Abstract
Background
Imatinib, a tyrosine kinase inhibitor, has been shown to restore blood–brain barrier integrity and reduce infarct size, haemorrhagic transformation and cerebral oedema in stroke models treated with tissue plasminogen activator. We evaluated the safety of imatinib, based on clinical and neuroradiological data, and its potential influence on neurological and functional outcomes.
Methods
A Phase II randomised trial was performed in patients with acute ischaemic stroke treated with intravenous thrombolysis. A total of 60 patients were randomly assigned to four groups [3 (active) : 1 (control)]; the active treatment groups received oral imatinib for 6 days at three dose levels (400, 600 and 800 mg). Primary outcome was any adverse event; secondary outcomes were haemorrhagic transformation, cerebral oedema, neurological severity on the National Institutes of Health Stroke Scale (NIHSS) at 7 days and at 3 months and functional outcomes on the modified Rankin scale (mRS).
Results
Four serious adverse events were reported, which resulted in three deaths (one in the control group and two in the 400 mg dose group; one patient in the latter group did not receive active treatment and the other received two doses). Non-serious adverse events were mostly mild, resulting in full recovery. Imatinib ameliorated neurological outcomes with an improvement of 0.6 NIHSS points per 100 mg imatinib (P = 0.02). For the 800 mg group, the mean unadjusted and adjusted NIHSS improvements were 4 (P = 0.037) and 5 points (P = 0.012), respectively, versus controls. Functional independence (mRS 0–2) increased by 18% versus controls (61 vs. 79; P = 0.296).
Conclusion
This Phase II study showed that imatinib is safe and tolerable, and may reduce neurological disability in patients treated with intravenous thrombolysis after ischaemic stroke. A confirmatory randomised trial is currently underway.
Keywords: Stroke, cerebral infarct, thrombolysis, imatinib, intracerebral haemorrhage, cerebral oedema
Introduction
Disruption of the blood–brain barrier (BBB) is a critical pathophysiological event following cerebral ischaemia [1–5], which may play a significant role in haemorrhagic transformation and cerebral oedema after treatment with tissue plasminogen activator (tPA) [6, 7]. Moreover, a leaky BBB may expose ischaemic tissue to infiltrating leukocytes [8]. Severe vascular disruption in ischaemia is associated with severe tissue injury [9]. Imatinib, a tyrosine kinase inhibitor, can restore the integrity of the BBB by blocking the signalling of platelet-derived growth factor alpha (PDGF-alpha) receptors on perivascular astrocytes [10]. PDGF-alpha receptors open the BBB after tPA-mediated activation of the latent form of PDGF subtype CC (PDGF-CC) [7, 11, 12]. Elevated plasma levels of PDGF-CC are associated with haemorrhagic transformation after treatment with tPA in ischaemic stroke patients [13]. In experimental stroke models, treatment with imatinib decreases BBB permeability, lowers the risk of haemorrhagic transformation and cerebral oedema and reduces infarct size [10, 14].
Despite intravenous thrombolysis (IVT) [15–23] and mechanical thrombectomy [24–28], up to 40% of patients are still functionally dependent or dead at follow-up [29]. Only a minority of all ischaemic stroke patients can be treated by reperfusion therapies because of the short time window available for treatment, contraindications and risk of haemorrhagic transformation.
The main aim of the present study was to evaluate the safety and tolerability of imatinib among ischaemic stroke patients treated with IVT. Secondary aims were to determine the effect of imatinib on haemorrhagic transformation, cerebral oedema and neurological and functional outcomes.
Methods
Study design
I-Stroke was a prospective, randomised, open label, blinded end-point evaluation (PROBE), dose escalation, regional multicentre clinical trial for patients with acute ischaemic stroke who were treated with IVT. After providing informed consent, a total of 60 patients were randomly assigned [3 (active) : 1 (control); using the web-based system Viedoc] within 1 h after the termination of reperfusion treatment, with the aim of creating four groups of equal size (n = 15 each), in three treatment phases (n = 20 in each phase). The effects of imatinib were tested at a daily dose of 400 mg (low dose) in the first phase, at 600 mg (medium dose) in the second phase and at 800 mg (high dose) in the third phase. Each dose-increase phase was preceded by clearance by an independent safety committee based on evaluation of the serious and non-serious adverse events. In case of a serious adverse event during the third phase of the study with high-dose imatinib the independent safety committee would have been involved but this did not occur. All patients received IVT in agreement with the accepted indications (Appendix 1). Mechanical thrombectomy was performed in patients with large artery occlusion who satisfied the local criteria for intervention (Appendix 2).
The study protocol was approved by the Ethics Committee of Karolinska Institutet and by the Medical Products Agency of Sweden. The study was registered with the European Clinical Trials Database (EUDRA CT NR 2010-019014-25).
Patients and participating centres
The study was conducted at five centres in Stockholm County. Patients for whom mechanical thrombectomy was considered had IVT initiated at the local hospital and were then transferred to Karolinska University Hospital at Solna. Patients were 18–85 years old, with acute ischaemic stroke onset causing a neurological deficit of 7 to 25 points on the National Institutes of Health Stroke Scale (NIHSS). A comprehensive list of exclusion criteria is shown in Appendix 3.
Treatment
If allocated to the active treatment group, imatinib was administered orally as soon as possible after randomisation (day 0) as tablets corresponding to doses of 400, 600 or 800 mg, depending on the phase of the study. From day 1 to day 5, tablets were given each morning, except at the highest dose level (800 mg) where tablets of 400 mg were given twice daily (morning and evening).
Outcome measures
Primary outcome
The primary outcome of this study was any serious or non-serious adverse event. The classification of adverse events, severity and likelihood of relation to treatment and clinical course was the local investigator’s responsibility and was documented in the case record form.
Secondary outcomes
In this study, secondary outcomes were the occurrence and severity of haemorrhagic transformation, cerebral oedema and infarct volume, neurological outcomes as measured by the NIHSS score and functional outcomes as measured by the modified Rankin scale (mRS) score at 3 months. The evaluation of infarct volume will be addressed in a future publication.
Clinical and radiological assessments
The NIHSS score was determined before IVT, at the start of study treatment (baseline), after 2 h (day 0), on day 1 and then daily until day 7. Adverse events were assessed daily from day 0 to day 7, and at 3 months. Blinded evaluations of the NIHSS and mRS scores were performed at 3 months at a different hospital from the one responsible for the acute treatment.
Computer tomography (CT) scans were performed at baseline, 24 h (accepted interval 22–36 h) and, optionally, at 7 days. Magnetic resonance imaging (MRI) was performed at 24 h (accepted interval as for CT) and at 7 days. CT and MRI examinations were evaluated blindly by two experienced neuroradiologists to reach a consensus.
Definitions of haemorrhagic transformation, such as haemorrhagic infarcts and intracerebral (parenchymatous) haemorrhages and cerebral oedema, have been described previously [17] (web-material W1).
Statistical analyses
Descriptive statistics for baseline, demographic and imaging data were used to compare the different treatment groups. Proportions were calculated for categorical values, dividing the number of events by the total number (excluding missing/unknown values). Median values were calculated for continuous variables and were compared using a median regression. For the calculation of differences between proportions, we used Fisher’s exact test. Potential associations between adverse events and treatment groups were analysed using Fisher’s exact test. The comparisons for creatinine, ALAT, ASAT and total white blood cell count were made using a two-sample t-test.
To analyse whether imatinib had any effect on the NIHSS score, the dose of imatinib was treated both as a categorical and a continuous variable. When looking at separate time points, a linear regression with robust standard errors was used while adjusting for thrombectomy status. When considering the mean effect over the entire time course, a linear mixed model was used with a random intercept and adjustment for thrombectomy status using robust standard errors and allowing the residuals to have an autoregressive 1 (AR1) correlation within individuals. The choice to use AR1 correlation was made by comparing the Akaike information criterion to a model without AR1 correlation. To model the median effect over the entire time course, we used a median regression with cluster robust standard errors [30]. Because of some missing NIHSS values, multiple imputations [31] were used.
When comparing the mean percentage NIHSS change from baseline, we used a linear regression adjusted for thrombectomy status with bootstrap confidence intervals. The shift analysis of the mRS after 90 days was conducted using three separate Mann–Whitney U-tests (comparing each imatinib group to the control). The association between treatment groups and functional independence was analysed using logistic regression. Statistical analyses were performed using Stata v.13 software (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
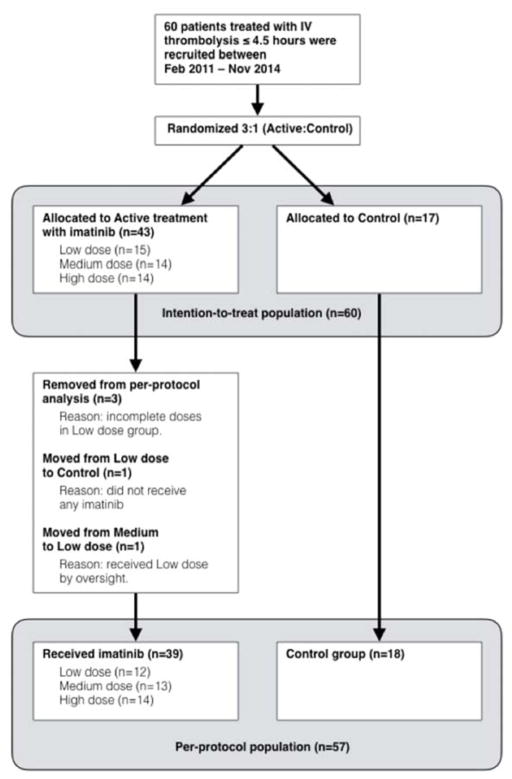
Sixty patients were recruited between February 2011 and November 2014 (20 patients for each dose level). In phase-I, 15 patients were randomised to low-dose and 5 to control, in phase-II, 14 patients to medium-dose and 6 to control, and in phase-III, 14 patients to high-dose and 6 to control. Baseline characteristics are shown in Table 1. All patients were included in the safety analysis (intention to treat). The remaining analyses were performed, according to actual treatment (per protocol) (Fig. 1).
Table 1.
Demographic and baseline data (intention-to-treat population)
| Control | Low dose | Medium dose | High dose | |
|---|---|---|---|---|
| Number of patients | 17 | 15 | 14 | 14 |
| Median age (years) | 70 | 76 | 73 | 72 |
| Male (%) | 65 | 53 | 79 | 43 |
| Hypertension (%) | 76 | 73 | 64 | 50 |
| Atrial fibrillation (%) | 29 | 33 | 43 | 36 |
| Diabetes (%) | 18 | 20 | 7 | 7 |
| Hyperlipidaemia (%) | 35 | 20 | 29 | 36 |
| Current smoker (%) | 18 | 7 | 29 | 0 |
| Previous smoker (%) | 35 | 40 | 50 | 29 |
| Previous stroke >3 months (%) | 12 | 7 | 0 | 7 |
| Previous TIA (%) | 12 | 13 | 7 | 0 |
| Median baseline NIHSS score | 13 | 12 | 11 | 13 |
| Intravenous thrombolysis (%) | 100 | 100 | 100 | 100 |
| Intra-arterial thrombolysis (%) | 0 | 7 | 0 | 0 |
| Mechanical thrombectomy (%) | 29 | 47 | 21 | 71 |
| Stenting (%) | 6 | 13 | 0 | 7 |
| Aspirin (%) | 31 | 47 | 36 | 21 |
| Warfarin (%) | 6 | 0 | 8 | 7 |
| Oral antidiabetic agent (%) | 7 | 7 | 0 | 7 |
| Insulin (%) | 6 | 0 | 8 | 0 |
| Statin (%) | 50 | 20 | 21 | 21 |
TIA, transient ischaemic attack; NIHSS, National Institutes of Health Stroke Scale.
Fig. 1.
Overview of study recruitment and populations included in the intention-to-treat (including all randomised patients) and in per-protocol analyses (including patients treated according to protocol).
Treatment
The median time from stroke onset to IVT for all participants (n = 60) was 86 min (range 35–269 min). For patients treated with mechanical thrombectomy, the median time from onset to the end of the intervention was 290 min (range 145–465 min; exact time missing for three participants). For patients allocated to imatinib (n = 41), the median time from stroke onset to the first dose of imatinib was 240 min (range 75–714 min).
Safety analysis: mortality
Three patients died during the study period. One patient, allocated to the low-dose group but not receiving active treatment, died because of a recurrent stroke. One control patient died because of an infection. The third patient, who received one dose of 400 mg imatinib at the day of onset and on day 1, died because of an infarct with intracerebral haemorrhage and oedema. The investigator and the independent safety committee found no likely connection between the study treatment and the patient’s clinical deterioration.
Safety analysis: adverse events
Serious adverse events
Four serious adverse events were reported, which resulted in the death of three patients (see above). The patient in the control group who deteriorated because of fever and infection also had a growing femoral haematoma at the site of the femoral artery puncture. There were no serious adverse events in the medium- and high-dose groups.
Non-serious adverse events
In total, 118 non-serious adverse events were reported in 41 patients (Table 2). Eleven cerebral haemorrhagic events were reported in the medium-dose group (10 were judged ‘unlikely’ to be related to the imatinib treatment, and one was classified ‘possible’). Severity was mild, moderate and severe in eight, two and one patients respectively. There were six reports of mild or moderate itching and skin reactions in the high-dose group; the relation to imatinib treatment was classified as ‘probable’, ‘possible’ or ‘expected’ in five cases and ‘unlikely’ in one.
Table 2.
Non-serious adverse events
| AEs | Control (n = 17) | Low dose (n = 15) | Medium dose (n = 14) | High dose (n = 14) | Total (n = 60) | P-value |
|---|---|---|---|---|---|---|
| Total number of AEs | 25 | 19 | 44 | 30 | 118 | |
| Fever and infection | 4(6) | 4(7) | 4(5) | 3(3) | 15(21) | 1 |
| Itching and skin reaction | 2(2) | 2(2) | 0(0) | 6(6) | 10(10) | 0.020 |
| Cerebral bleeding | 3(3) | 0(0) | 9(11) | 1(1) | 13(15) | <0.001 |
| Arrhythmia and tachy/bradycardia | 3(3) | 1(1) | 3(4) | 2(2) | 9(10) | 0.483 |
| Nausea and vomiting | 0(0) | 1(2) | 2(6) | 4(4) | 7(12) | 0.063 |
| Fall | 1(1) | 0(0) | 2(3) | 1(1) | 4(5) | 0.528 |
| Dyspnoea | 2(2) | 0(0) | 1(1) | 1(1) | 4(4) | 0.780 |
| Systemic bleeding | 1(3) | 0(0) | 1(1) | 2(3) | 4(7) | 0.528 |
| Fainting | 0(0) | 0(0) | 0(0) | 2(2) | 2(2) | 0.103 |
| Other | 3(5) | 5(7) | 9(13) | 5(7) | 22(32) | 0.067 |
Data show number of patients with an event (total number of events) in the intention-to-treat population. AE, adverse event.
The severity of non-serious adverse events, plasma concentrations of imatinib and laboratory values are shown in the web-material (W2, W3 and W4 respectively).
Haemorrhagic transformation and cerebral oedema
A total of 21 haemorrhagic infarctions (six HI1, 15 HI2) were observed in ths study: six each in the control, low-dose and medium-dose groups and three in the high-dose group. Three intracerebral haemorrhages were reported (two PH1, one PH2). One PH1-type haemorrhage occurred in the control group and one in the high-dose group; the PH2-type haemorrhage occurred in the medium-dose group.
In addition, four remote intracerebral haemorrhages of a milder grade (PHr1) were reported (one each in the low- and medium-dose groups and two in those receiving the high dose).
No haemorrhagic transformations (0 of 5) occurred when imatinib treatment was initiated within 5 h after stroke onset (Table 3) or when reperfusion treatment (IVT alone or in combination with thrombectomy) was completed within 4.5 h (0 of 6) in the high-dose group.
Table 3.
Haemorrhagic transformations
| Any haemorrhagic transformation | Control n = 18 | Low dose n = 12 | Medium dose n = 13 | High dose n = 14 |
|---|---|---|---|---|
| Stroke onset to imatinib treatment <5 h | 7/18 | 4/8 | 4/9 | 0/5 |
| Stroke onset to imatinib treatment ≥5 h | 3/4 | 4/4 | 6/9 | |
| Stroke onset to end of reperfusion 0–4.5 h | 5/16 | 5/9 | 6/11 | 0/6 |
| Stroke onset to end of reperfusion >4.5 h | 2/2 | 2/3 | 2/2 | 6/8 |
Data show number of patients with haemorrhagic transformations, relative to the total number of patients, in the per-protocol population.
Results for cerebral oedema are available as web-material (W5). Overall, there were 33 cases of cerebral oedema in the study: 28 mild (n = 7, 4, 7 and 10 in the control and low-, medium- and high-dose groups respectively), three moderate (n = 2 and 1 in the low- and medium-dose groups respectively) and two severe oedema (both in the control).
Neurological outcomes
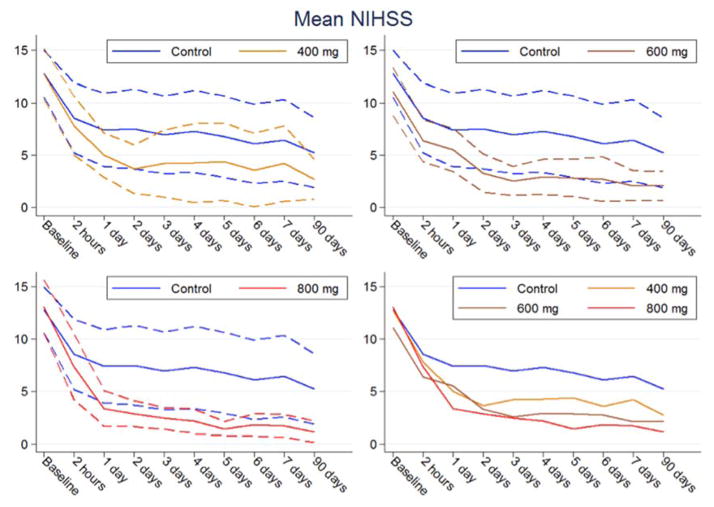
The mean unadjusted improvement in the NIHSS score (Fig. 2) compared to controls over all time points after baseline was 2 points for the low-dose group (P = 0.283), 3 points for the medium-dose group (P = 0.084) and 4 points for the high-dose group (P = 0.037). After adjustment for thrombectomy, the corresponding mean improvement compared to controls was 2 points (P = 0.259), 3 points (P = 0.106) and 5 points (P = 0.012).
Fig. 2.
National Institutes of Health Stroke Scale (NIHSS) score per treatment group and time point (solid lines, means; dashed lines, 95% CIs; per-protocol population).
For the three protocol-specified time points, there were significant improvements in the adjusted mean NIHSS scores in patients treated with high doses of imatinib compared to controls: 5 points (−5.28; 95% CI −9.60 to −0.96; P = 0.018) on day 1; 6 points (−5.68; 95% CI −10.38 to −0.98; P = 0.019) on day 7; and 5 points (−4.81; 95% CI −10.39 to −0.98; P = 0.019) at 3 months. There were also significant improvements with the high dose for all other individual time points except 2 h.
When stratified by treatment with mechanical thrombectomy, the improvement in the mean NIHSS score between controls and the high-dose group at day 7 was 4 points for patients who underwent thrombectomy and 5 points for those who did not. Compared to baseline, patients treated with thrombectomy in the control group (n = 6) showed an improvement in the mean NIHSS of 10 points from baseline to 7 days (16 to 6). In the high-dose group (n = 10), the corresponding improvement was 12 points (14 to 2). For non-thrombectomy patients, the corresponding improvement was 5 points (11 to 6) in the control group (n = 12) and 10 points (11 to 1) in the high-dose group (n = 4).
Neurological outcomes improved with increasing doses of imatinib throughout the study period. The improvement in the mean unadjusted NIHSS score compared to controls was 0.5 points per 100 mg imatinib (−0.5; 95% CI −0.98 to −0.02; P = 0.04). After adjustment for thrombectomy, the improvement was 0.6 score points per 100 mg imatinib (−0.59; 95% CI −1.08 to −0.09; P = 0.02).
Neurological outcome expressed as the median, as improvement of ≥8 NIHSS points and as a percentage of the baseline value at different time points is shown in web-material (W6).
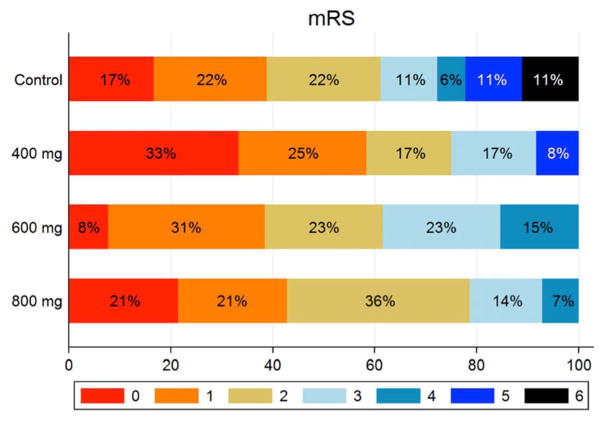
Functional outcomes
Functional outcomes on the mRS are shown in Fig. 3. Functional independence (mRS 0–2) was observed in 61% of the control group and 72% of all imatinib-treated patients. For the high-dose group, there was an 18% absolute increase in the proportion of functional independence and a higher odds ratio adjusted for thrombectomy compared to control (2.33; 95% CI 0.48 to 11.44; P = 0.296).
Fig. 3.
Distribution of modified Rankin scale (mRS) scores in the per-protocol population. Scores ranged from 0 to 6: 0, no symptoms; 1, no clinically significant disability; 2, slight disability (patients able to look after their own affairs without assistance but unable to perform all previous activities); 3, moderate disability (patients require some help but able to walk without assistance); 4, moderately severe disability (patients unable to attend to bodily needs or walk without assistance); 5, severe disability (patients require constant nursing care and attention); 6, death.
Discussion
Treatment with all doses of imatinib daily for 6 days in acute ischaemic stroke patients who received IVT was safe and well tolerated. Although any efficacy outcome should be interpreted with caution considering that the trial was a Phase II study of limited size, the effect of imatinib on neurological outcome increased dose dependently, with a 0.6-point improvement in the NIHSS score per 100 mg imatinib. Only the high-dose imatinib treatment resulted in a statistically significant improvement for the protocol-specified neurological outcomes at day 7 and at 3 months compared to controls. The improvement was consistent for the whole study period and for each individual time point later than 2 h after the initiation of thrombolysis. The neurological improvement with the high-dose versus control was clinically relevant, as it was comparable to the effect of mechanical thrombectomy [24, 27]. This effect was added to the improvement achieved by IVT and, when indicated, mechanical thrombectomy. High-dose imatinib was also associated with 18% improvement of functional independency versus control, although these results should be interpreted with extreme caution because of the small numbers of patients in the study groups.
Imatinib is primarily used in the treatment of haematological cancers with an acceptable rate of adverse events in long-term follow-up [33] but has not previously been used for the treatment of stroke. For its usual indication, the dose is titrated from a lower dose to an individually adapted level up to a recommended maximum of 800 mg daily. In the present study we started immediately with a dose of 400, 600 or 800 mg daily, with evaluation by an independent safety committee between each dose increase. We did not observe any serious adverse events with imatinib treatment. Non-serious events were mild or only moderately severe and tolerable. Itching and skin reactions, and possibly also nausea (results were non-significant), may be related to treatment.
Although our findings on neurological outcome in this clinical study are preliminary, they correlate well with observations made in experimental studies [6–14]. There are several conceivable mechanisms for an effect of imatinib, including the restoration of the BBB with a reduction in the subsequent inflammatory response, reduced oedema and preserved nutritional supply to the ischaemic area. The inflammatory response to ischaemic injury includes the activation of microglia, the production of pro-inflammatory mediators and the infiltration of inflammatory cells, events that contribute to ischaemic brain injury [8, 32]. The peak infiltration of inflammatory cells occurs during the first days after acute stroke. Restriction of the invasion of cells from the blood vessels to the ischaemic brain may provide an explanation for an effect of imatinib on the neurological outcomes.
An expected effect of BBB restoration would be a reduced risk of haemorrhagic transformation after IVT [21–23]. Interestingly, this increased risk of haemorrhagic transformation observed before IVT is greater in patients with higher levels of PDGF-CC on admission and at 24 h after stroke [13]. In our study, there were no marked differences between the treatment groups regarding the total occurrence of haemorrhagic transformation. One explanation may be that imatinib treatment was administered too late to exert an overall influence on the haemorrhage risk; of note, no haemorrhagic transformation occurred in patients treated with 800 mg imatinib within the first 5 h after stroke onset. Currently, imatinib is only available in tablet form for oral administration. An intravenous form of imatinib could potentially enhance very early administration in patients with acute stroke. In patients with suspected difficulty in swallowing, it is possible to administer imatinib by nasogastric tube after dispersion of the tablets in water. A reduction in the haemorrhage risk with early imatinib treatment would be clinically important because the risk of bleeding is a serious concern during the use of IVT and may restrict its use for some patients. Early high-dose imatinib treatment also seemed to reduce the occurrence of cerebral oedema. These observations were consistent with data from experimental studies in which imatinib was administered 1 h after the induction of stroke and thrombolysis with tPA was administered 5 h after stroke induction [10].
Because of its effects in ischaemic stroke, as shown in preclinical studies [10, 14] and preliminary clinical results from our pilot study, the potential benefit of imatinib in haemorrhagic stroke (intracerebral and subarachnoid haemorrhage) warrants further preclinical and clinical studies.
This study had several limitations. First, although evaluations of imaging scans and 3-month outcomes were blinded, this was an open-label study. Secondly, the higher frequency of mechanical thrombectomy in the high-dose group could potentially influence the results. However, the results were adjusted for mechanical thrombectomy and, when stratified by thrombectomy, the result was fairly robust, although the number of patients was small. Third, a limited number of patients were enrolled in the study (n = 60).
In conclusion, imatinib treatment is safe and tolerable and may reduce neurological disability in patients with acute ischaemic stroke who received thrombolysis. The findings of this Phase II study open a new strategy for acute stroke treatment, complementary to the existing evidence-based treatments, thrombolysis and thrombectomy. Our findings need confirmation in a larger trial comparing high-dose imatinib with placebo. One such trial is in preparation.
Acknowledgments
Funding sources
This study was funded by the Swedish Agency for Innovation Systems (Vinnova) and private donations through the Swedish Heart and Lung Foundation and Karolinska Institutet.
The funders had no role in the design of the study, data collection, analysis and interpretation or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Appendix 1 Main criteria for intravenous thrombolysis (IVT)
Clinical diagnosis of acute ischaemic stroke and computed tomography scan exclusion of haemorrhage
Symptoms must be distinguishable from an episode of generalised ischaemia (i.e. syncope), seizure or migraine disorder
Treatment initiated within 4.5 h of stroke onset
Exclusion criteria
Severe stroke as assessed clinically (e.g. NIHSS score >25) and/or developing infarct extending into more than one-third of the middle cerebral artery territory or half of other vascular territories
Administration of heparin within 48 h preceding the onset of stroke with an elevated activated thromboplastin time (aPTT) at presentation, or corresponding low-molecular weight heparin
Platelet count below 100,000/mm3. Significant bleeding disorder at stroke onset or within the past 6 months, known haemorrhagic diathesis.
Patients receiving oral anticoagulants, e.g. warfarin sodium (INR >1.7) or direct oral anticoagulation: dabigatran (Pradaxa, aPTT >40 s), apixaban, rivaroxaban
History, evidence or suspicion of intracranial haemorrhage including subarachnoid haemorrhage
Systolic blood pressure >185 mmHg or diastolic blood pressure >110 mmHg, in spite of repeated doses of i.v. medication to reduce blood pressure to below these limits
Any history of central nervous system damage (i.e. neoplasm, aneurysm, intracranial or spinal surgery) or neurodegenerative disorder
Haemorrhagic retinopathy, or other haemorrhagic ophthalmic conditions
Patients of childbearing potential, if pregnancy cannot be excluded by pregnancy test and/or interview
Major surgery or significant trauma in previous 10 days (including any trauma associated with current acute myocardial infarction), recent trauma to head or cranium
Appendix 2 Main criteria for thrombectomy according to local guidelines
Confirmed diagnosis on computed tomography angiography of persistent occlusion of the proximal middle cerebral artery, terminal carotid artery or basilar artery consistent with the clinical symptoms
NIHSS ≥6 or aphasia
Anticipated life expectancy of at least 6 months
Initiation of endovascular procedure (defined as start with groin puncture) within 8 h of symptom onset
Exclusion criteria
Known significant pre-stroke disability (mRS ≥2)
Extended early ischaemic changes
Severe comorbidities such as dementia, terminal illness and other medical conditions
Appendix 3 Inclusion and exclusion criteria specific for the study drug in addition to criteria for intravenous thrombolysis (IVT) and thrombectomy
Inclusion criteria
Clinical diagnosis of acute ischaemic stroke causing a measurable neurological deficit defined as impairment of language, motor function and cognition, including dysphasia and neglect, gaze and vision. Ischaemic stroke is defined as an event characterised by sudden onset of acute focal neurological deficit, presumed to be caused by cerebral ischaemia, after CT scan exclusion of haemorrhage
Neurological deficit corresponding to ≥7 points on the National Institutes of Health Stroke Scale (NIHSS), at the time of randomisation
Age 18–85 years
IVT is indicated on clinical grounds and has been initiated within 4.5 h of stroke onset
Patients should be randomly assigned to treatment as soon as possible but not later than 1 h after completion of IVT, or if applicable, within 1 h after completion of additional intra-arterial intervention
Patients are competent to make a decision and have provided informed consent with regard to participation in the study, retrieval and storage of data and follow-up procedures
Exclusion criteria
Severe stroke as assessed clinically (e.g. NIHSS >25) and/or developing infarct extending into more than one-third of the middle cerebral artery territory or half of other vascular territories
Acute pancreatitis
Severe hepatic dysfunction, including hepatic failure, cirrhosis, portal hypertension (oesophageal varices) and active hepatitis
Ongoing treatment with chemotherapy
Drugs that may increase the plasma concentration of imatinib: ketoconazole, itraconazole, erythromycin and clarithromycin
Drugs that may decrease the plasma concentration of imatinib: dexamethasone, phenytoin, carbamazepine, rifampicin, phenobarbital, fosphenytoin, primidone and St John’s wort (Hypericum performatum)
Contraindications against magnetic resonance imaging
Footnotes
Conflict of interest statement
Nils Wahlgren and Ulf Eriksson have applied for a patent for the use of imatinib in acute stroke.
All other authors have no conflicts of interest.
Details regarding study coordination, contribution to the manuscript, members of the safety committee, study investigators and the research nurses responsible for patient recruitment are available as web-material (W7).
References
- 1.Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol ExpTher. 1999;289:668–675. [PubMed] [Google Scholar]
- 2.Fischer S, Clauss M, Wiesnet M, Renz D, Schaper W, Karliczek GF. Hypoxia induces permeability in brain microvessel endothelial cells via VEGF and NO. Am J Physiol. 1999;276:C812–820. doi: 10.1152/ajpcell.1999.276.4.C812. [DOI] [PubMed] [Google Scholar]
- 3.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Latour LL, Kang DW, Ezzeddine MA, Chalela JA, Warach S. Early blood-brain disruption in human focal brain ischemia. Ann Neurol. 2004;56:468–477. doi: 10.1002/ana.20199. [DOI] [PubMed] [Google Scholar]
- 5.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischaemic stroke. Neurobiology of Disease. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y, Rosenberg GA. Blood brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 2011;42:3323–3328. doi: 10.1161/STROKEAHA.110.608257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su EJ, Fredriksson L, Schielke GP, Eriksson U, Lawrence DA. Tissue plasminogen activator-mediated PDGF signaling and neurovascular coupling in stroke. J Thromb Haemost. 2009;(Suppl 1):155–158. doi: 10.1111/j.1538-7836.2009.03402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin R, Yang G, Li G. Inflammatory mechanisms in ischaemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen B, Friedman B, Cheng Q, et al. Severe blood-brain disruption and surrounding tissue injury. Stroke. 2009;40:e666–e674. doi: 10.1161/STROKEAHA.109.551341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su EJ, Fredriksson L, Geyer M, et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischaemic stroke. Nat Med. 2008;14:731–737. doi: 10.1038/nm1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fredriksson L, Li H, Fieber C, Li X, Eriksson U. Tissue plasminogen activator is a potent activator of PDGF-CC. EMBO J. 2004;23:3793–3802. doi: 10.1038/sj.emboj.7600397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fredriksson L, Ehnman M, Fieber C, Eriksson U. Structural requirements for activation of latent platelet-derived growth factor CC by tissue plasminogen activator. J Biol Chem. 2005;280:26856–26862. doi: 10.1074/jbc.M503388200. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez-Gonzalez R, Blanco M, Rodriguez-Yáñes M, Moldes O, Castillo J, Sobrino T. Platelet derived growth factor-CC isoform is associated with haemorrhagic transformation in ischaemic stroke patients treated with plasminogen activator. Atherosclerosis. 2013;226:165–171. doi: 10.1016/j.atherosclerosis.2012.10.072. [DOI] [PubMed] [Google Scholar]
- 14.Merali Z, Leung J, Mikulis D, Silver F, Kassner A. Longitudinal assessment of Imatinib’s effect on the blood-brain barrier after ischemia/reperfusion injury with permeability MRI. Translational Stroke Research. 2015;6:39–49. doi: 10.1007/s12975-014-0358-6. [DOI] [PubMed] [Google Scholar]
- 15.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischaemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 16.Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS) JAMA. 1995;274:1017–1025. [PubMed] [Google Scholar]
- 17.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 18.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischaemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 19.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase 3–4. 5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303–1309. doi: 10.1016/S0140-6736(08)61339-2. [DOI] [PubMed] [Google Scholar]
- 20.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP., Jr Expansion of the time window for treatment of acute ischaemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40:2945–2948. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 22.Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke – a meta-analysis of individual patients data from randomised trials. Lancet. 2014;384:1929–1935. doi: 10.1016/S0140-6736(14)60584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischaemic stroke. The New England Journal of Medicine. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 25.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischaemic stroke. The New England Journal of Medicine. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 26.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischaemic stroke with perfusion-imaging selection. The New England Journal of Medicine. 2015;372:1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 27.Saver JL, Goyal M, Bonafé A, et al. Stent-Retriever Thrombectomy after Intravenous t-PA vs. t-PA Alone in Stroke. New England Journal of Medicine. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]
- 28.Jovin TG, Chamorro Á, Cobo E, et al. Thrombectomy within 8 Hours after Symptom Onset in Ischaemic Stroke. New England Journal of Medicine. 2015;372:2296–2306. doi: 10.1056/NEJMoa1503780. [DOI] [PubMed] [Google Scholar]
- 29.Wahlgren N, Moreira T, Michel P, et al. Mechanical thrombectomy in acute ischaemic stroke: Consensus statement by ESO-Karolinska Stroke Update 2014/2015 by ESO, ESMINT, ESNR and EAN. International Journal of Stroke. 2016;11:134–147. doi: 10.1177/1747493015609778. [DOI] [PubMed] [Google Scholar]
- 30.Parente PMDC, Santos Silva JMC. Quantile Regression with Clustered Data. Journal of Econometric Methods. 2016;5:1–15. [Google Scholar]
- 31.Bottai M, Zhen H. Multiple imputation based on conditional quantile estimation. Epidemiol Biostat Pub Health. 2013;10:e8758–1. doi: 10.2427/8758. [DOI] [Google Scholar]
- 32.Lopes Pinheiro MA, Kooij G, Mizee MR, et al. Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke. Biochimica et biophysica acta. 2015;1862:461–471. doi: 10.1016/j.bbadis.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. New England Journal of Medicine. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]