Abstract
Cognitive decline and anxiety symptoms commonly co-occur in later life, but the temporal order of changes on these two attributes is unclear. Specifically, it is unknown if greater anxiety leads to subsequent declines in cognitive performance or if worse cognitive performance leads to increased anxiety. In this study, we sought to elucidate the temporal dynamics between anxiety symptoms and cognitive performance across old age, that is, the extent to which level and change in one variable influence subsequent changes in a second variable. We examined data from 721 non-demented participants from the Swedish Adoption/Twin Study of Aging. Participants completed as many as eight assessments of cognitive performance and anxiety over a 26-year period. Bivariate dual change score models were fit to examine the dynamic association between anxiety and cognitive performance. Bidirectional associations between anxiety and cognitive performance were found among measures of processing speed, attention, and memory, but not visuospatial abilities. Higher anxiety was associated with greater declines in processing speed over the duration of six years and worsening attention over a span of three years. The reverse direction was also significant in that slower processing speed, worse attention, and poorer nonverbal and working memory performance were associated with larger increases in anxiety three years later. These findings highlight that in cognitively intact older adults, the association between anxiety and worse cognitive performance is bidirectional and complex.
Keywords: Anxiety, cognition, longitudinal, structural equation modeling
Dementia and cognitive decline are common in later life (Hebert, Weuve, Scherr, & Evans, 2013), debilitating, and carry a significant public health cost (Hurd, Martorell, & Langa, 2013). As no significantly effective treatments currently exist for dementia, there is a focus on identifying potentially modifiable risk factors in order to develop successful interventions to prevent or delay decline.
Anxiety symptoms and disorders are the most prevalent psychiatric conditions in later life. As many as 14% of older adults meet diagnostic criteria for an anxiety disorder, and 32% experience clinically significant symptoms of anxiety (Braam et al., 2014; Wolitzky-Taylor, Castriotta, Lenze, Stanley, & Craske, 2010). Higher anxiety in later life is associated with adverse health outcomes such as myocardial infarction (Annelieke et al., 2012), death ideation (Van Orden, Simning, Conwell, Skoog, & Waern, 2013), functional impairment (Brenes et al., 2008; Porensky et al., 2009), greater use of healthcare resources (Porensky et al., 2009; Vasiliadis et al., 2012), and placement in a nursing home (Gibbons et al., 2002).
Not only are both cognitive decline and anxiety symptoms common in later life, they also often co-occur (Beaudreau & O’Hara, 2008). This co-occurrence is important as anxiety may serve as a potentially modifiable risk factor for declining cognitive performance. The cognitive domains most associated with anxiety are processing speed, attention, memory, and executive functioning. Cross-sectional examinations with community dwelling older adults document higher state anxiety symptoms being associated with worse learning and memory (Bierman, Comijs, Jonker, & Beekman, 2005) and executive functioning (Booth, Schinka, Brown, Mortimer, & Borenstein, 2006). Similarly, Beaudreau and O’Hara (2009) found that increased symptoms of anxiety were associated with slower processing speed and worse executive functioning, independent of depressive symptoms. The association between anxiety and worse cognitive performance has also been found in physically frail, homebound older adults (Petkus, Gum, & Wetherell, 2013).
Longitudinal studies provide further evidence that anxiety symptoms and disorders are associated with poorer cognitive performance, as well as greater declines over time. Compared with those not experiencing anxiety symptoms, community dwelling older adults experiencing mild worry symptoms had worse performance on list learning tasks, as well as greater declines in that domain over the following two years (Pietrzak et al., 2012). Anxiety symptoms and disorders also appear to be associated with increased risk of developing neurocognitive disorders, such as mild cognitive impairment or dementia (Burton, Campbell, Jordan, Strauss, & Mallen, 2013; Yaffe et al., 2010; Petkus et al., 2016). Taken together, the evidence from cross-sectional and longitudinal studies documents anxiety symptoms and disorders to be negatively associated with cognitive performance and possible risk factors for future cognitive decline.
Many longitudinal studies make inferences of directionality, though they are not designed to yield these conclusions. Thus, the directionality of the association between anxiety and cognitive performance remains unclear. One hypothesis is that higher anxiety leads to worse cognitive performance with the other hypothesis being that worse cognitive performance leads to higher anxiety. Individuals with anxiety disorders may exhibit a disruption of the hypothalamic-pituitary-adrenal axis (Mantella et al., 2008). HPA axis dysfunction is frequently associated with changes in cortisol levels, which may lead to damage of the hippocampus (Lupien et al., 1998), resulting in deficits in learning and memory. Treatment for late life anxiety may decrease putative HPA-axis overactivation (Lenze et al., 2011), and decreases in cortisol have been found to be correlated with improvements in memory (Lenze et al., 2012). On the other hand, cortisol levels have also been associated with other cognitive abilities such as executive function and processing speed (Franz et al., 2011). Anxiety symptoms have also predicted increased rates of atrophy of the insular cortex, an important brain structure associated with memory, in individuals with amnestic mild cognitive impairment (Mah et al., 2015). Anxiety may moderate the negative effect of Alzheimer’s disease pathology, specifically amyloid-beta; in a longitudinal study, individuals with both mild anxiety symptoms and high amyloid-beta accumulation experienced greater declines in cognitive functioning compared to individuals who had only one of these risk factors (Pietrzak et al., 2015). Taken together, these studies support the hypothesis that anxiety may lead to declines in cognitive performance over time.
Reverse causality is also possible in that worsening cognitive performance may lead to subsequent increases in anxiety. Declining abilities may become a source of worry and anxiety in older adults. Older adults are more likely to worry about developmentally salient factors, such as physical and cognitive health (Gould & Edelstein, 2010; Wetherell, Le Roux, & Gatz, 2003). Additionally, problem solving is an important type of cognitive functioning (Burton, Strauss, Bunce, Hunter, & Hultsch, 2009; Burton, Strauss, Hultsch, & Hunter, 2006) directly related to stress and anxiety. Deficits in problem solving may result in decreased ability to effectively resolve current problems or prevent stressors from arising in the future. This inability to adequately solve problems may in turn result in the development of anxiety. Research supports this hypothesis in that neuroticism, a personality trait associated with anxiety, was increased in individuals subsequent to new-onset mild cognitive impairment (Waggel et al., 2016). It is also possible that a bi-directional association is present in that anxiety leads to subsequent declines in cognitive performance while worse cognitive performance leads to subsequently higher anxiety. Even the putative cortisol-cognition association may be bi-directional. For example, Franz et al. (2011) found that cortisol levels were negatively associated with cognitive performance in midlife, but they also showed that general cognitive ability at age 20 predicted midlife cortisol levels 35 years later.
Anxiety may have a cumulative negative effect over time, thus, making the length of follow-up period important for studies examining anxiety and cognitive performance across later life. The effect of possible mechanisms linking anxiety and cognition explained previously (e.g. HPA dysfunction, inflammation, amyloid beta) are most likely small, but could exert a cumulative effect over time. The negative effect of anxiety on cognitive performance may be too small to detect over a short period; however, the effect may become larger with a greater length of time and extended exposure. Studies examining the association between depressive symptoms and risk of dementia find a stronger association when examined over a longer follow-up period compared to a shorter period (Byers & Yaffe, 2011). A similar relationship may be found with anxiety symptoms. In a previous analysis from our group, we found that anxiety symptoms in healthy, older Swedish twins were associated with increased risk of dementia over a 28-year follow-up period and cognitive decline when examined over a 26-year follow-up period (Petkus et al., 2016). However, in an earlier report examining the same data when only 6-years of follow-up data were available there was no significant longitudinal association (Wetherell, Reynolds, Gatz, & Pedersen, 2002). Statistical explanations are also possible: greater individual variability in cognitive changes over a longer period of time may result in greater likelihood of detecting an association between anxiety and cognitive changes.
In summary, past research, including from our group, has found an association between anxiety symptoms and cognitive performance in later life. This past research has not elucidated the directionality of this association. Past research also has not directly tested the question regarding length of follow-up period. Given these unanswered questions, we examined change in anxiety and cognitive performance over multiple short (3-year) and long (6-year) time periods using a method that is designed specifically to address the temporal order of these changes. The 3-year follow-up period corresponds to change over one wave, which the 6-year follow-up corresponds to change over two waves of assessment. This question has remained unresolved because it can only be answered with a long-term longitudinal study with multiple assessments over time. Here we examine such a study.
Methods
Participants
Participants included 721 twins from the Swedish Adoption/Twin Study of Aging (SATSA; Finkel & Pedersen, 2004). SATSA contains all twins from the Swedish registry who were separated and reared apart before the age of 11, as well as twins reared together who were matched with the twins reared apart on gender, county of birth, and age. In 1986, a subsample of those twins from SATSA age 50 or older completed an in-person assessment of cognitive ability, referred to as IPT1 (first in-person testing) one. Participants completed subsequent IPT follow-up assessments approximately every three years. Participants in SATSA who were younger than 50 years old at the first in-person assessment but turned 50 during the follow-up period were invited to complete an IPT assessment upon their 50th birthday. The assessment schedule was as follows: IPT1:1986–1988, IPT2: 1989–1991, IPT3: 1992–1994, IPT5: 1999–2001, IPT6: 2002–2004, IPT7:2005–2007, IPT8: 2008–2010, and IPT9:2010–2012. Due to gaps in funding, IPT4 was conducted over the phone and therefore no cognitive or anxiety data were collected. Anxiety was not measured at IPT1 so participants may have completed up to seven anxiety assessments and eight cognitive assessments over the span of 26-year study period. Our interest in SATSA was the longitudinal nature of the study, having multiple waves of assessment over a long follow-up period. In the present report, we focus on phenotypic associations between anxiety and cognitive performance, not questions of heritability. SATSA was approved by the Ethics Committee at Karolinska Institutet. All participants provided informed consent for participation.
Measures
Anxiety
Anxiety symptoms were measured using an anxiety scale called the Anxiety Personality Questionnaire (APQ; Petkus, Gatz, Reynolds, Kremen, & Wetherell, 2016). The measure of anxiety available in the IPTs was the neuroticism scale from the Eysenck Personality inventory (EPI; Eysenck & Eysenck, 1968). The APQ was derived via Rausch data harmonization between the EPI and the state anxiety scale from the State Trait Personality Inventory (STPI; Spielberger, 1979) to identify an anxiety scale comprised of items from the EPI best measuring anxiety. The phenotypic mean and genetic and environmental contributions to the APQ were similar to the STPI suggesting that the APQ is a valid measure of anxiety (Petkus et al., 2016). The correlation between the APQ and STPI was 0.57.
Cognitive measures
Tests of processing speed, attention, and working memory were the main cognitive measures of interest. The cognitive battery also consisted of tests of non-verbal memory and visuospatial abilities, which were included in our analyses.
Processing speed
Processing speed was measured with the Symbol Digit Test and the Figure Identification Test. For the Symbol Digit Test participants were presented with symbols and asked to verbally report digits that correspond to the symbols (Pedersen, Plomin, Nesselroade, & McClearn, 1992). The Figure Identification test (Dureman, Kebbon, & Osterberg, 1971) measures processing speed and attention. It consists of a 60-item pattern matching test and participants report which pattern.
Memory
The Thurstone Picture Memory test was used to measure non-verbal episodic memory (Dureman et al., 1971). Participants were presented with 28 drawings of items for five seconds each. They were then asked to recognize which items they had previously seen.
Attention/Working memory
Attention and working memory were assessed using the Digit Span test (Jonsson & Molander, 1964). Participants are asked to repeat strings of 3–9 digits forward and backward. The final score is the sum of the highest number of digits the participant can recall forward and backward. Digit Span Forward is a measure of attention whereas Digit Span Backwards measures both attention and working memory (Ramsay & Reynolds, 1995). In this study we analyzed Digit Span Forward and Digit Span Backward separately.
Visuospatial abilities
The Koh’s Block Design test (Dureman et al., 1971), and the Card Rotations test (Ekstrom, French, & Harman, 1976) were administered to assess visuospatial abilities. The Block Design task is similar to the WAIS Block Design task. Participants use blocks to create seven designs. Each item is scored from 0–6 based on the amount of time taken to complete the design. For Card Rotations, participants are given a target design followed by four items. Participants then determine which of the items was a rotated form of the target. Possible scores range from 0 to 112. The whole Card Rotation test was not administered during IPT9 so data is only available for seven measurement points.
Physical illness
Physical illness was indexed by a count of the number of organ systems affected by medical illness at the first assessment. Scores range from 0–13 and include disease in organ systems including cardiovascular, respiratory, musculoskeletal, and central nervous system. This score has been used in prior SATSA studies (Harris, Pedersen, McClearn, Plomin, & Nesselroade, 1992).
Depressive symptoms
Depressive symptoms were measured using the Center for Epidemiological Studies Depression Scale at the first time this was assessed (CESD; Radloff, 1977).
Statistical analysis
Description of dual change score models
Advances in structural equation modeling allow for the examination of the longitudinal dynamic association between two variables (McArdle & Hamagami, 2003). These models enable quantification of how the level of one variable is associated with changes in another variable. In this study, we fit bivariate dual change score models (DCSMs) to investigate the extent to which level of anxiety was associated with change in cognitive performance over time. The reverse was also examined, i.e., the extent to which level of cognitive performance led to changes in anxiety over time. DCSMs have been used in other longitudinal examinations investigating the association between processing speed and other cognitive domains (Finkel, Reynolds, McArdle, & Pedersen, 2007), openness to experience and cognition (Sharp, Reynolds, Pedersen, & Gatz, 2010), and depression and cognitive performance (Jajodia & Borders, 2011). Because participants were twins, data within each twin pair are correlated and the assumption of independence of data was violated. To account for the twin pair dependency, clustering of participants within twin pairs was accounted for across all models. Clustering of data does not impact parameter estimates but does impact the standard errors of each parameter estimate, typically resulting in underestimation. Hence, we applied robust maximum likelihood estimation (MLR) that produced maximum likelihood parameter estimates with robust standard errors calculated with a sandwich estimator.
Data preparation
To aid in the interpretation of the resulting parameters, the APQ scores and cognitive data were standardized into T-scores. The mean and standard deviation at the first time these variables were measured were used for standardization. Standardizing both the APQ and the cognitive variables on the same scale aids in the interpretation of the dynamic coupling between the two variables and is common practice in DCSM analyses (Finkel, Reynolds, McArdle, Hamagami, & Pedersen, 2009; Finkel et al., 2007; Infurna, Gerstorf, Ryan, & Smith, 2011). All participants with at least one anxiety and cognitive assessment were included in each analysis. In all models, age was modeled as a time variable. Three-year age intervals were used to examine age trajectories. This time period was chosen largely due to the structure of the data collection protocol because participants completed assessments approximately every three years. Additionally, prior SATSA investigations have utilized a 3-year interval to maximize the age range for which trajectories can be examined (Finkel, Reynolds, McArdle, Gatz, & Pedersen, 2003; Finkel et al., 2009; Finkel et al., 2007). Thus, age was modeled as 50–52.99, 53–55.99, 56–58.99, and so on, up to 86–88.99. If participants were assessed twice within one age interval, the first assessment was utilized and the second assessment was dropped. Lastly, possible confounding variables (education, sex, depressive symptoms, and physical health) were added as covariates in the models and regressed upon the latent intercept and slope factors. Models were run with and without these covariates regressed upon the intercept and slope factors to see the impact of the covariates on the parameter estimates from the models. Regressing the intercept and slope on these covariates adjusted the initial level and constant linear change of anxiety and cognitive performance by these variables.
DCSM model parameters
Univariate DCSM models were fit to examine the average trajectory and individual differences around change in anxiety and cognitive performance variable separately over age. Bivariate DCSMs were fit next to examine the dynamic associations between anxiety and cognitive performance. Figure 1 provides a conceptual illustration of the full bivariate DCSM. In the univariate models, for the APQ and each cognitive test, linear and nonlinear proportional change was estimated. In the univariate DCSM models, constant linear change is represented with α, the slope of the latent factor is represented as ys, and β represents the proportional or non-linear change from one time point to the next. The equation representing change in cognitive performance at age A, without taking into account anxiety, can thus be written as follows: Δcog[A] = α*cogs + βcog*(cog[A−1]). The equation representing change in anxiety at age A, without taking into cognitive performance into consideration, is written as: Δanx[A] = α*anxs + βanx*(anx[A−1]). In these models, α is set to a value of 1 while parameter β is estimated. DCSM investigations assume that α and β parameters are consistent across time (Finkel et al., 2009; Finkel et al., 2007). The univariate DCSMs also estimate the intercepts (anx0 or cog0), slopes (anxs or cogs), variance of the intercepts (anxб0 or cogб0) and slopes (anxбs or cogбs), error deviation (anxб or cogб), as well as correlation between the latent intercept and slope factors. Sex, education, depression, and physical illness were covariates of interest and regressed on the intercept and slope factors.
Figure 1.
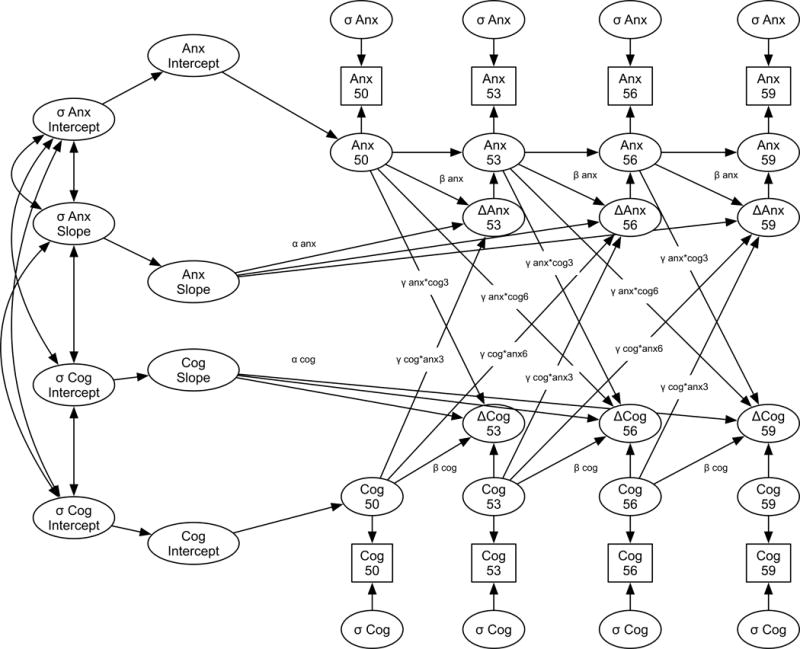
Diagram of the full Bivariate Dual Change Score model examining changes in anxiety and changes in cognitive performance. Note that this model only depicts predicted level and change of anxiety and each cognitive score from ages 50 through 59. The full model estimated continues through age 86–89 years old.
The bivariate DCSM models contain the same parameter estimates as described in the univariate models. The bivariate DCSM models also estimate four additional coupling parameters, designated by γ, which link the APQ and cognitive performance variables. In bivariate models the coupling parameters are estimates of how level of one variable is associated with subsequent change in the other variable. The first coupling parameter estimated how anxiety symptoms were associated with subsequent change in cognitive performance over the following three years (γanx*cog3). We then added a second coupling parameter, which is an extension of the original DCSM model (McArdle & Hamagami, 2003). The second coupling parameter was added to test the length of follow-up period hypothesis. The second coupling parameter estimated how anxiety was associated with changes in cognitive performance over the six years subsequent to the cognitive assessment (γanx*cog6). The third coupling parameter estimates the reverse association: the association between cognitive performance and change in anxiety over the subsequent three years (γcog*anx3). The last coupling parameter estimated the association between cognitive performance and change in anxiety over the six years subsequent to the anxiety assessment (γcog*anx6). The equation representing the modeling of change in cognitive performance in relation to prior levels of anxiety can be written as: Δcog[A] = α*cogs + βcog*cog[A−1]+γanx*cog3*anx[A−1]+ γanx*cog6*anx[A−2]. The equation representing the modeling of the change in anxiety in relation to prior levels of cognitive performance can be written as: Δanx[A]=α*anxs+ βanx*anx[A−1]+γcog*anx3*cog[A−1]+ γcog*anx6*cog[A−2]. Similar to the α and β parameters, the γ parameters are assumed to be constant across all ages. The bivariate DCSMs also estimate the intercepts (anx0 & cog0), slopes (anxs & cogs), variance of the intercepts (anxб0 & cogб0) and slopes (anxбs & cogбs), error deviations of each variable (anxб and cogб), as well as the correlation between all latent growth factors. Covariates of interest, sex, education, depression, and physical illness were regressed on the intercept and slope factors.
DCSM model comparisons
To examine the significance of parameters, both the probability of parameter estimates and model testing were conducted. A total of seven nested models were compared in a stepwise manner for each anxiety and cognitive domain combination. First, we examined a full model estimating all four coupling parameters γanx*cog6, γcog*anx6, γanx*cog3, γcog*anx3. Second, we ran an omnibus test of the coupling parameters by removing all coupling parameters and comparing the nested model fit to the full model. The omnibus test of coupling tests if there is any significant association between anxiety and cognitive performance. The third model examined change in anxiety in association with level of cognitive performance three years prior by constraining the γcog*anx3 to zero, while the fourth model examined change in cognition in association with level of anxiety three years prior γanx*cog3. Model five examined change in cognitive performance as a function of level of anxiety six years prior by removing the coupling parameter γanx*cog6. Model six examined change in anxiety as a function of level of cognitive performance six years prior γcog*anx6. Model comparisons were conducted using the log likelihood difference test with the MLR correction for scaling factors (Satorra & Bentler, 2001). The MLR correction for scaling factors has to be used because the MLR log likelihood is not distributed as a chi-square. All DCMS models were conducted using the structural equation modeling program MPLUS (Muthen & Muthen, 2010).
Results
Just over half (58.7%; N = 423) of the participants were women. Table 1 presents the number of participants who contributed data at each age interval. Participants completed an average of 3.5 (SD = 1.9) assessments of anxiety. At initial assessment the average CESD score was 10.3 (SD = 7.8) and participants had an average of 2.17 (SD = 1.6) medical conditions.
Table 1.
Number of participants who provided anxiety and cognitive data at each age group (N= 721).
| Age (Years) | APQ (N) | Symbol Digit (N) | Figure Id (N) | Thurstone (N) | Digit Span (N) | Blocks (N) | Rotations (N) |
|---|---|---|---|---|---|---|---|
| 50.00–52.99 | 76 | 87 | 87 | 88 | 88 | 88 | 88 |
| 53.00–55.99 | 108 | 155 | 156 | 156 | 158 | 156 | 157 |
| 56.00–58.99 | 137 | 188 | 190 | 191 | 193 | 190 | 187 |
| 59.00–61.99 | 163 | 233 | 235 | 233 | 238 | 236 | 229 |
| 62.00–64.99 | 236 | 309 | 299 | 311 | 317 | 314 | 287 |
| 65.00–67.99 | 304 | 381 | 366 | 383 | 385 | 382 | 350 |
| 68.00–70.99 | 301 | 365 | 355 | 364 | 378 | 371 | 333 |
| 71.00–73.99 | 289 | 362 | 340 | 361 | 376 | 370 | 315 |
| 74.00–76.99 | 296 | 318 | 293 | 317 | 330 | 321 | 264 |
| 77.00–79.99 | 235 | 239 | 222 | 236 | 255 | 248 | 185 |
| 80.00–82.99 | 181 | 189 | 184 | 184 | 203 | 200 | 144 |
| 83.00–85.99 | 136 | 130 | 110 | 121 | 150 | 136 | 70 |
| 86.00–88.99 | 93 | 89 | 71 | 74 | 107 | 100 | 38 |
| One | 16.4 (118) | 6.4 (44) | 5.8 (42) | 6.0 (43) | 3.2 (23) | 4.1 (29) | 7.5 (54) |
| Two | 19.1 (138) | 13.0 (94) | 11.5 (83) | 11.8 (85) | 10.5 (76) | 11.6 (83) | 16.8 (121) |
| Three | 15.8 (114) | 20.9 (151) | 26.9 (194) | 23.6 (170) | 23.6 (170) | 22.6 (161) | 25.5 (184) |
| Four | 15.8 (114) | 18.0 (130) | 18.2 (131) | 18.3 (132) | 18.9 (136) | 18.2 (130) | 18.0 (130) |
| Five | 14.0 (101) | 16.0 (115) | 14.0 (101) | 15.7 (113) | 16.6 (120) | 15.7 (112) | 15.0 (108) |
| Six | 12.2 (88) | 9.0 (65) | 13.7 (99) | 9.9 (71) | 11.0 (79) | 10.2 (73) | 11.1 (80) |
| Seven | 6.7 (48) | 11.5 (83) | 9.9 (71) | 10.4 (75) | 10.8 (78) | 11.8 (84) | 5.1 (37) |
| Eight | 0.0 (0) | 5.1 (37) | 0.0 (0) | 4.4 (32) | 5.4 (39) | 5.8 (41) | 0.0 (0) |
Notes: Individual participants contribute data to more than one age interval
Univariate DCSM
The parameter estimates and overall model fit from the univariate DCSM models are presented in table 2. Constant linear change in anxiety symptoms was not statistically significant, although individual differences around this change was present. Proportional and constant linear change was significant for all cognitive variables, except for digit span forwards and backwards. As a whole, compared to anxiety symptoms, cognitive performance variables exhibited greater mean change and larger individual differences around that change.
Table 2.
Parameter estimates and goodness-of-fit statistics from the full univariate dual change score models.
| Parameters & Fit | Anxiety | Symbol Digit | Figure Identification | Thurstone |
|---|---|---|---|---|
| Constant change | =1 | =1 | =1 | =1 |
| Proportional change | .05 (.20) | 0.11 (0.02)** | .16 (.04)** | .41 (.07)** |
| Mean Intercept | 48.95 (.79)** | 58.72 (.61)** | 58.63 (.86)** | 55.45(.43)** |
| Mean Slope | −2.19 (9.87) | −7.07 (.97)** | −9.80 (2.21)** | −22.98 (3.65)** |
| Intercept deviation | 36.49 (10.66)** | 47.09 (4.42)** | 58.76 (5.25)** | 35.15 (3.03)** |
| Slope deviation | .67 (.27)* | .85 (.19)** | 1.89 (.63)** | 6.07 (2.03)** |
| Error deviation | 43.80 (3.52)** | 18.12 (1.03)** | 28.97 (1.42)** | 20.78 (1.05)** |
| Misfit index: -2LL/parameters | −9033/15 | −9693/15 | −9879/15 | −9690/15 |
| Parameters & Fit | Digits Forward | Digits Backward | Blocks | Rotations |
| Constant change | =1 | =1 | =1 | =1 |
| Proportional change | .13 (.08) | .17 (.09) | .24 (.03)** | .16 (.04)** |
| Mean Intercept | 53.28 (.76)** | 52.91 (.69)** | 57.59 (.53)** | 52.59 (.66)** |
| Mean Slope | −7.27 (4.04) | −9.36 (4.74)* | −14.20 (1.44)** | −8.82 (2.06)** |
| Intercept deviation | 58.97 (6.07)** | 40.99 (5.35)** | 50.95 (3.49)** | 59.98 (4.85)** |
| Slope deviation | 1.22 (1.14) | 1.36 (.27) | 3.17 (.70)** | 1.81 (.84)* |
| Error deviation | 47.59 (1.74)** | 52.93 (2.52)** | 14.82 (.79)** | 26.74 (1.17)** |
| Misfit index: -2LL/parameters | −11254/15 | −11288/15 | −9632/15 | −8861/15 |
denotes p <0.01
denotes p < 0.05
Omnibus test of coupling parameters
Across all cognitive measures, the fully adjusted models were not substantially different than the zero order models without covariates. Therefore, we only present the results from the models adjusting for covariates (see tables 3–9). Fit indices from the full models estimating all four coupling parameters are also presented in these tables. We first conducted an omnibus test of coupling by removing all four coupling parameters for each dynamic model. Across all models removing these coupling parameters resulted in significantly worse fit compared to the full model. This degradation in fit suggests that a significant dynamic association existed between anxiety and cognitive performance.
Table 3.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Symbol Digit score.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/ΔParameter |
|---|---|---|---|---|
| 1. Full model | −17154/38 | 1.72 | – | – |
| CFI = 0.93 | ||||
| RMSEA = 0.03 | ||||
| 2. No coupling | −17165/34 | 1.75 | 1 | 14.00/4** |
| Test 3-year coupling | ||||
| 3. Set Symd➔Anx=0 | −17161/37 | 1.79 | 1 | 11.73/1** |
| 4. Set Anx ➔Symd=0 | −17155/37 | 1.66 | 1 | .43/1 |
| Test 6-year coupling | ||||
| 5.Set Symd➔Anx=0 | −17156/37 | 1.66 | 1 | .43/1 |
| 6. Set Anx ➔Symd=0 | −17158/37 | 1.75 | 1 | 14.08/1** |
|
| ||||
| Anxiety | Symbol Digit | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.05 | .17 | .10 | .02 |
| 3-year coupling | ||||
| Coupling Anx➔Sym | – | – | .08 | .12 |
| Coupling Sym➔Anx | −.06 | .02** | – | – |
| 6-year coupling | ||||
| Coupling Anx➔Sym | – | – | −.03 | .01** |
| Coupling Sym➔Anx | −.01 | .01 | – | – |
| Mean Intercept | 50.15 | .66** | 60.45 | .64** |
| Mean Slope | 6.03 | 8.80 | −9.37 | 6.80 |
| Intercept deviation | 14.45 | 4.58** | 39.27 | 3.74** |
| Slope deviation | .55 | .52 | .70 | .30* |
| Error deviation | 16.65 | 1.32** | 14.56 | .83** |
Notes: Coupling Anx ➔ Sym = coupling parameter γANX*SYMBOL DIGIT; Coupling SD ➔ Anx = coupling parameter γSYMBOL DIGIT*ANX. The three-year coupling represents change over a three year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Table 9.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Rotations performance.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/Parameter |
|---|---|---|---|---|
| 1. Full model | −16653/38 | 1.59 | – | – |
| CFI = 0.89 | ||||
| RMSEA = 0.04 | ||||
| 2. No coupling | −16662/34 | 1.66 | 1 | 17.09/4** |
| Test 3 Year coupling | ||||
| 2. Set Rota➔Anx =0 | −16662/37 | 1.66 | 1 | 14.87/1** |
| 3. Set Anx ➔Rota=0 | −16653/37 | 1.57 | 1 | .04/1 |
| Test 6 Year coupling | ||||
| 4. Set Rota➔Anx =0 | −16653/37 | 1.61 | 1 | .02/1 |
| 5.Set Anx ➔Rota =0 | −16653/37 | 1.60 | 1 | .06/1 |
|
| ||||
| Anxiety | Rotations | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.09 | .16 | .16 | .05** |
| Three-year coupling | ||||
| Coupling Anx➔Rota | – | – | .02 | .13 |
| Coupling Rota➔Anx | −.10 | .03** | – | – |
| Six-year coupling | ||||
| Coupling Anx➔Rota | – | – | −.01 | .02 |
| Coupling Rota➔Anx | −.01 | .01 | – | – |
| Mean Intercept | 50.11 | .66** | 52.46 | .86** |
| Mean Slope | 9.95 | 8.84 | −7.18 | 7.63 |
| Intercept deviation | 14.08 | 4.99** | 59.39 | 4.99** |
| Slope deviation | 1.13 | .82 | 1.71 | .85* |
| Error deviation | 16.68 | 1.34** | 26.80 | 1.16** |
Notes: Coupling Anx ➔ Rota = coupling parameter γANX*ROTATIONS; Coupling Rota ➔ Anx = coupling parameter γROTATIONS*ANX. The three-year coupling represents change over a three-year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Anxiety and subsequent changes in cognitive performance
Higher anxiety was associated with greater declines in processing speed during six years after anxiety was measured. This was true for both Symbol Digit (γanx*sym dig6 = −0.03, Δfit = 14.08, Δdf = 1, p < 0.01) and Figure Identification (γanx*fig id6 = −0.05. Δfit = 4.88, Δdf = 1, p < 0.05) performance. Higher anxiety was also associated with greater declines in attention over the subsequent three years but not six years as measured by Digit Span Forward performance (γanx*digits forward3 = −0.12, Δfit = 12.43, Δdf = 1, p < 0.01). Setting all anxiety on cognitive performance coupling parameters to zero did not significantly reduce model fit for all the other cognitive measures. Anxiety was not significantly associated with subsequent changes in picture memory, block design, or card rotation (visuospatial) performances.
Cognitive performance and subsequent changes in anxiety
We next examined the coupling parameters testing the hypothesis that cognitive performance was associated with subsequent changes in anxiety. With the exception of Card Rotations (visuospatial), worse cognitive performance was associated with increases in anxiety across the next three years. The largest effects between worse performance and higher anxiety were seen on measures of attention (γdigits forward*anx3 = −0.29, Δfit = 33.27, Δdf = 1, p < 0.01), nonverbal memory (γthurstone*anx3 = −0.18, Δfit = 9.49, Δdf = 1, p < 0.01), and working memory (γdigits backward*anx3 = −0.28, Δfit = 33.50, Δdf = 1, p < 0.01). Across all cognitive tests the six year coupling between cognitive performance and changes in cognition over the subsequent six years was not significant (γanx*cognition6, p > 0.05).
To visualize the associations between anxiety and each measure of processing speed, we graphed the estimated means of each variable at each age under different scenarios. We first graphed the estimated trajectory of anxiety from age 50 to 86 for individuals at the average, low (−1.5 SD), and high (+1.5 SD) symbol digit performance. We also estimated the trajectory of performance on each respective cognitive measure for individuals at different levels of initial anxiety (average anxiety, no anxiety, and high anxiety (+1.5 SD). Figure 2 presents the estimated reported anxiety and each cognitive variable by age.
Figure 2.
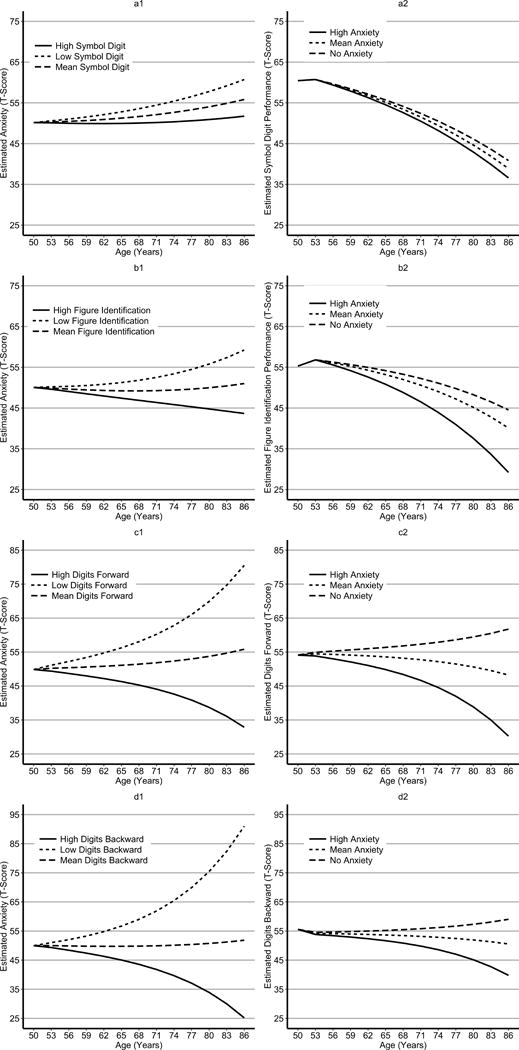
Graphs of the estimated APQ and cognitive scores from each of the most parsimonious dual change score model. Panel A displays the estimated trajectories for anxiety (a1) and symbol digit performance (a2), panel B displays the estimated trajectories for anxiety (b2) and figure identification (b2) performance, panel C displays the estimated trajectories for anxiety (c1) and digit span forward performance (c2), panel D displays the estimated trajectories for anxiety (d1) and digit span backwards performance (d2). Each graph presents estimated trajectories for individuals who scored low (minus one SD), at the mean, or high (plus one SD) on either anxiety or respective cognitive measure.
Discussion
In order to elucidate the temporal dynamics of the association between cognitive performance and anxiety symptoms, bivariate dual change score models were fit to longitudinal data to examine the dynamic relationship between these two variables across older adulthood. Bidirectional associations were present between anxiety symptoms and the cognitive domains of processing speed and attention. Slower processing speed was associated with subsequently larger increases in anxiety three years later, whereas higher anxiety was associated with declines in processing speed between the years three and six after the assessment of anxiety. Similarly, for measures of attention, we found that anxiety was a leading indicator of change in attention with higher anxiety being associated with worsening attention over the following three years. We also found that worse performance on measures of processing speed, attention, working memory, nonverbal memory, and visuospatial ability were associated with increasing anxiety three years later, with largest effects for nonverbal and working memory. These findings were independent of sex, baseline depressive symptoms, and physical health. The hypothesis of a bidirectional association between cognitive ability and anxiety symptoms was generally supported, with the duration of time being important. Differential associations were present based on length of follow-up period, with poorer cognitive performance being associated with higher anxiety over a shorter follow-up period while anxiety symptoms were associated with worse cognitive performance over a longer period.
The results from the univariate models were consistent with other research suggesting that anxiety symptoms exhibited little mean change with age (Wetherell et al., 2001); although, significant individual differences in extent of change were present. The univariate models also demonstrated a larger mean change with greater variability for most cognitive performance variables over age compared to anxiety symptoms. The absence of systematic change in anxiety over age makes it more likely that short-term associations would be detected between cognitive performance and very individual changes in anxiety symptoms. The absence of change in anxiety symptoms also made it less likely to detect long-term associations between cognitive performance and changes in anxiety. The presence of systematic change in cognitive performance with significant individual differences around this change over age increased the likelihood that levels of anxiety would be associated with changes in cognitive performance. If differences in the amount of change were solely driving these associations, the levels of anxiety would predict change in cognitive performance over a three-year interval, given that there was more change in cognitive performance to be explained. Differences between the variables in change over age are a plausible explanation for the different associations between anxiety symptoms and cognitive performance over age; however, this explanation is unlikely.
With respect to cognitive domains, we found that anxiety was associated with greater declines in processing speed and attentional abilities, consistent with other research (Beaudreau & O’Hara, 2009). The finding that anxiety is associated with worse processing speed when examined over a longer follow-up period is consistent with past research finding that the effect of emotional distress and cognitive performance is more robust when examined over a longer period of time (Beaudreau & O’Hara, 2008; Byers, Covinsky, Barnes, & Yaffe, 2012). This association suggests that the negative physiological effect of anxiety on brain health may have a modest effect over a short period of time but may accumulate over longer time periods.
Psychological processes may explain the association between worse cognitive performance and future increases in anxiety symptoms. Noticeable declines in cognitive ability may become a source of worry and distress leading to increased anxiety three years later. Declines in cognitive performance may affect social functioning, as these declines may contribute to feeling anxious about performance in social settings. This anxiety may result in avoidance of these situations, thereby reinforcing concerns.
Biological processes may also explain the association between cognition and subsequent increases in anxiety. Possible processes include neurodegenerative processes, cardiovascular disease, or proinflammatory processes that affect cognitive functioning and are followed by anxiety later in the disease process. Recent research has shown that individuals with first onset of anxiety disorders after the age of 50 have elevated proinflammatory processes compared to individuals without anxiety disorders (Vogelzangs, Beekman, de Jonge, & Penninx, 2013). Multi-morbidity, especially disorders characterized by vascular dysfunction and increased inflammation, has also been shown to be associated with increased risk for anxiety in older adults (Gould, O’Hara, Goldstein, & Beaudreau, 2016). Genetic factors may also play a role. Prior research has found that genes common to risk of dementia and anxiety were partially mediating the association between higher anxiety and increased risk of dementia (Petkus et al., 2016). Other recent reviews discuss the many genes in common to both cognitive performance and emotional distress such as anxiety and depression (Rodrigues, Petersen, & Perry, 2014). Future research needs to identify what these specific genetic factors are, and the role that they play in explaining this association.
The findings from this study have important implications for cognitive aging research. They highlight the importance of assessing anxiety when conducting cross-sectional and longitudinal studies of cognitive aging. Most studies screen and assess depressive symptoms and dementia; however, few studies adequately assess anxiety symptoms. In a review of 51 longitudinal studies funded by the National Institute on Aging (NIA), anxiety was not identified as a frequently measured component of health and functioning (Stanziano et al., 2010). Similarly, when searching “anxiety” on the NIA’s database of longitudinal studies, only three studies were identified as having data on anxiety (compared to eight with depression, nine with personality, and 47 with dementia). Our findings highlight the importance of measuring anxiety in future research on cognitive aging.
Our findings also have important clinical implications such as the importance of psychoeducation of cognitive aging and providing interventions to decrease anxiety in older age. If older adults are educated about normal cognitive aging they may be less likely to worry about perceived declines in their own current level of functioning. Research suggests that older adults are satisfied with and can improve knowledge through programs designed to increase knowledge about cognitive aging (Norrie et al., 2011). Another clinical implication includes the potential importance of identifying, preventing, and reducing anxiety and how this may potentially slow the decrease that was seen in processing speed and attention over age. Although it is unclear what physiological mechanisms are driving this association successful treatment may influence possible physiological mechanisms such as HPA hyperactivation and cardiovascular dysfunction. Successfully treating anxiety may lower cortisol levels (Lenze et al., 2011) and these changes in cortisol may be beneficial for cognitive performance (Lenze et al., 2012). Treatment aimed at reducing anxiety might also have beneficial effects on cardiovascular function (e.g., helping to reduce blood pressure), and it is well known that hypertension and other cardiovascular conditions can have negative impacts on brain and cognition (Fennema-Notestine et al., 2016). Important future research includes examining the long-term impact of treating anxiety on cognitive performance over time and the mechanisms underlying the association. Although it will be important to examine the association between anxiety and cognitive performance in clinically anxious populations, our results indicate that even relatively low levels of anxiety in community-based samples can have deleterious effects on cognitive aging.
Despite the strengths of this study such as a large sample size, number of assessment points, and population-based sample, some limitations should be noted. First, issues exist with the assessment of both anxiety and cognitive performance. The APQ was created via a Rasch harmonization analysis with a more established measure of anxiety (the State Trait Personality Inventory). A more established state-like measurement of anxiety symptoms, such as the anxiety subscale of the Brief Symptom Inventory, may have been more sensitive in capturing the dynamic association over time. Alternatively, it is also possible however that the negative impact of anxiety on cognitive performance may not have been present with a state-like measure of anxiety given that this association was only detected when examined over a longer period of time of six years. Although the cognitive battery is both reliable and valid, it did not contain measures of verbal memory, problem solving, or executive functions. These domains are domains have been associated with anxiety and the lack of these in the cognitive battery is a limitation. Lastly, past research has found interactions between anxiety and depressive symptoms on cognitive performance (Beaudreau & O’Hara, 2009), but modeling the interaction between anxiety and depressive symptoms with cognitive performance proved impossible.
In sum, we examined the temporal dynamics between anxiety and cognitive performance in later life. We found evidence of a bidirectional association between anxiety and cognitive performance. For processing speed, anxiety was not associated with declines in cognitive performance three years after the assessment but was associated with more delayed changes seen six years later. These findings have important clinical implications and highlight the possible usefulness of psychoeducation efforts regarding anxiety in older adults experiencing objective cognitive decline. These findings also highlight the potential importance of treating anxiety as a way to minimize future cognitive performance declines.
Table 4.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Figure Identification score.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/ΔParameter |
|---|---|---|---|---|
| 1. Full model | −17395/38 | 2.01 | – | – |
| CFI = 0.87 | ||||
| RMSEA = 0.04 | ||||
| 2. No coupling | −17406/34 | 2.00 | 1 | 10.96/4* |
| Test 3-year coupling | ||||
| 3. Set Fig id➔Anx =0 | −17400/37 | 2.04 | 1 | 12.2/1** |
| 4. Set Anx ➔Fig id =0 | −17395/37 | 2.01 | 1 | .04/1 |
| Test 6-year coupling | ||||
| 5.Set Fig id➔Anx =0 | −17395/37 | 2.03 | 1 | 1.21/1 |
| 6. Set Anx ➔Fig id =0 | −17400/37 | 2.12 | 1 | 4.88/1* |
|
| ||||
| Anxiety | Figure Identification | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.05 | .23 | .15 | .06* |
| 3-year coupling | ||||
| Coupling Anx➔Fig id | – | – | .07 | .36 |
| Coupling Fig id➔Anx | −.06 | .02** | – | – |
| 6-year coupling | ||||
| Coupling Anx➔Fig id | – | – | −.05 | .02* |
| Coupling Fig id➔Anx | .01 | .01 | – | – |
| Mean Intercept | 50.06 | .73 | 55.26 | .95 |
| Mean Slope | 5.70 | 12.47 | −10.18 | 21.22 |
| Intercept deviation | 14.55 | 4.98 | 50.14 | 4.46 |
| Slope deviation | .56 | .71 | 1.50 | 1.27 |
| Error deviation | 16.72 | 1.33 | 23.90 | 1.14 |
Notes: Coupling Anx ➔ Fig id = coupling parameter γANX*FIGURE ID; Coupling Fig id ➔ Anx = coupling parameter γFIGURE ID*ANX. The three-year coupling represents change over a three-year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Table 5.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Thurstone score.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/ΔParameter |
|---|---|---|---|---|
| 1. Full model | −17485/38 | 1.61 | – | – |
| CFI = 0.88 | ||||
| RMSEA= 0.03 | ||||
| 2. No coupling | −17492/34 | 1.62 | 1 | 9.65/4* |
| Test 3 Year coupling | ||||
| 3. Set Thur➔Anx =0 | −17491/37 | 1.62 | 1 | 9.49/1** |
| 4. Set Anx ➔Thur =0 | −17485/37 | 1.59 | 1 | .43/1 |
| Test 6 Year coupling | ||||
| 5.Set Thur➔Anx =0 | −17486/37 | 1.62 | 1 | .95/1 |
| 6. Set Anx ➔Thur =0 | −17485/37 | 1.64 | 1 | 2.13/1 |
|
| ||||
| Anxiety | Thurstone | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.06 | .20 | .41 | .07** |
| Three-year coupling | ||||
| Coupling Anx➔Thur | – | – | .04 | .15 |
| Coupling Thur➔Anx | −.18 | .09** | – | – |
| Six-year coupling | ||||
| Coupling Anx➔Thur | – | – | −.01 | .01 |
| Coupling Thur➔Anx | .01 | .01 | – | – |
| Mean Intercept | 50.13 | .65 | 55.07 | .58 |
| Mean Slope | 12.29 | 13.18 | −23.93 | 9.28 |
| Intercept deviation | 14.59 | 4.70 | 35.56 | 2.99 |
| Slope deviation | 1.31 | 1.26 | 5.88 | 1.96 |
| Error deviation | 16.60 | 1.34 | 20.82 | 1.06 |
Notes: Coupling Anx ➔ Thur = coupling parameter γANX*THURSTONE; Coupling Thurstone ➔ Anx = coupling parameter γTHURSTONE*ANX. The three-year coupling represents change over a three-year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Table 6.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Digits Forward score.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/ΔParameter |
|---|---|---|---|---|
| 1. Full model | −18673/38 | 1.51 | – | – |
| CFI = 0.91 | ||||
| RMSEA= 0.03 | ||||
| 2. No coupling | −18682/34 | 1.60 | 1 | 22.09/4** |
| Test 3 Year coupling | ||||
| 3. Set Dig F➔Anx =0 | −18681/37 | 1.54 | 1 | 33.27/1** |
| 4. Set Anx ➔Dig F=0 | −18674/37 | 1.55 | 1 | 12.43/1** |
| Test 6 Year coupling | ||||
| 5.Set Dig F➔Anx =0 | −18673/37 | 1.53 | 1 | .02/1 |
| 6. Set Anx ➔Dig F =0 | −18673/37 | 1.53 | 1 | .21/1 |
|
| ||||
| Anxiety | Digits Forward | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.10 | .15 | .12 | .08 |
| Three-year coupling | ||||
| Coupling Anx➔Dig F | – | – | −.12 | .10 |
| Coupling Dig F➔Anx | −.29 | .12* | – | – |
| Six-year coupling | ||||
| Coupling Anx➔Dig F | – | – | −.01 | .02 |
| Coupling Dig F➔Anx | −.01 | .01 | – | – |
| Mean Intercept | 49.86 | .62** | 54.11 | .90** |
| Mean Slope | 21.04 | 10.52* | −.16 | 7.5 |
| Intercept deviation | 14.13 | 4.70** | 46.96 | 4.70** |
| Slope deviation | 3.93 | 2.98 | .97 | .82 |
| Error deviation | 16.56 | 1.34** | 37.78 | 1.34** |
Notes: Coupling Anx ➔ Dig F = coupling parameter γANX*DIGITS FORWARD; Coupling Dig F ➔ Anx = coupling parameter γDIGITS FORWARD*ANX. The three-year coupling represents change over a three-year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Table 7.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Digits Backward score.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/Δrameter |
|---|---|---|---|---|
| 1. Full model | −18642/38 | 1.59 | – | – |
| CFI = 0.85 | ||||
| RMSEA = 0.03 | ||||
| 2. No coupling | −18654/34 | 1.71 | 1 | 41.58/1** |
| Test 3 Year coupling | ||||
| 3. Set Dig B➔Anx =0 | −18652/37 | 1.65 | 1 | 33.50/1** |
| 4. Set Anx ➔Dig B=0 | −18643/37 | 1.61 | 1 | 1.11/1 |
| Test 6 Year coupling | ||||
| 5.Set Dig B➔Anx =0 | −18642/37 | 1.61 | 1 | .29/1 |
| 6. Set Anx ➔Dig B =0 | −18642/37 | 1.61 | 1 | .63/1 |
|
| ||||
| Anxiety | Digits Backwards | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.08 | .14 | .20 | .10* |
| Three-year coupling | ||||
| Coupling Anx➔Dig B | – | – | −.08 | .10 |
| Coupling Dig B➔Anx | −.28 | .11** | – | – |
| Six-year coupling | ||||
| Coupling Anx➔Dig B | – | – | .03 | .01 |
| Coupling Dig B➔Anx | −.01 | .01 | – | – |
| Mean Intercept | 49.97 | .63** | 55.57 | .65** |
| Mean Slope | 19.60 | 10.21 | −8.46 | 7.20 |
| Intercept deviation | 13.29 | 4.52** | 30.38 | 3.74** |
| Slope deviation | 2.59 | 1.94 | 1.38 | 1.16 |
| Error deviation | 16.35 | 1.33** | 40.55 | 1.92** |
Notes: Coupling Anx ➔ Dig B = coupling parameter γANX* DIGITS BACKWARD; Coupling Dig B ➔ Anx = coupling parameter γDIGITS BACKWARD*ANX. The three-year coupling represents change over a three-year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Table 8.
Parameter estimates and goodness-of-fit statistics from the full bivariate dual change score model of Anxiety Personality Questionnaire and Block Design performance.
| Model | Misfit/Parameters | Scaling Factor | Compare | Δfit/Parameter |
|---|---|---|---|---|
| 1. Full model | −17414/38 | 1.61 | – | – |
| CFI = 0.94 | ||||
| RMSEA = 0.03 | ||||
| 2. No couplings | −17421/34 | 1.66 | 1 | 12.79/4* |
| Test 3 Year coupling | ||||
| 3. Set Block➔Anx =0 | −17419/37 | 1.64 | 1 | 27.15/1** |
| 4. Set Anx ➔Block=0 | −17416/37 | 1.60 | 1 | 2.06/1 |
| Test 6 Year coupling | ||||
| 5.Set Block➔Anx =0 | −17414/37 | 1.63 | 1 | .04/1 |
| 6. Set Anx ➔Block =0 | −17414/37 | 1.63 | 1 | .68/1 |
|
| ||||
| Anxiety | Blocks | |||
| Parameter | Est | SE | Est | SE |
|
| ||||
| Constant Change | =1 | – | =1 | – |
| Proportional Change | −.05 | .17 | .25 | .03** |
| Three-year coupling | ||||
| Coupling Anx➔Block | – | – | .11 | .07 |
| Coupling Block➔Anx | −.09 | .03** | – | – |
| Six-year coupling | ||||
| Coupling Anx➔Block | – | – | −.01 | .01 |
| Coupling Block➔Anx | .01 | .01 | - | – |
| Mean Intercept | 50.10 | .62** | 57.26 | .68** |
| Mean Slope | 7.65 | 9.80 | −19.67 | 4.28** |
| Intercept deviation | 15.04 | 4.70** | 51.03 | 3.57** |
| Slope deviation | .74 | .64 | 3.48 | .82 |
| Error deviation | 16.67 | 1.32** | 14.77 | .79** |
Notes: Coupling Anx ➔ Block = coupling parameter γANX*BLOCKS; Coup Block ➔ Anx = coupling parameter γBLOCKS*ANX. The three-year coupling represents change over a three-year period and six-year coupling represents change over a six-year period. Est represents parameter estimate.
denotes p <0.01
denotes p < 0.05
Acknowledgments
SATSA was supported by grants R01 AG04563, R01 AG10175, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council For Working Life and Social Research (FAS) (97:0147:1B, 2009-0795) and Swedish Research Council (825-2007-7460, 825-2009-6141).
This work was also partially supported by NIA grants R01AG018396, RO1AG005095, RO1AG022381, and R01AG037985
Dr. Petkus was partially supported by a Ruth L. Kirschstein National Research Service Award (NRSA) fellowship awarded by the National Institute on Aging (1F31AG042218-01).
References
- Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. American Journal of Geriatric Psychiatry. 2008;16:790–803. doi: 10.1097/JGP.0b013e31817945c3. [DOI] [PubMed] [Google Scholar]
- Beaudreau SA, O’Hara R. The association of anxiety and depressive symptoms with cognitive performance in community-dwelling older adults. Psychology and Aging. 2009;24:507–512. doi: 10.1037/a0016035. doi: 2009-08094-022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman EJ, Comijs HC, Jonker C, Beekman AT. Effects of anxiety versus depression on cognition in later life. American Journal of Geriatric Psychiatry. 2005;13:686–693. doi: 10.1176/appi.ajgp.13.8.686. [DOI] [PubMed] [Google Scholar]
- Booth JE, Schinka JA, Brown LM, Mortimer JA, Borenstein AR. Five-factor personality dimensions, mood states, and cognitive performance in older adults. Journal of Clinical and Experimental Neuropsychology. 2006;28:676–683. doi: 10.1080/13803390590954209. [DOI] [PubMed] [Google Scholar]
- Braam AW, Copeland JR, Delespaul PA, Beekman AT, Como A, Dewey M, Skoog I. Depression, subthreshold depression and comorbid anxiety symptoms in older Europeans: Results from the EURODEP concerted action. Journal of Affective Disorders. 2014;155:266–272. doi: 10.1016/j.jad.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Brenes GA, Penninx BW, Judd PH, Rockwell E, Sewell DD, Wetherell JL. Anxiety, depression and disability across the lifespan. Aging & Mental Health. 2008;12:158–163. doi: 10.1080/13607860601124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton C, Campbell P, Jordan K, Strauss V, Mallen C. The association of anxiety and depression with future dementia diagnosis: a case-control study in primary care. Family Practice. 2013;30:25–30. doi: 10.1093/fampra/cms044cms044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Bunce D, Hunter MA, Hultsch DF. Functional abilities in older adults with mild cognitive impairment. Gerontology. 2009;55:570–581. doi: 10.1159/000228918. [DOI] [PubMed] [Google Scholar]
- Burton CL, Strauss E, Hultsch DF, Hunter MA. Cognitive functioning and everyday problem solving in older adults. Clinical Neuropsychologist. 2006;20:432–452. doi: 10.1080/13854040590967063. [DOI] [PubMed] [Google Scholar]
- Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. American Journal of Geriatric Psychiatry. 2012;20:664–672. doi: 10.1097/JGP.0b013e31822001c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dureman I, Kebbon L, Osterberg E. Manual of the DS-Battery. Stokholm, Sweden: Psykologi Forlaget; 1971. [Google Scholar]
- Ekstrom RB, French J, Harman H. Manual for Kit of Factor-Referenced Cognitive Tests. Princeton, NJ: Educational Testing Service; 1976. [Google Scholar]
- Eysenck HJ, Eysenck SBG. Manual for the Eysenck Personality Inventory. San Diego, CA: Educational and Industrial Testing Service; 1968. [Google Scholar]
- Fennema-Notestine C, McEvoy LK, Notestine R, Panizzon MS, Wai-Ying W, Franz CE, Lyons MJ, Eyler LT, Neale MC, Xian H, McKenzie RE, Kremen WS. White matter disease in midlife is heritable, related to hypertension, and shares some genetic influence with systolic blood pressure. Neuroimage: Clinical. 2016;12:737–745. doi: 10.1016/j.nicl.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11:325–345. doi: 10.1080/13825580490511152. [DOI] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Developmental Psychology. 2003;39:535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Hamagami F, Pedersen NL. Genetic variance in processing speed drives variation in aging of spatial and memory abilities. Developmental Psychology. 2009;45:820–834. doi: 10.1037/a0015332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Age changes in processing speed as a leading indicator of cognitive aging. Psychology and Aging. 2007;22:558–568. doi: 10.1037/0882-7974.22.3.558. [DOI] [PubMed] [Google Scholar]
- Franz CE, Lyons MJ, O’Brien R, Panizzon MS, Kim K, Bhat R, Grant MD, Toomey R, Eisen S, Xian H, Kremen WS. A 35-year longitudinal assessment of cognition and midlife depression symptoms: The Vietnam Era Twin Study of Aging. American Journal of Clinical Psychiatry. 2011;19:559–570. doi: 10.1097/JGP.0b013e3181ef79f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons L, Teri L, Logsdon R, McCurry S, Kukull W, Bowen J, Larson E. Anxiety symptoms as predictors of nursing home placement in patients with Alzheimer’s disease. Journal of Clinical Geropsychology. 2002;8:335–342. [Google Scholar]
- Gould CE, Edelstein BA. Worry, emotion control, and anxiety control in older and young adults. Journal of Anxiety Disorders. 2010;24:759–766. doi: 10.1016/j.janxdis.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Gould CE, O’Hara RO, Goldstein MK, Beaudreau SA. Multimorbidity is associated with anxiety in older adults in the Health and Retirement Study. International Journal of Geriatric Psychiatry. 2016;31:1105–1115. doi: 10.1002/gps.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR, Pedersen NL, McClearn GE, Plomin R, Nesselroade JR. Age differences in genetic and environmental influences for health from the Swedish Adoption/Twin Study of Aging. Journal of Gerontology. 1992;47:P213–220. doi: 10.1093/geronj/47.3.p213. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Langa KM. Monetary costs of dementia in the United States. New England Journal of Medicine. 2013;369:489–490. doi: 10.1056/NEJMc1305541. [DOI] [PubMed] [Google Scholar]
- Infurna FJ, Gerstorf D, Ryan LH, Smith J. Dynamic links between memory and functional limitations in old age: longitudinal evidence for age-based structural dynamics from the AHEAD study. Psychology and Aging. 2011;26:546–558. doi: 10.1037/a0023023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jajodia A, Borders A. Memory predicts changes in depressive symptoms in older adults: a bidirectional longitudinal analysis. Journal of Gerontology Series B: Psychological Sciences. 2011;66:571–581. doi: 10.1093/geronb/gbr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson C, Molander L. Manual till CVB-sklan [Manual of the CVB-Scales] Stokholm, Sweden: Psykologi Forlaget; 1964. [Google Scholar]
- Lenze EJ, Dixon D, Mantella RC, Dore PM, Andreescu C, Reynolds CF, 3rd, Butters MA. Treatment-related alteration of cortisol predicts change in neuropsychological function during acute treatment of late-life anxiety disorder. International Journal of Geriatric Psychiatry. 2012;27:454–462. doi: 10.1002/gps.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, Rollman BL. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. American Journal of Geriatric Psychiatry. 2011;19:482–490. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Mah L, Binns MA, Steffens DC, Alzheimer’s Disease Neuroimaging, Initiative Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. American Journal of Geriatric Psychiatry. 2015;23:466–476. doi: 10.1016/j.jagp.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantella RC, Butters MA, Amico JA, Mazumdar S, Rollman BL, Begley AE, Lenze EJ. Salivary cortisol is associated with diagnosis and severity of late-life generalized anxiety disorder. Psychoneuroendocrinology. 2008;33:773–781. doi: 10.1016/j.psyneuen.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle JJ, Hamagami F. Structural equation models for evaluating dynamic concepts within longitudinal twin analyses. Behavioral Genetics. 2003;33:137–159. doi: 10.1023/a:1022553901851. doi: 460863. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User’s Guide. Sixth. Los Angeles, CA: 2010. [Google Scholar]
- Norrie LM, Diamond K, Hickie IB, Rogers NL, Fearns S, Naismith SL. Can older “at risk” adults benefit from psychoeducation targeting healthy brain aging? International Psychogeriatrics. 2011;23:413–424. doi: 10.1017/S1041610210001109. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A quantitative genetic analysis of cognitive abilities during the second half of the life span. Psychological Science. 1992;3:346–353. [Google Scholar]
- Petkus AJ, Gatz M, Reynolds CA, Kremen WS, Wetherell JL. Stability of Genetic and Environmental Contributions to Anxiety Symptoms in Older Adulthood. Behavior Genetics. 2016;46:492–505. doi: 10.1007/s10519-015-9772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Gum AM, Wetherell JL. Anxiety and cognitive impairment in homebound older adults. International Journal of Geriatric Psychiatry. 2013;28:989–990. doi: 10.1002/gps.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Pedersen NL, Gatz M. Anxiety is associated with increased risk of dementia in older Swedish twins. Alzheimer’s & Dementia. 2016;12:399–406. doi: 10.1016/j.jalz.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Lim YY, Neumeister A, Ames D, Ellis KA, Harrington K, Lifestyle Research, Group Amyloid-beta, anxiety, and cognitive decline in preclinical Alzheimer disease: a multicenter, prospective cohort study. Journal of the American Medical Association: Psychiatry. 2015;72:284–291. doi: 10.1001/jamapsychiatry.2014.2476. [DOI] [PubMed] [Google Scholar]
- Pietrzak RH, Maruff P, Woodward M, Fredrickson J, Fredrickson A, Krystal JH, Darby D. Mild worry symptoms predict decline in learning and memory in healthy older adults: a 2-year prospective cohort study. American Journal of Geriatric Psychiatry. 2012;20:266–275. doi: 10.1097/JGP.0b013e3182107e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porensky EK, Dew MA, Karp JF, Skidmore E, Rollman BL, Shear MK, Lenze EJ. The burden of late-life generalized anxiety disorder: effects on disability, health-related quality of life, and healthcare utilization. American Journal of Geriatric Psychiatry. 2009;17:473–482. doi: 10.1097/jgp.0b013e31819b87b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin O, Forget H, Grenier S, Preville M, Hudon C. Anxiety, depression, and 1-year incident cognitive impairment in community-dwelling older adults. Journal of the American Geriatrics Society. 2011;59:1421–1428. doi: 10.1111/j.1532-5415.2011.03521.x. [DOI] [PubMed] [Google Scholar]
- Radloff L. The CES-D scale: A self report depression scale for research in the general population’. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ramsay MC, Reynolds CR. Separate digits tests: a brief history, a literature review, and a reexamination of the factor structure of the Test of Memory and Learning (TOMAL) Neuropsychological Review. 1995;5:151–171. doi: 10.1007/BF02214760. [DOI] [PubMed] [Google Scholar]
- Rodrigues R, Petersen RB, Perry G. Parallels between major depressive disorder and Alzheimer’s disease: role of oxidative stress and genetic vulnerability. Cell and Molecular Neurobiology. 2014;34:925–949. doi: 10.1007/s10571-014-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A, Bentler PM. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/s11336-009-9135-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp ES, Reynolds CA, Pedersen NL, Gatz M. Cognitive engagement and cognitive aging: Is openness protective? Psychology and Aging. 2010;25:60–73. doi: 10.1037/a0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) Tamps, FL: University of South Florida; 1979. [Google Scholar]
- Stanziano DC, Whitehurst M, Graham P, Roos BA. A review of selected longitudinal studies on aging: Past findings and future directions. Journal of the American Geriatric Society. 2010;58:S292–S297. doi: 10.1111/j.1532-5415.2010.02936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden KA, Simning A, Conwell Y, Skoog I, Waern M. Characteristics and comorbid symptoms of older adults reporting death ideation. American Journal of Geriatric Psychiatry. 2013;21:803–810. doi: 10.1016/j.jagp.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliadis HM, Dionne PA, Preville M, Gentil L, Berbiche D, Latimer E. The excess healthcare costs associated with depression and anxiety in elderly living in the community. American Journal of Geriatric Psychiatry. 2012 doi: 10.1097/JGP.0b013e318248ae9e. [DOI] [PubMed] [Google Scholar]
- Vogelzangs N, Beekman AT, de Jonge P, Penninx BW. Anxiety disorders and inflammation in a large adult cohort. Translational Psychiatry. 2013;3:e249. doi: 10.1038/tp.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychology and Aging. 2001;16:187–195. doi: 10.1037//0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Le Roux H, Gatz M. DSM-IV criteria for generalized anxiety disorder in older adults: distinguishing the worried from the well. Psychology and Aging. 2003;18:622–627. doi: 10.1037/0882-7974.18.3.622. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. Journals of Gerontology Series B: Psychological Science and Social Science. 2002;57:P246–255. doi: 10.1093/geronb/57.3.p246. [DOI] [PubMed] [Google Scholar]
- Wolitzky-Taylor KB, Castriotta N, Lenze EJ, Stanley MA, Craske MG. Anxiety disorders in older adults: A comprehensive review. Depression and Anxiety. 2010;27:190–211. doi: 10.1002/da.20653. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, Marmar C. Posttraumatic stress disorder and risk of dementia among US veterans. Archives of General Psychiatry. 2010;67:608–613. doi: 10.1001/archgenpsychiatry.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]


