentration determined by ICP-MS (Fig 3B) were obtained in Balb/c mice, not in C57BL/6 mice used as a control group for assessment of age-dependent changes of cerebral 64Cu radioactivity in Atp7b−/− knockout mice. We used Balb/c mice for sequential [18F]FDG and [64Cu]CuCl2 PET/CT imaging in this study because it was difficulty to conduct tail vein injection in C57BL/6 mice and frequently there was leakage of radiotracer at tail vein injection site after multiple attempts of repeated tail vein injection. It was technically less challenging to conduct repeated tail vein injection in Balb/c mice than in C57BL/6 mice, therefore, we used Balb/c mice for sequential [18F]FDG and [64Cu]CuCl2 PET/CT imaging in this study. There might be a small difference of [18F]FDG and 64Cu accumulation in the brain of Balb/c and C57BL/6 mice after intravenous injection of [18F]FDG and [64Cu]CuCl2 respectively. This should not limit use of the data obtained from sequential [18F]FDG and [64Cu]CuCl2 PET/CT imaging of Balb/c mice to determine feasibility and use of 64CuCl2 as a radiotracer for assessment of age-dependent changes of cerebral 64Cu radioactivity in Atp7b−/− knockout mice with PET/CT. The data of 64Cu radioactivity measured from manually drawn mouse brain ROIs was subjected to spatial resolution error in view of small size of mouse brain and limited spatial resolution of a PET scanner. Additionally, biodistribution of 64CuCl2 in rodents might be different from that in humans considering possible difference of copper metabolism between rodents and human. Nevertheless, the findings of age-dependent changes of 64Cu accumulation in Atp7b−/− knockout mice support further study of age-dependent changes of cerebral copper fluxes in WD patients using [64Cu]CuCl2-PET/CT imaging. Radiation dosimetry of [64Cu]CuCl2 estimated from preclinical biodistribution and radiation dosimetry studies in rodents [7, 8, 32] and clinical use of [64Cu]CuCl2 as a radiopharmaceutical for diagnostic imaging of prostate cancer in human [33] support safe use of [64Cu]CuCl2 as a tracer for evaluation of cerebral copper metabolism imbalance in WD patients. In view of a tiny amount of copper ions (< 0.04 ng/μCi of 64CuCl2) in a tracer dose of [64Cu]CuCl2, there is little concern of copper toxicity on neurons when a tracer dose of [64Cu]CuCl2 is administered for PET/CT imaging in human.
The data from this study demonstrated age-dependent changes of 64Cu accumulation in the brains of Atp7b−/− knockout mice, which are encouraging and support further investigation of age-dependent changes of copper metabolism in WD patients who suffer from psychiatric and neurological symptoms, using [64Cu]CuCl2-PET/CT imaging in conjunction with [18F]FDG PET [34, 35] and MRI [35, 36]. Delineation of age-dependent changes of copper metabolism in WD patients is expected to contribute to a better understanding of molecular mechanism of neurodegeneration in WD. In summary, 64CuCl2-PET/CT is a promising new tool for assessment of the changes of cerebral copper metabolism in WD patients who present with psychiatric and neurological symptoms and may set the stage for individualized treatment of WD [17, 18, 37].
Conclusion
Age-dependent changes of cerebral 64Cu radioactivity in the brains of Atp7b−/− knockout mice were detected by PET/CT imaging after intravenous injection of [64Cu]CuCl2 as a tracer, supporting further development of [64Cu]CuCl2 PET/CT as a tool for noninvasive assessment of age-dependent changes of cerebral copper metabolism in WD patients.
Acknowledgments
The authors thank Xiankai Sun for use of ICP-MS instrument. This research project was partially supported by National Institutes of Health (1R21NS074394-01A1 and 1R21AG047953-01 to F.P). The production of [64Cu]CuCl2 at Washington University School of Medicine was supported by the NIH/NCI grant R24 CA86307.
Footnotes
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest. The authors alone are responsible for the content and writing of the paper
References
- 1.Olivares M, Uauy R. Copper as an essential nutrient. Am J Clin Nutr. 1996;63:791S–6S. doi: 10.1093/ajcn/63.5.791. [DOI] [PubMed] [Google Scholar]
- 2.Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr. 1998;67:952S–9S. doi: 10.1093/ajcn/67.5.952S. [DOI] [PubMed] [Google Scholar]
- 3.Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–37. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, Devoto M, Peppercorn J, Bush AI, Sternlieb I, Pirastu M, Gusella JF, Evgrafov O, Penchaszadeh GK, Honig B, Edelman IS, Soares MB, Scheinberg IH, Gilliam TC. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344–50. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Heiny ME, Gitlin JD. Isolation and characterization of a human liver cDNA as a candidate gene for Wilson disease. Biochem Biophys Res Commun. 1993;197:271–7. doi: 10.1006/bbrc.1993.2471. [DOI] [PubMed] [Google Scholar]
- 6.Buiakova OI, Xu J, Lutsenko S, Zeitlin S, Das K, Das S, et al. Null Mutation of the Murine ATP7B (Wilson Disease) Gene Results in Intracellular Copper Accumulation and Late-Onset Hepatic Nodular Transformation. Hum Mol Genet. 1999;8:1665–1671. doi: 10.1093/hmg/8.9.1665. [DOI] [PubMed] [Google Scholar]
- 7.Peng F, Lutsenko S, Sun X, Muzik O. Positron emission tomography of copper metabolism in the Atp7b−/− knock-out mouse model of Wilson’s disease. Mol Imaging Biol. 2012;14:70–8. doi: 10.1007/s11307-011-0476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng F, Lutsenko S, Sun X, Muzik O. Imaging copper metabolism imbalance in Atp7b−/− knockout mouse model of Wilson’s disease with PET-CT and orally administered 64CuCl2. Mol Imaging Biol. 2012;14:600–7. doi: 10.1007/s11307-011-0532-0. [DOI] [PubMed] [Google Scholar]
- 9.Gray LW, Peng F, Molloy SA, Pendyala VS, Muchenditsi A, Muzik O, Lee J, Kaplan JH, Lutsenko S. Urinary copper elevation in a mouse model of Wilson’s Disease is a regulated process to specifically decrease the hepatic copper load. PLoS One. 2012;7:e38327. doi: 10.1371/journal.pone.0038327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Som P, Atkins HL, Bandoypadhyay D, Fowler JS, MacGregor RR, Matsui K, Oster ZH, Sacker DF, Shlue CY, Turner H, Wan CN, Wolf AP, Zabinski SV. A fluorinated glucose analog, 2-fluoro-2-deoxy-D-glucose (F-18): nontoxic tracer for rapid tumor detection. J Nucl Med. 1980;21:670–5. [PubMed] [Google Scholar]
- 11.Toyama H, Ichise M, Liow JS, Vines DC, Seneca NM, Modell KJ, Seidel J, Green MV, Innis RB. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal PET. Nucl Med Biol. 2004;31(2):251–6. doi: 10.1016/S0969-8051(03)00124-0. [DOI] [PubMed] [Google Scholar]
- 12.Fueger BJ, Czernin J, Hildebrandt I, Tran C, Halpern BS, Stout D, Phelps ME, Weber WA. Impact of Animal Handling on the Results of 18F-FDG PET Studies in Mice. J Nucl Med. 2006;47:999–1006. [PubMed] [Google Scholar]
- 13.Peng F, Muzik O, Gatson J, Kernie SG, Diaz-Arrastia R. Assessment of Traumatic Brain Injury by Increased 64Cu Uptake on 64CuCl2 PET/CT. J Nucl Med. 2015;56:1252–7. doi: 10.2967/jnumed.115.154575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faa G, Lisci M, Caria MP, Ambu R, Sciot R, Nurchi VM, Silvagni R, Diaz A, Crisponi G. Brain copper, iron, magnesium, zinc, calcium, sulfur and phosphorus storage in Wilson’s disease. J Trace Elem Med Biol. 2001;15:155–160. doi: 10.1016/S0946-672X(01)80060-2. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Shi SS, Chen S, Ni W, Zhu M, Wu ZY. The discrepancy between the absence of copper deposition and the presence of neuronal damage in the brain of Atp7b−/− mice. Metallomics. 2015;7:283–8. doi: 10.1039/c4mt00242c. [DOI] [PubMed] [Google Scholar]
- 16.Chuang N, Mori S, Yamamoto A, Jiang H, Ye X, Xu X, Richards LJ, Nathans J, Miller MI, Toga AW, Sidman RL, Zhang J. An MRI-based atlas and database of the developing mouse brain. Neuroimage. 2011;54:80–9. doi: 10.1016/j.neuroimage.2010.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 18.Lorincz MT. Neurologic Wilson’s disease. Ann N Y Acad Sci. 2010;1184:173–87. doi: 10.1111/j.1749-6632.2009.05109.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M, Dong Y, Ni W, Wu ZY. Defective roles of ATP7B missense mutations in cellular copper tolerance and copper excretion. Mol Cell Neurosci. 2015;67:31–36. doi: 10.1016/j.mcn.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Lutsenko Svetlana. Modifying factors and phenotypic diversity in Wilson’s disease. Ann N Y Acad Sci. 2014;1315:56–63. doi: 10.1111/nyas.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutsenko Svetlana. Atp7b−/− mice as a model for studies of Wilson’s disease. Biochem Soc Trans. 2008;36:1233–1238. doi: 10.1042/BST0361233. [DOI] [PubMed] [Google Scholar]
- 22.Terwel D, Löschmann YN, Schmidt HH, Schöler HR, Cantz T, Heneka MT. Neuroinflammatory and behavioural changes in the Atp7b mutant mouse model of Wilson’s disease. J Neurochem. 2011;118:105–12. doi: 10.1111/j.1471-4159.2011.07278.x. [DOI] [PubMed] [Google Scholar]
- 23.Przybyłkowski A, Gromadzka G, Wawer A, Bulska E, Jabłonka-Salach K, Grygorowicz T, Schnejder-Pachołek A, Członkowski A. Neurochemical and behavioral characteristics of toxic milk mice: an animal model of Wilson’s disease. Neurochem Res. 2013;38:2037–45. doi: 10.1007/s11064-013-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi BS, Zheng W. Copper transport to the brain by the blood-brain barrier and blood-CSF barrier. Brain Res. 2009;1248:14–21. doi: 10.1016/j.brainres.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang LM, Becker JS, Wu Q, Oliveira MF, Bozza FA, Schwager AL, Hoffman JM, Morton KA. Bioimaging of copper alterations in the aging mouse brain by autoradiography, laser ablation inductively coupled plasma mass spectrometry and immunohistochemistry. Metallomics. 2010;2:348–53. doi: 10.1039/c003875j. [DOI] [PubMed] [Google Scholar]
- 26.Nomura S, Nozaki S, Hamazaki T, Takeda T, Ninomiya E, Kudo S, Hayashinak E, Wada Y, Hiroki T, Fujisawa C, Kodama H, Shintaku H, Watanabe Y. PET imaging analysis with 64Cu in disulfiram treatment for aberrant copper biodistribution in Menkes disease mouse model. J Nucl Med. 2014;55:845–51. doi: 10.2967/jnumed.113.131797. [DOI] [PubMed] [Google Scholar]
- 27.Zhou B, Gitschier J. hCTR1: A human gene for copper uptake identified by complementation in yeast. Proc Natl Acad Sci USA. 1997;94:7481–86. doi: 10.1073/pnas.94.14.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen E, Gitlin JD. Copper and iron disorders of the brain. Annu Rev Neurosci. 2007;30:317–37. doi: 10.1146/annurev.neuro.30.051606.094232. [DOI] [PubMed] [Google Scholar]
- 29.Lutsenko S, Bhattacharjee A, Hubbard AL. Copper handling machinery of the brain. Metallomics. 2010;2:596–608. doi: 10.1039/c0mt00006j. [DOI] [PubMed] [Google Scholar]
- 30.Litwin T, Gromadzka G, Szpak GM, Jabłonka-Salach K, Bulska E, Członkowska A. Brain metal accumulation in Wilson’s disease. J Neurol Sci. 2013;329:55–8. doi: 10.1016/j.jns.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 31.Zhou XX, Li XH, Qin H, Li GD, Huang HW, Liang YY, Liang XL, Pu XY. Diffusion tensor imaging of the extracorticospinal network in the brains of patients with Wilson disease. J Neurol Sci. 2016;362:292–298. doi: 10.1016/j.jns.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 32.Manrique-Arias JC, Carrasco-Hernández J, Reyes PG, Ávila-Rodríguez MA. Biodistribution in rats and estimates of doses to humans from 64CuCl2, a potential theranostic tracer. Appl Radiat Isot. 2016;115:18–22. doi: 10.1016/j.apradiso.2016.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Capasso E, Durzu S, Piras S, Zandieh S, Knoll P, Haug A, Hacker M, Meleddu C, Mirzaei S. Role of 64CuCl2 PET/CT in staging of prostate cancer. Ann Nucl Med. 2015;29(6):482–8. doi: 10.1007/s12149-015-0968-4. [DOI] [PubMed] [Google Scholar]
- 34.Hawkins RA, Mazziotta JC, Phelps ME. Wilson’s disease studied with FDG and positron emission tomography. Neurology. 1987;37(11):1707–11. doi: 10.1212/wnl.37.11.1707. [DOI] [PubMed] [Google Scholar]
- 35.Hermann W1, Barthel H, Hesse S, Grahmann F, Kühn HJ, Wagner A, Villmann T. Comparison of clinical types of Wilson’s disease and glucose metabolism in extrapyramidal motor brain regions. J Neurol. 2002;249(7):896–901. doi: 10.1007/s00415-002-0756-7. [DOI] [PubMed] [Google Scholar]
- 36.Hermann W. Morphological and functional imaging in neurological and non-neurological Wilson’s patients. Ann N Y Acad Sci. 2014;1315:24–9. doi: 10.1111/nyas.12343. [DOI] [PubMed] [Google Scholar]
- 37.Roberts EA, Schilsky ML. Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47:2089–111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]